?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Growing mixed-sex Nile tilapia, Oreochromis niloticus in earthen ponds to table size is a major challenge due to its early maturity and prolific breeding. This study determined the effects of two medicinal plants; Aspilia plant, Aspilia mossambicensis and Neem tree, Azadirachta indica on hatchlings production, growth performance, feed utilization, survival and haematology of O. niloticus. Experimental diets were prepared by adding 1.0, 2.0, 4.0 and 8.0 g of either A. mossambicensis or A. indica leaf powders into a kg of the control diet subsequently administered daily to twenty triplicates of O. niloticus for three months. Both A. mossambicensis and A. indica leaf powder at the used doses, reduced significantly hatchlings production of O. niloticus when compared to the control (P < .05). The lowest value of hatchlings count was found in A. indica dose 8.0 g kg−1 (P < .05). The use of A. mossambicensis leaf powder at a dose of 4.0 g kg−1 improved significantly growth performance and feed utilization (P < .05). In contrast, survival rate was not affected significantly by the two plants (P > .05). Both plants differentially increased significantly haematological parameters such as Hb concentration, packed cell volume (PCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), white blood cells (WBC), monocyte and lymphocytes while reduced significantly neutrophils and eosinophils (P < .05). In conclusion, A. mossambicensis and A. indica leaf powders control prolific breeding of O. niloticus, modulate its growth performance and feed utilization. The two plants also modulate haematological parameters of O. niloticus indicating immunological response towards stress or intoxication, however, the values obtained were not beyond the recommended range for healthy fish.
1 Introduction
Nile tilapia, Oreochromis niloticus is one of the most popular freshwater fish species for aquaculture worldwide. Its suitability for culture is attributed by its neutral taste, ability to tolerate a wide range of environmental conditions and utilization of food from the lowest trophic level [Citation1]. However, growing mixed-sex O. niloticus in ponds to table size is a major challenge due to its early maturity and prolific breeding [Citation2–Citation4]. Consequently, ponds become overpopulated with O. niloticus of varying sizes which makes management aspects such as feeding and water quality difficult to perform because of size-dependent requirements. Accordingly, water quality deteriorates, competition for food and space increases and O. niloticus diverts energy towards reproduction causing slow growth [Citation5,Citation6]. Synthetic hormones have been used as the popular and favoured techniques in order to overcome its early maturity and prolific breeding [Citation5]. However, their higher cost in addition to their environmental and human health concerns, limit their use [Citation7,Citation8]. A number of medicinal plants have been explored as natural remedy, safe and affordable alternatives to control prolific breeding of O. niloticus [Citation7,Citation9,Citation10].
Aspilia mossambicensis also known as Wild sunflower is a medicinal plant which belongs to the family Compositae (Asteraceae) within the genus Aspilia. It is widespread in central and Eastern tropical Africa from Ethiopia, through East Africa, the Congo, Zambia, Zimbabwe, Malawi, Mozambique and Transvaal to Natal [Citation11]. In Tanzania, the plant is found along Lake Victoria [Citation12], Kigoma and Tanga Regions [Citation13]. Based on its medicinal properties, A. mossambicensis is used by herbalists and local people to treat several ailments including malaria, bacterial infection and human immunodeficiency virus (HIV) [Citation12,Citation14,Citation15]. The plant is also known to alleviate menstrual cramps as well as uterotonic agent capable of inducing uterine contraction and labour in pregnant women [Citation12,Citation16].
On the other hand, Azadirachta indica popularly known as “Neem tree” is a member of the mahogany family, called Meliaceae, which is a broad-leaved evergreen plant that grows up to 30 m tall and 2.5 m girth [Citation17]. It is native to Burma, Nigeria, India and Pakistan, growing in tropical and semi-tropical regions [Citation18]. In East Africa it is also known as ‘the plant of the 40’ because it has been suggested to treat at least 40 different diseases [Citation18]. A. indica is known to have medicinal properties such as antimicrobial, anti-inflammatory, antipyretic, spermicidal effect, immuno-contraceptive, anti-fertility activity and abortificient [Citation19,Citation20]. Based on their medicinal properties, the two plants have the potential to control prolific breeding of O. niloticus.
Studies conducted on A. mossambicensis are limited to domestic animals such cattle and goats [Citation21] where it has been shown to stimulate growth. Furthermore, A. mossambicensis was reported to improve survival, weight gain and immunological parameters in HIV patients [Citation14]. To date, the ability of A. mossambicensis in controlling prolific breeding and its effects on growth performance and haematological parameters of fish are unknown. On the other hand, A. indica have been subjected to extensive research in various animal species based on its medicinal properties. However, most studies on A. indica used extracts which require technical know-how during their preparation beyond the reach of most fish farmers at large scale production [Citation22–Citation25]. It is known that, medicinal plants modulate physiological functioning of fish in a positive or negative way depending on the type of the plant and dose administered [Citation26]. Higher growth performance, survival rate of cultured animals and feed utilization are primary goals of fish farmers. Moreover, haematological evaluation is useful in monitoring the health status of fish [Citation27].
This study was therefore conducted to determine the effect of various doses of A. mossambicensis and A. indica leaf powders on hatchlings production, growth performance, feed utilization, survival rate and haematological parameters of O. niloticus.
2 Materials and methods
2.1 Ethical statement
The study was carried out in accordance with the Tanzanian laws and Sokoine University of Agriculture guidelines for the care of experimental animals. All procedures of the current work were approved by the Committee of the College of Agriculture of the Sokoine University of Agriculture (SUA).
2.2 Experimental fish and their management
Juvenile O. niloticus males and females weighing between 30 and 50 g (mean weight 41.5 ± 3.1 g) were collected from SUA ponds located in Morogoro region, Tanzania. The fish were acclimatized for two weeks before the start of the experiment. After the acclimatization period, three replicates of 20 fish (10 females and 10 males) were stocked and raised in 3.6 m3 experimental tanks for three months. Each culture tank was supplied with 2700 L clean water with optimum quality of dissolved oxygen, pH and water temperature recommended for O. niloticus farming [Citation28]. Water quality parameters were monitored on a daily basis and in each tank, a complete replacement of water was done once every week. Dissolved oxygen, pH and temperature during the entire study ranged from 6.0–7.8 mg L−1, 8.0–8.4 and 26.7–27.2 °C, respectively.
2.3 Plants collection and preparations
The plant leaves were collected based on ethno-botanical knowledge using available literature, visual observations and identification by a botanist according to guidelines by Smith [Citation13] and Styles and White [Citation29]. The leaves of A. mossambicensis were collected from Magamba village located at Lushoto district in Tanga region whereas A. indica leaves were collected from Morogoro municipal. The collected leaves were thoroughly washed and shade dried in a dry room at room temperature for two weeks. The dried leaves were ground into fine powders by using a Lab Mill (Serial number 19911, Christy Hunt Engineering, LTD, England) fitted with 1.0 mm screen. The powders were then kept in dry containers and stored at room temperature pending feeds formulations.
2.4 Feed formulation and feeding regimes
The control diet (250 crude protein g kg−1) was formulated using Pearson’s square by including 300 g kg−1 fishmeal (sardines) and 700 g kg−1 maize bran. Eight experimental diets were formulated by adding 1.0, 2.0, 4.0 and 8.0 g of either A. mossambicensis (AM1, AM2, AM4 & AM8, respectively) or A. indica (AI1, AI2, AI4, and AI8, respectively) to a kilogram of the control diet. Proximate composition of the control diet and plants used is the present study are given in . The diets prepared were fed to fish twice a day (10.00 and 17.00 h) at a rate of 3% body weight per day for three months.
Table 1 Proximate composition (dry weight basis) of control diet, Aspilia mossambicensis and Azadirachta indica (g kg−1).
2.5 Hatchlings count
After every two weeks, number of hatchlings produced by O. niloticus was counted from each experimental tank and hatchlings count (HC) was recorded as described before[Citation30].
2.6 Fish growth performance, feed utilization and percentage survival
All O. niloticus were weighed and their individual initial weights (g) recorded to the nearest 0.01 g by using a sensitive weighing balance before stocking in the tanks. Subsequent weighing of O. niloticus individuals was conducted every 14 days by scooping out the fish using a scoop net and their weights determined as described before. Feed rations were adjusted based on fish body weight obtained after every two weeks. After 90 days of culture, all O. niloticus were removed, counted for final mean body weight (FMW) and percentage survival determination. Growth performance (specific growth rate; SGR, weight gain; WG, and daily weight gain; DWG), feed utilization (feed conversion ratio; FCR and feed conversion efficiency; FCE) and percentage survival (Sr) were calculated at the end of the experiment using the following formulae according to Hopkins [Citation31] and Silva and Anderson [Citation32].
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4)
(5)
(5)
(6)
(6)
2.7 Fish haematological parameters
Blood was collected from caudal vein and then directly transferred into the ethylenediaminetetraacetic acid (EDTA) coated bottles. Packed cell volume (PCV) was determined by micro-haematocrit reader (Sigma 201-M) after centrifuging at 3000 r.p.m. Red blood cells (RBC), white blood cells (WBC) and haemoglobin concentration (Hb) were determined following standard procedures as described by Ispır et al. [Citation33] and Gabriel et al. [Citation34]. The Hb, PCV and RBC values were used to calculate MCV, MCHC and MCHC indices using the following formulae:
(7)
(7)
(8)
(8)
(9)
(9)
Differential count of leucocytes (neutrophils, lymphocytes, eosinophils, monocytes, basophils) were determined under the light microscope (Olympus BH-2)) at 100× magnification [Citation33,Citation35].
2.8 Statistical analysis
Results are presented as means ± standard deviation and data were tested for normality and homogeneity of variances using Kolmogorov-Sminorv and Levene’s tests, respectively. Thereafter, one-way analysis of variance (ANOVA) was used to test for significant differences in the growth performance, feed utilization, survival and haematological parameters measured among the different diets for each medicinal plant. When significant differences were detected, Tukey’s post hoc test was performed to determine specific differences among treatments. Pearson correlation was used to establish the relationship between hatclings production and plant doses and haematological parameters and plants doses. All statistical analyses were performed using SPSS version 20 (IBM, Armonk, NY, USA) for Windows. Results with P ≤ 0.05 were considered statistically significant for all tests except some Pearson correlations results which used P ≤ 0.010.
3 Results
3.1 Fish hatchlings production
The results on hatchlings production of O. niloticus post-exposure to various doses of A. mossambicensis and A. indica indicated variations between the two plants and among doses (). Both A. mossambicensis and A. indica leaf powders reduced significantly hatchlings production of O. niloticus when compared to the control (P < .05; ). The O. niloticus fed on A. indica (2.0–8.0 g kg−1) had significantly reduced hatchlings production than those fed on A. mossambicensis (2.0–8.0 g kg−1) (P < .05). The O. niloticus fed A. indica at higher dose (8.0 g kg−1) had significantly less values of hatchlings production than those fed the lower dose (1.0 g kg−1). In contrast, O. niloticus fed the lower and higher doses of A. mossambicensis leaf powder had similar hatchlings production (P > .05). The lowest values of hatchlings production (62.50), was found in O. niloticus fed 8.0 g kg−1 A. indica. The hatchlings production decreased significantly as the doses increased for O. niloticus supplemented with A. indica (r = −0.566, P < .05) while they decreased insignificantly for those fed on A. mossambicensis (r = −0.389, P > .05).
Table 2 Hatchlings production for Oreochromis niloticus fed Asipilia mossambicensis (AM) and Azadirachta indica (AI) at different doses.
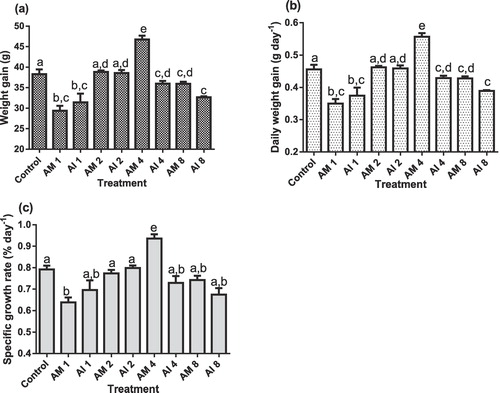
3.2 Fish growth performance
The results on growth performance of O. niloticus post-exposure to various doses of A. mossambicensis and A. indica indicated variations between the two plants and doses as depicted in (a–c). Feeding A. mossambicensis and A. indica at the lower (1.0 g kg−1) and higher (8 g kg−1) doses reduced significantly WG, SGR, DWG of treated O. niloticus than the control (P < .05). Significantly higher WG, DWG and SGR (46.8 g, 0.56 gday−1 and 0.84 %day−1, respectively) of O. niloticus were obtained at a dose of 4.0 g kg−1 A. mossambicensis when compared to the remaining groups fed both plants and the control (P < .05). Moreover, O. niloticus fed on A. indica diet containing 2 g kg−1 (AI2) had significantly higher growth performance compared to those fed on AI1 and AI8 (P < .05).
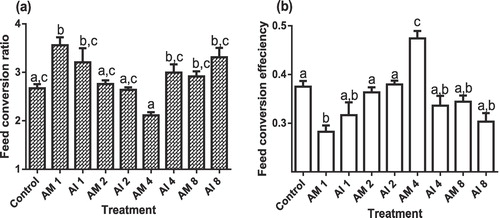
3.3 Feed utilization
Results for FCR and FCE of O. niloticus administered various doses of A. mossambicensis and A. indica indicated plant and dose specific effects as shown in (a and b). Inclusion of A. mossambicensis and A. indica at 1.0 g kg−1 and 8.0 g kg−1 doses, respectively, increased significantly FCR of O. niloticus compared to control (P < .05). A. mossambicensis at an inclusion level of 4.0 g kg−1 reduced significantly FCR (2.11) value when compared to > 2.50 obtained from AM1, AI1, AI4, AM8 and AI8 (P < .05). In contrast, A. mossambicensis at an inclusion level of 4.0 g kg−1 revealed the highest FCE (0.47) of all the other doses for both plants and the control group.
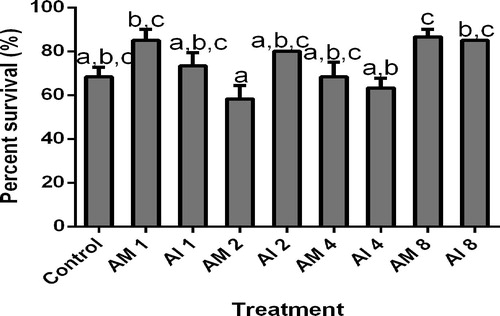
3.4 Survival (%)
Similarly, plant and dose specific effects were obtained on percentage survival (). Both plants did not significantly affect the percentage survival when compared to control (P > .05). However significant differences were obtained among different doses in the treated groups. Significantly higher percentage survivals were found at higher doses (8.0 g kg−1) for A. mossambicensis (86.7%) and A. indica (85%) when compared to the lower survival (58.3%) obtained from fish fed A. mossambicensis at a lower dose of 2.0 g kg−1 (P < .05).
3.5 Haematological parameters
The results on haematological parameters of O. niloticus fed various doses of A. mossambicensis and A. indica indicated immunological modulation (). The Hb concentration, PCV, MCH, MCHC and WBC of O. niloticus fed A. mossambicensis and A. indica were significantly higher than control (P < .05). The O. niloticus fed the diets supplemented with both medicinal plants containing doses ranging from 2.0 g kg−1 to 8.0 g kg−1 recorded the highest levels of PCV, MCH, MCHC and WBC. In addition, the O. niloticus fed A. mossambicensis at 4.0 g kg−1 (AM4) and 8.0 g kg−1 (AM8) inclusion levels had significantly higher MCV values than control (P < .05). Moreover, Hb, PCV, MCV and MCH of O. niloticus fed A. mossambicensis had slightly higher values than A. indica supplemented ones at similar doses. The RBC values were generally statistically similar among all O. niloticus fed the two plants and the control group (P > .05).
Table 3 Haematological parameters of Oreochromis niloticus fed on Aspilia mossambicensis and Azadirachta indica doses during the study period.
Differential leukocyte count for O. niloticus fed A. mossambicensis and A. indica doses revealed significant increase in the percentage of monocytes when compared to the control (P < .05). However, neutrophils for both plants and eosinophils of A. indica decreased significantly when compared to the control (P < .05). The highest monocyte values (17.5 and 16.5%) were found in O. niloticus fed 4.0 g kg−1 A. indica and A. mossambicensis, respectively. In contrast, the highest value (31%) for neutrophils was noticed in O. niloticus fed the control diet whereas the lowest values (18.5 and 16.5%) were obtained in O. niloticus fed the higher doses (8.0 g kg−1) for both A. indica and A. mossambicensis, respectively. Conversely, eosinophils values were generally low in O. niloticus fed the control diet (1.5%) and those fed on diets supplemented with 1.0 and 4.0 g kg−1 A. mossambicensis (1.0%). Eosinophils were below detection level in any of O. niloticus fed the A. indica diets. The results indicated higher levels of lymphocytes (62–68%) followed by neutrophils (16.5–22.5%), monocytes (11.5–17.5%) and eosinophils (1.0–1.5%).
3.6 Relationship between haematological parameters and the plants doses
The results on correlation between haematological parameters and the plant doses are shown in . The O. niloticus Hb concentration (r = 0.684 and r = 0.834), PCV (r = 0.727 and r = 0.752) and monocyte (r = 0.923 and r = 0.737) increased positively as the doses of the plants increased for A. indica and A. mossambicensis, respectively (P < .05), while only WBC (r = 0.548), MCV (r = 0.609) and lymphocytes (r = 0.686) were positively correlated for O. niloticus supplemented with A. mossambicensis (P < .010 except WBC, P < 0.05). On contrary, neutrophils (r = −0.735 and r = −0.775) and eosinophils (r = −0.663 and r = −0.629) decreased negatively as the doses increased for O. niloticus supplemented with A. indica and A. mossambicensis (P < .05), respectively.
Table 4 Correlation between Aspilia mossambicensis and Azadirachta indica doses and haematological parameters of Oreochromis niloticus.
4 Discussion
The present study intended to determine the effects of A. mossambicensis and A. indica leaf powders on some physiological parameters of O. niloticus when used to control its prolific breeding. The two plants were able to control prolific breeding partially by reducing significantly hatchlings production of O. niloticus. Similar reduction in the number of hatchings has also been obtained after feeding O. niloticus with A. indica ethanol extracts [Citation10] and A. indica saponin [Citation36]. Contrary to the findings obtained from this study, the former and the latter noticed completely inhibition of hatchlings production using doses between 1.0 g kg−1 and 4.0 g kg−1 of A. indica leaf extract and A. indica saponins, respectively. In this study, hatchlings production was not completely inhibited even at the highest dose (8.0 g kg−1). This deviation can be explained by the differences in the forms of A. indica leaves experimented. In this study, leaf powder was used without any further purification whereas in previous studies extracts and pure phytocompound (saponin) were used. Reduction of hatchlings is possibly because of antifertility phytocompounds such as saponins, flavonoids and alkaloids present in these plants [Citation12,Citation37]. The results also revealed that A. indica was more effective in controlling prolific breeding of O. niloticus, presumably due to its spermicidal effect [Citation20,Citation38] which has lead the plant to be used for contraceptive purposes in variety forms including pills, vaginal foam or creams [Citation39]. In general, farmers can use the two plants to partially control prolific breeding depending on availability. However, A. indica controls prolific breeding more effective than A. mossambicensis at similar doses.
Results revealed significant dose-specific effects of the two plants on growth performance, feed utilization and feed conversion efficiency. Low WG, SGR, DWG, FCE and high FCR values which is an indication of slow growth and low feed utilization were noticed in O. niloticus fed the lower and higher doses of A. mossambicensis and A. indica. These results are in agreement with those obtained by Obaroh and Nzeh [Citation24] which revealed negative effect of A. indica (crude extract) on O. niloticus WG, SGR and FCR at higher doses (4.0 and 8.0 g kg−1). Similar findings were also reported by Jegede and Fagbenro [Citation40] in Tilapia zilii after exposure to A. indica leaf powder doses (1.0–2.0 g kg−1). Furthermore, dose dependent retardation of growth in T. zilii was shown by Omoregie and Okpanachi [Citation41] following exposure to variety doses of A. indica (0.78 and 1.56 mg L−1) bark crude extract. In contrast, saponin extracted from A. indica did not show any negative effect on O. niloticus growth performance and feed utilization even at higher doses of 4.0 and 8.0 g kg−1 [Citation5].
Findings from this study demonstrate that the two plants influence growth performance differently depending on the type of the plant and dose used. Better growth performance and feed utilization were obtained in O. niloticus supplemented with A. mossambicensis compared to A. indica groups. Previous studies conducted on A. mossambicensis are restricted to domestic animals and human being [Citation14,Citation21]. The use of A. mossambicensis has been shown to influence positively growth performance in terrestrial animals such as cattle and goats [Citation21]. Based on its growth enhancement effects and medicinal properties, farmers from Kenya preferred using it as a fodder crop for livestock. On the contrary, it has been documented that A. indica caused depressed growth performance in cattle [Citation20]. The differences might be contributed by variations in protein content between the two plants. Proximate analysis of the plants in the present study showed that A. mossambicensis has higher crude protein compared to A. indica (). Furthermore, A. mossambicensis prepared by herbalists in Tanzania against HIV patients was found to improve survival and weight gain in the treated patients [Citation14]. These results imply that O. niloticus farmers interested in controlling prolific breeding using the two plants should use dosages below 4 g kg−1 and 2 g kg−1 for A. mossambicensis and A. indica, respectively.
The two plants increased significantly haematological values for Hb, PCV, MCH, MCHC, WBC, lymphocytes and monocytes, but did not affect RBC. The significant increase in Hb and PCV obtained in the present study is in agreement with the findings reported by Obaroh et al. [Citation42] after feeding O. niloticus with diets containing Mangifera indica doses (0.5–8 mg kg−1). Similarly, Gabriel et al. [Citation34] found no significant effect of Aloe vera supplemented diets on O. niloticus RBC. However, the results obtained in the present study are contrary to those obtained by Fafioye [Citation23] on O. niloticus fed on A. indica doses (0.1–0.5 g L−1) which revealed significant decrease in Hb concentration, RBC, PCV and MCH values. Similarly, Saravanan et al. [Citation22] reported significant decrease in Hb, PCV, MCV, MCH and MCHC values from C. mrigala after exposure to A. indica (1.0 g L−1). In addition, sharp decrease in PCV was noticed in O. niloticus subjected to higher doses (> 1.2 g L−1) of A. indica water extract [Citation43].
Haematological parameters such as Hb, PCV, MCV, MCH and MCHC are particularly known to indicate erythrocyte status and oxygen carrying capability in fish [Citation34]. Therefore, the increased levels in these parameters indicate the stimulation of erythropoiesis, hence increasing the capacity of oxygen transport and strengthening the defense mechanisms against physiological stress [Citation34]. Generally, these results imply that feeding O. niloticus with diets containing A. mossambicensis and A. indica at the dosage used improve immune system because most values of haematological parameters obtained from this study are within the ranges for healthy O. niloticus cultured under semi intensive system as described by Bittencourt et al. [Citation44]. The ranges described by Bitterncourt et al. [Citation44] are 0.7–28 × 106 Μl−1mm−3, 6.58–15.98 g dL−1 and 15–45% for RBC, Hb and PCV, respectively. In addition, Clauss et al. [Citation45] also showed that, the accepted range of PCV for fish is between 20 and 45%. Fish with PCV values above 45% and below 20% are considered to have polycythaemia as a result of dehydration and anaemia, respectively. Most of the PCV values in the present study were above 20% and below 45% indicating the fish were in good health. However, the surprising increase in most RBC indices while RBC values were the same among treatments suggest the possibility of regenerative anaemia. This condition occurs when demand for RBC is higher such that reticulocytes are prematurely released from the bone marrow into circulation [Citation46].
This study indicated increase in WBC of O. niloticus in the two plants for most doses. Significant increase in WBC of O. niloticus supplemented with the highest dose of A. mossambicensis concurs with the findings obtained by Saravanan et al. [Citation22] in Cirrhinus mrigala after the exposure to A. indica leaf extract at various doses (0.25–1.50 mg L−1). The increase in WBC is a defensive mechanism of O. niloticus due to inclusion of the medicinal plants in its diets [Citation25,Citation47,Citation48]. The body of O. niloticus treated with the medicinal plants stimulated the immune system which reacted by producing disease or foreign particles fighting cells.
The results further showed that, both plants increased the percentage of lymphocytes and monocytes while they decreased neutrophils and eosinophils in a dose-dependent manner. The higher percentage of lymphocytes followed by neutrophils, monocytes and eosinophils obtained from this study concurs with findings reported by Gabriel et al. [Citation49] and Martins et al. [Citation50] in O. niloticus. Increase in monocytes and lymphocytes percentages from O. niloticus treated with A. mossambicensis and A. indica is in agreement with findings obtained by Martins et al. [Citation50] after subjecting fish to stressors. In general, this study revealed high percentage of monocytes compared to previous studies on O. niloticus while testing variety of medicinal plants [Citation34,Citation51] presumably due to variations in medicinal properties of the different plants used. The monocytes increased significantly as the doses increased for both plants.
The increase in lymphocytes and monocytes and the decrease in neutrophils and eosinophils are due to immunological modulation by the two medicinal plants. In the present study, the levels of neutrophils and eosinophils decreased significantly as the doses increased for both medicinal plants. Differential count of leukocytes is very essential in vertebrates including fishes because each of the five leucocytes (lymphocyte, monocyte, neutrophil, eosinophil and basophil) has a specific immunological function. Neutrophils are primary phagocytes in response to disease, stress or inflammation whereas lymphocyte has immunological functions including the production of immunoglobulin and modulation of immune defense. Eosinophils respond to inflammation process and defense against parasites. Monocytes also known as phagocytic cells are responsible for defense against infections and bacteria [Citation52]. Accordingly, in normal conditions each group exists at a certain percentage, however, they can be altered in response to infection, disease, toxic substances or stress [Citation35]. Ultimately, increase in lymphocytes and monocytes indicates that the cells in the treated groups were protecting the body of O. niloticus from introduced foreign particles of the medicinal plants contained in the diets. This was confirmed by the decreased levels of neutrophils and eosinophils which indicate stress, intoxication or over production of certain specific steroids in the body, such as cortisol, due to the use of the two medicinal plants. The results on differential count of leucocytes suggest immunological modulation of O. niloticus due to application of the two medicinal plants. However, the two medicinal plants did not alter the immunity beyond the normal healthy ranges. According to Davis et al. [Citation52] neutrophils and lymphocytes account for about 80% of total leucocytes whereas monocytes, eosinophil and basophils fall in the remaining 20%. These ranges were similar to the values obtained in this study.
The existence of significant positive correlation in WBC, MCV and lymphocytes for only O. niloticus treated with A. mossambicensis indicates immunomodulation stimulation differences between the two medicinal plants. Apparently, A. mossambicensis has higher immune modulation effects than A. indica at similar inclusion levels. Possibly, this might be the reason for the faster growth performance and enhanced feed utilization obtained for O. niloticus supplemented with A. mossambicensis, an observation which requires further studies.
5 Conclusions
The findings from this study indicate that, both A. mossambicensis and A. indica leaf powders controlled partially prolific breeding of O. niloticus. In general, A. indica is more effective in controlling prolific breeding when compared to A. mossambicensis. The two plants also revealed plant-specific modulation on fish growth performance and feed utilization. Based on the present experimental conditions, for better growth and feed utilization of O. niloticus, the dose inclusion limit should be 2.0 g kg−1 and 4.0 g kg−1 for A. indica and A. mossambicensis, respectively. Haematological findings indicate that the two medicinal plants improved immune system as a response towards stress, intoxication or over production of certain specific steroids but was within the body cells to counteract. In general, for most measured parameters, higher values were recorded for fish fed on diets supplemented with A. mossambicensis compared to those fed on A. indica indicating plant-specific effects. To our knowledge, this is the first study reporting the effects of A. mossambicensis on growth performance and haematological parameters in fish.
Competing interests
There is no conflict of interest to declare.
Acknowledgement
The Tanzania Commission for Science and Technology (COSTECH) is acknowledged for sponsoring this study as part of PhD research.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
Part of abstract of the manuscript was published at https://www.was.org/meetings/ShowAbstract.aspx?Id=47248 during the World Aquaculture 2017 – Meeting Abstract as part of the conference proceedings.
References
- V.SureshR.C.BhujelTilapiasJ.S.LucasP.C.SouthgateAquaculture: Farming Aquatic Animals and Plants2012Wiley-Blackwell Publishing CompanyUnited Kingdom338364
- A.P.ShokoS.M.LimbuH.D.J.MrossoY.D.MgayaReproductive biology of female Nile tilapia Oreochromis niloticus (Linnaeus) reared in monoculture and polyculture with African sharptooth catfish Clarias gariepinus (Burchell)SpringerPlus42015275
- S.M.LimbuA.M.ShokoH.A.LamtaneE.D.ShirimaM.A.Kishe-MachumuH.F.MganaEffect of initial stocking size of the predatory African sharptooth catfish (Clarias gariepinus) on recruits, growth performance, survival and yield of mixed-sex Nile tilapia (Oreochromis niloticus) in concrete tank culture systemInt Aquat Res720156373
- M.MbiruS.M.LimbuS.W.ChenyambugaH.A.LamtaneR.TamatamahN.A.MadallaComparative performance of mixed-sex and hormonal-sex-reversed Nile tilapia Oreochromis niloticus and hybrids (Oreochromis niloticus × Oreochromis urolepis hornorum) cultured in concrete tanksAquacult Int242015557566
- I.ObarohG.NzehS.OguntoyeD.BawaGrowth response of Oreochromis niloticus (L) fed crude extract of Azadirachta indica saponinsIOSR J Pharm Biol Sci320144448
- K.CowardN.R.BromageSpawning frequency, fecundity, egg size and ovarian histology in groups of Tilapia zilli maintained upon two distinct food ration sizes from first-feeding to sexual maturityAquat Living Resour1219991122
- T.JegedeControl of reproduction in Oreochromis niloticus (Linnaeus 1758) using Hibiscus rosa-sinensis (Linn.) leaf meal as reproduction inhibitorJ Agr Sci22010149151
- T.StadtlanderB.Levavi-SivanZ.KeremK.DweikM.QutobS.Abu-LafiEffects of a saponin fraction extracted from Trigonella foenum-graecum L. and two commercially available saponins on sex ratio and gonad histology of Nile tilapa fry, Oreochromis niloticus (L.)J Appl Ichthyol292013265267
- F.F.KhalilF.H.FarragA.I.MehrimM.M.A.RefaeyPawpaw (Carica papaya) seeds powder in Nile tilapia (Oreochromis niloticus) diets: Liver status, sexual hormones and histological structure of the gonadsEgypt Aqua Biol Fish18201497113
- I.O.ObarohG.C.NzehAntifertility effect of some plant leaf extracts on the prolific breeding of Oreochromis niloticusAcad J Interdiscipl Stud220138794
- R.A.NortonD.Q.HuangE.RodriguezAspilia mossambicensis: In vitro propagation and production of antibiotic polyacetylenes by root culturesY.P.S.BajajMedicinal and aromatic plants V Biotechnology in agriculture and forestryvol. 241993SpringerBerlin, Heidelberg
- D.M.MusyimiJ.A.OgurP.M.MuemaPhytochemical compounds and antimicrobial activity of extracts of Aspilia plant (Aspilia mossambicensis) (Oliv) WildInt J Bot420085661
- A.R.SmithEuphorbiaceae (Part 1)R.M.PolhillFlora of Tropical East Africa1987Royal Botanic GardensEngland185199
- Elmar T. Herbal treatment for HIV-patients in Tanzania, In: Médicaments et aliments: l'approche ethnopharmacologique. Actes du 2e Colloque Européen d’Ethnopharmacologie et de la 11e Conference internationale d’Ethnomedecine, Heidelberg 24–27 mars; 1993. p. 167.
- M.J.MoshiD.F.OtienoP.K.MbabaziA.WeisheitThe Ethnomedicine of the Haya people of Bugabo ward, Kagera Region, north western TanzaniaJ Ethnobiol Ethnomed5200915
- C.GruberO’Brien M. Uterotonic plants and their bioactive constituentsPlanta Med772011207220
- N.D.NdodoJ.A.AnukaU.G.EsomonuJ.E.OnuR.U.OkoloC.OnwuchekwaThe effects of Neem (Azadirachta indica) leaves extracts, on some haematological indices of Wistar ratsIOSR J Pharm320132428
- D.A.SilayoH.R.KiwangoManagement of invasive plants in tropical forest ecosystems: Trials of control methods of Azadirachta indicaWorld Appl Sci J10201014141424
- G.PriyaK.SaravananC.RenukaMedicinal plants with potential antifertility activity- A review of sixteen years of herbal medicine research (1994–2010)Int J Pharmtech R42012481494
- K.BiswasI.ChattopadhyayB.K.BanerjeeU.BandyopadhyayBiological activities and medicinal properties of neem (Azadirachta indica)Curr Sci82200213361345
- R.L.RoothaertS.FranzelFarmers’ preferences and use of local fodder trees and shrubs in KenyaAgrofor Syst322001239252
- M.SaravananM.RameshA.MalarvizhiR.PetkamToxicity of Neem leaf extracts (Azadirachta indica A. Juss) on some haematological, ionoregulatory, biochemical and enzymological parameters of Indian major carp, Cirrhinus mrigalaJ Trop Forest Environ120111426
- O.O.FafioyeAcute and sub-acute toxicities of five plant extracts on white tilapia, Oreochromis niloticus (Trewavas)Int Res J Agricult Sci Soil Sci22012525530
- I.O.ObarohG.C.NzehEffect of crude extract of Azadirachta indica leaves at controlling prolific breeding in Oreochromis niloticusIndian J Agric Res52011277282
- A.D.TalpurM.IkhwanuddinAzadirachta indica (neem) leaf dietary effects on the immunity response and disease resistance of Asian seabass, Lates calcarifer challenged with Vibrio harveyiFish Shellfish Immunol342013254264
- G.PrasadS.MukthirajEffect of methanolic extract of Andrographis paniculata (Nees) on growth and haematology of Oreochromis mossambicus (Peters)World J Fish Mar Sci32011473479
- T.M.ClaussA.D.M.DoveJ.E.ArnoldHematologic disorders of fishVet Clin Exot Anim112008445462
- M.H.BahnasawyT.E.Abdel-BakyG.A.Abd-AllahGrowth performance of Nile tilapia (Oreochromis niloticus) fingerlings raised in an earthen pondArch Pol Fish112003277285
- B.T.StylesF.WhiteFlora for Tropical East AfricaR.M.PolhillMeliaceae1991CRC PressRoyal Botanic Gardens, Kew, UK116
- G.de GraafOptimization of the pond rearing of nile tilapia (Oreochromis niloticus niloticus L.): the impact of stunting processes and recruitment control2004Wageningen University179
- K.D.HopkinsReporting fish growth: A review of the basics1J World Aquacult Soc231992173179
- S.S.D.SilvaA.AndersonFish nutrition in aquaculture1995Chapman and HallLondon, UK319
- U.IspırM.E.YonarO.B.OzEffect of dietary vitamin E supplementation on the blood parameters of Nile tilapia (Oreochromis niloticus)J Anim Plant Sci212011566569
- N.N.GabrielJ.QiangJ.HeX.Y.MaM.D.KpundehP.XuDietary Aloe vera supplementation on growth performance, some haemato-biochemical parameters and disease resistance against Streptococcus iniae in tilapia (GIFT)Fish Shellfish Immunol442015504514
- R.Silveira-CoffignyaA.Prieto-TrujilloaF.Ascencio-VallebEffects of different stressors in haematological variables in cultured Oreochromis aureus SComp Biochem Physiol C Toxicol Pharmacol1392004245250
- I.O.ObarohG.C.NzehS.O.OguntoyeControl of reproduction in Oreochromis niloticus (L) using crude extract of Azadirachta indica saponinAdv Environ Biol6201213531356
- J.L.Harry-AsobaraOkon S.Eno-ObongComparative study of the phytochemical properties of Jatropha curcas and Azadirachta indica plant extractsJ Poisonous Med Plants Res2201402024
- G.PriyaK.SaravananC.RenukaMedicinal plants with potential antifertility activity-A review of sixteen years of herbal medicine research (1994–2010)Int J Pharm Tech Res42012481494
- K.BalaM.AryaD.P.KatareHerbal contraceptive: an overviewWorld J Pharm Pharm Sci320143051326
- Jegede T, Fagbenro O. Dietary neem (Azadirachta indica) leaf meal as reproduction inhibitor in redbelly tilapia, Tilapia zillii. In: 8th International symposium on tilapia in aquaculture 2008; 2008.
- E.OmoregieM.A.OkpanachiGrowth of Tilapia zilli exposed to sublethal concentrations of crude extracts of Azadirachta indicaActa Hydrobiol Sin341992281286
- I.O.ObarohJ.N.KetaD.Y.BawaHaematological indices of Oroechromis niloticus fed crude extract Mangifera indica leafEuropean J Biotechnol Biosci220141520
- E.Oyoo-OkothC.C.NgugiV.Chepkirui-BoitPhysiological and biochemical responses of Nile tilapia (Oreochromis niloticus) exposed to aqueous extracts of Neem (Azadirachta indica)J Appl Aquacult232011177186
- N.L.R.BittencourtL.M.MolinariD.O.ScoarisR.B.PedrosoC.V.NakamuraT.U.NakamuraHaematological and biochemical values for Nile tilapia, Oreochromis niloticus cultured in semi-intensive systemActa Sci Anim Sci252003385389
- T.M.ClaussA.D.M.DoveJ.E.ArnoldHematologic disorders of fishVet Clin North Am Exot Anim Pract112008445462
- Alemu Y, Atomsa A, Sahlemariam Z. Haematology, ed. Jimma University in collaboration with the Ethiopia Public Health Training Initiative TCC, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Ethiopia: Ethiopia Public Health Training Initiative; 2006. p. 541.
- M.L.MartinsJ.L.P.MouriñoG.V.AmaralF.N.VieiraG.DottaA.M.B.JatobáHaematological changes in Nile tilapia experimentally infected with Enterococcus spBraz J Biol682008657661
- G.PandeyM.SharmaY.P.SahniBeneficial effects of certain herbal supplements on the health and disease resistance of fishNovel Sci Int J Pharm Sci12012497500
- U.U.GabrielO.A.AkinrotimiF.EseimokumoHaematological responses of wild Nile tilapia (Oreochromis niloticus) after acclimation to captivityJordan Biol Sci42011225230
- M.L.MartinsD.T.NomuraD.M.Y.MyiazakiF.PilarskyK.RibeiroM.P.de CastroPhysiological and haematological response of Oreochromis niloticus (Osteichthyes: Cichlidae) exposed to single and consecutive stress of captureActa Sci Anim Sci262004449456
- G.DottaA.BrumG.T.JeronimoM.MaraschinM.L.MartinsEffect of dietary supplementation with propolis and Aloe barbadensis extracts on hematological parameters and parasitism in Nile tilapiaBraz J Vet Parasitol2420156671
- A.K.DavisD.L.ManeyJ.C.MaerzThe use of leukocyte profiles to measure stress in vertebrates: A review for ecologistsFunct Ecol222008760772