Abstract
Indiscriminate use of organophosphate acaricides especially among livestock and dog owners in the control of ticks and other ectoparasites has taken a worrisome dimension. In the present study, we investigated, the effects of acute dermal exposure in the form of acaricides baths of coumaphos at different concentrations on the haematology, blood pressure and liver functions in local mongrel dogs.
Twenty-four, male mongrel dogs of about 8 months of age with an average weight of 9.88 ± 0.4 kg were used for the study. The dogs were divided into four groups consisting of six dogs per group. Group A (control) was bathed with ordinary water, while group B was bathed with the recommended concentration of 0.016% (160 ppm) Coumaphos in water. Groups C and D were bathed with 10 and 20 times the recommended dose (1600 ppm and 3200 ppm), respectively.
Significant leucopenia, increased plasma urea and decreased low density lipoprotein (LDL) values were observed at 8 h post exposure, which worsened with time. At 24 and 36 hrs post exposure, normochromic normocytic anaemia, pan leucopenia, bloody diarrhoea, retching, vomiting and paddling were observed in affected animals. Post mortem examination revealed severe lungs, liver and stomach congestion. Multifocal areas of necrosis in the liver and kidney, serosal and mucosal haemorrhages and haemorrhagic meningitis were also observed.
The use of excessively high concentration of organophosphate as acaricides bath is associated with severe anticholinesterase poisoning, which may result in death of affected animals.
1 Introduction
Indiscriminate and incessant use of organophosphates in the developing world in the control of ectoparasites in domestic animals has been on the rise. It has also been associated with majority of self-poisoning cases in humans with a case fatality rate of about 15% in developing countries worldwide [Citation1]. Many of them, from this writer’s personal observation, are even marketed in bits and pieces on the road, local markets and in street corners. They are then used as baits for killing of large animals without recourse to the toxic effects of these insecticides on the final user of the baited animal [Citation2], nor consider the effects of these chemicals when they find their ways into the environment, especially when they seep into water bodies, thereby resulting in bioaccumulation in fish and other aquatic organisms with its attendant effects on final consumers of the aquatic products [Citation3]. Although the use of organophosphate and other acaricides is a cheap and effective method of control of arthropod vectors on livestock and companion animals including dogs and cats, their use is nonetheless associated with considerable signs of toxicity and eventual death in affected animals [Citation4].
Organophosphate insecticides act by inhibition of acetyl cholinesterase at the synaptic junction, thereby resulting in the accumulation of acetylcholine and subsequent prolongation of the muscarinic and nicotinic effects of acetylcholine in the autonomic nervous system, CNS and in neuromuscular junctions [Citation5]. They have also been reported to cause neurotoxicity, even at concentrations below those capable of inhibiting acetyl cholinesterase ((sub) micromolar concentrations) by concentration-dependent inhibition of depolarization-evoked Ca2+ influx at the synaptic bulb [Citation6].
The general clinical manifestation of organophosphate toxicity is well documented and summarized by Eddleston et al. [Citation1] in their review of management of acute organophosphates poisoning. They included signs of overstimulation of the muscarinic acetylcholine receptors in the parasympathetic nervous system such as bronchospasm, bronchorrhea, miosis, lachrymation, urination, diarrhoea, hypotension, bradycardia, vomiting and salivation. Overstimulation of nicotinic acetylcholine receptors in sympathetic nervous system on the other hand results in tachycardia, mydriasis, hypertension and sweating. In the CNS overstimulation of both nicotinic and muscarinic acetylcholine receptors results in confusion, agitation, coma or respiratory failure while muscle weakness, paralysis and fasciculations are the hallmark of excessive stimulation of nicotinic acetylcholine receptors at the neuromuscular junction [Citation7].
Toxicity of organophosphates is both dose and route dependent, but major study emphasis has been placed on oral ingestion and or inhalation in self and accidental poisoning cases [Citation8], with less or no attention on dermal exposure. In facts, several studies have been conducted on the toxic effects of ingestion of organophosphates alone or potentiated by other insecticides [Citation9,Citation10], but little studies have been reported in the literature on dermal exposure through acaricides baths, especially in the domestic dogs and cats. Yet, the tendency to overuse and the use of higher concentrations than normal is very high due to development of resistance to organophosphates including coumaphos [Citation11,Citation12].
Coumaphos is an acetyl cholinesterase inhibitor, also known as Asuntol, is a 3-chloro-7-diethoxyphosphinothioyloxy-4-methylchromen-2-one, a slightly brownish crystal with a slight sulfurous odor. It is used for the control of a wide variety of livestock insects including cattle grubs, lice, scabies, flies, and ticks; the common ectoparasites of sheep, goats, horse, swine, and poultry as well as for screw worms [Citation13]. It has CAS and EP numbers of 56-72-4 and 200-285-3 as identifier numbers on ChemIDPlus, EPADSStox and European Chemical Agency – ECHA (https://pubchem.ncbi.nlm.nih.gov/compound/coumaphos#section=Names-and-Identifiers). The present study was therefore designed to evaluate the effects of dermal exposure through acaricides control baths using Coumaphos at different concentrations and document its implication in the local dogs.
2 Materials and methods
2.1 Ethical approval
All international, national, and institutional guidelines for the care and use of animals were followed. The study was also approved by the Animal Care and Use and Research Ethics Committee of University of Ibadan, Ibadan, Nigeria.
2.2 Chemicals and reagents
All chemicals and reagents used are of analytical grade. Commercial reagent kits for the assay of alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferases (ALT), Creatinine phosphokinase (CPK), total cholesterol (TC), total protein (TP), triglyceride (TAG), and high-density lipoprotein (HDL), Low density lipoprotein (LDL), Urea, Creatinine were as supplied by Randox Diagnostics, Crumlin, United Kingdom.
2.3 Experimental animals
Twenty-four, eight months old local male mongrel breed of dogs were obtained from a local animal market in Ibadan, Oyo State, Nigeria. The dogs were housed in well-ventilated kennels at the Veterinary Teaching Hospital, University of Ibadan, Ibadan, Nigeria. They were allowed to acclimatize for a period of 14 days before the commencement of treatments. During this period, the animals were treated against endoparasites and ectoparasites with Ivermectin. The dogs also had free access to standard feed and clean water ad libitum and were handled in accordance with relevant institutional and ethical guidelines as approved for scientific study.
2.4 Experimental design
The twenty-four experimental animals were grouped into four groups consisting of six animals per group. All animals were examined and ensured that they had intact skin with no laceration, abrasion or any skin infection or lesion. The animals in group B, were bathed with 0.016% (160 ppm) coumaphos, which is the manufacturer’s recommended dose of coumaphos, in water while those in groups C and D were bathed with 10× (0.16%) and 20× (0.32%) the recommended doses, which corresponded to 1600 ppm and 3200 ppm, respectively, according to the report of Davey and George [Citation14] and personal field observation of excessive and indiscriminate use of coumaphos. Group A served as the control and was bathed with water only. The bath lasted for only 5 min and each animal was thoroughly rinsed with fresh water. The animals were then monitored individually for signs of toxicity.
2.5 Sample collection
Body temperature was taken before and after bath while blood samples for haematology were collected two hours after bath through the cephalic vein into Lithium heparinized tubes. Blood samples were then centrifuged at 5000g after which the plasma was collected from each sample and stored at −20 °C until when they were needed for further analysis. Blood samples were also collected from groups C and D at 36 and 24 hrs, respectively when signs of toxicity were observed in those dogs.
2.6 Blood pressure
Blood pressure was determined before and two hours after bath on each animal using oscillometric blood pressure monitor, Hartmann Tensoval Duo, Germany.
2.7 Haematology
Haematological parameters – packed cell volume (PCV), haemoglobin concentrations (Hb), red blood cell counts (RBC), white blood cell counts (WBC) and platelet counts as well as differential leucocytes counts were determined by Sysmex Kx-21 (Canada) automatic haematology analyzer. While haematimetric indices, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentrations (MCHC) were determined from the PCV, Hb and RBC values.
2.8 Plasma biochemistry
The levels of plasma total protein (TP) was determined by the Biuret method as described by Okutucu et al. [Citation15]. Plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lipid profile including total cholesterol (TC), triglyceride (TAG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were determined using Randox assay kits, Randox Diagnostics, Crumlin, United Kingdom. Plasma electrolytes including; sodium, potassium, chloride, and bicarbonate were determined by potentiometry method at 8, 24 and 36 hrs after exposure [Citation16].
2.9 Postmortem and histopathological examinations
All the five animals that died in the course of the study (after 3 days post exposure) were taken to the Department of Veterinary Pathology, Veterinary Teaching Hospital, University of Ibadan, for post mortem examination and collection of samples for histopathology.
Samples for histopathology, including liver, lungs, kidney, brain, intestines and skin sections were fixed in 10% neutral buffered formalin (containing 10% formalin in 0.08 M Sodium phosphate at pH 7.4). Samples were prepared for histology by fixation in 10% buffered formalin, dehydrated in graded alcohol and embedded in paraffin at 60 °C inside labeled embedding mold or cassettes. The embedded tissues were then sectioned using a microtome at 5 μm thickness and each section floated in 45 °C water bath to allow crinkled part to spread before being floated on the glass microscope slide for proper adherence unto the glass slide. The slides were stained with haematoxylin and counterstained with eosin using standard protocol. Slides were examined using Olympus BX 63 light microscope.
2.10 Statistical analysis
All data are presented as mean ± SD. Mean values were compared by One-way ANOVA, with Tukey’s post hoc test for multiple comparisons between groups, using GraphPad Prism statistical software, version 5.01 for Windows, GraphPad Software, San Diego California, USA (https://www.graphpad.com). A probability value of 0.05 and below was considered to be significant.
3 Results
3.1 Clinical signs
Following exposure of the treatment groups to coumaphos organophosphate bath, excessive cholinergic activities were observed in those animals in groups C and D that received 10× and 20× the normal concentration of coumaphos at 36 hrs and 24 hrs after bath, respectively. These observed signs included: vomiting, urination, salivation, defecation, gasping, dyspnea, shivering and rough tarry coats (). Gastrointestinal enteritis with bloody feaces and retching was observed in one of the animals exposed to 20× the normal coumaphos concentration 36 hrs after organophosphate bath. During this stage, the temperature fell to 36.2 °C. The blood pressure also showed signs of fluctuation at 132/110, 124/105 and 153/111. The animal was treated with atropine and the body temperature rose to 37 °C. Paddling was also observed in one of the animals in group D, with considerable fall in body temperature from 37.2 °C to as low as 34.8 °C, which was restored to 38.6 °C after treatment with atropine.
Table 1 Clinical signs shown by dogs 24 h after organophosphate bath with varying concentrations of Coumaphos.
3.2 Body temperature and blood pressure
As shown in , no significant change in body temperature before coumaphos bath and after was observed in all the treated animals. However, the systolic blood pressure (SBP) in group B that were bathed with normal concentration (0.016%) of coumaphos had significantly higher (P < 0.05) SBP than those bathed with 10x the normal concentration (group C). It was also higher than the unbathed control (group A) and those bathed with 20× the normal concentration (group D) although, non-significantly. The diastolic blood pressure (DBP) on the other hand showed considerable difference across the groups. For example, DBP value of 112.90 ± 27.49 recorded from the dogs in group B was significantly higher (P < 0.001) than the values of 75.0 ± 7.76, 77.20 ± 7.61 and 95.57 ± 15.49 recorded from groups A, C and D, respectively. Diastolic blood pressure in the unbathed control group (group A) was also found to be significantly lower (P < 0.001) than the values from both C and D groups.
Table 2 Body temperature and blood pressure of local mongrel dogs exposed to various concentrations of Coumaphos acaricides bath.
3.3 Haematology and Plasma biochemistry findings
shows the effects of varied concentrations of coumaphos organophosphate bath on the erythrocyte parameters in the local mongrel dogs, at 8 hrs after the coumaphos organophosphate bath. As shown in , the mean corpuscular volume (MCV) and mean corpuscular haemoglobin (MCH) values in those animals in group D were significantly lower than the control dogs that were not exposed to organophosphate bath. The mean PCV value was also slightly lower in this group, though non-significantly, than the PCV in the control group. Total red blood cell counts and haemoglobin concentrations were similar in the treated and untreated animals.
Table 3 Erythrocytic indices of local dogs at 8 hours following exposure to different concentrations of coumaphos organophosphate as acaricides bath.
But for the differential monocytes, eosinophil and basophil counts that were significantly lower (P < 0.001) in the treated groups than that of the control (), all other leucocyte parameters and platelet counts did not show any significant difference when compared with the control that were not exposed to organophosphate bath.
Table 4 Leucocyte and platelet parameters of local dogs at 8 hours following exposure to different concentrations of coumaphos organophosphate as acaricides bath.
As shown in , the plasma electrolytes and metabolites were also evaluated at 8 hrs after exposure to coumaphos as acaricides bath. However, only plasma urea concentration of the dogs in group D was significantly higher (P < 0.05) than that of the control dogs. The plasma sodium, potassium, bicarbonate and creatinine did not vary when compared across the groups. Low-density lipoprotein (LDL) values in those dogs that were in group D was significantly lower (P < 0.001) than the control dogs that were not bathed with the organophosphate. It was also significantly lower (P < 0.05) than those in group B that were bathed with the recommended coumaphos concentration of 0.016% and group C (P < 0.001) that were bathed with 10 times the recommended concentration of coumaphos ().
Table 5 Plasma electrolyte and metabolite values of local dogs at 8 hours following exposure to different concentration of organophosphate – coumaphos acaricides bath.
Table 6 Plasma protein, enzymes and lipoprotein values of local dogs at 8 hours following exposure to different concentration of organophosphate – coumaphos acaricides bath.
At 24 and 36 hrs after the organophosphate bath, animals in group D and C, respectively started to manifest signs of toxicity including retching, paddling and bloody diarrhoea. They were however treated with atropine sulphates. Blood samples were collected and the results are presented as Cx and Dx in –. Red blood cells, monocytes, eosinophil and basophil counts in the group D with signs of toxicity after 24 h (Dx) were significantly lower (P < 0.001) than those of the control group. Group C after 36 h with signs of toxicity (Cx) also had lower monocytes, eosinophil and basophil counts than those of the control.
Table 7 Erythrocytic indices of local dogs at 24 and 36 h after coumaphos organophosphate acaricides bath.
Table 8 Leucocyte parameters of local dogs at 24 and 36 h after coumaphos organophosphate acaricides bath.
As shown in , plasma urea in this (Cx) group was higher (P < 0.01) than those of the control. Plasma urea level in the Dx group also showed marginal increase after 24 h, although not statistically significant. The low-density lipoprotein (LDL) level was also significantly lower (P < 0.001) in Dx than in the control ().
Table 9 Plasma electrolyte and metabolite parameters of local dogs at 24 and 36 h after coumaphos organophosphate acaricides bath.
Table 10 Plasma protein, enzymes and lipoprotein values of local dogs at 24 and 36 h after coumaphos organophosphate acaricides bath.
3.4 Post mortem findings
Post mortem examination of five dogs that died after 36 hrs post exposure to the organophosphate bath showed different signs and of organophosphate poisoning at different degrees, despite the fact that the probable site of absorption was the skin. The findings included; paleness of the mucous membrane, jaundice, linear haemorrhages in the colon, ecchymotic haemorrhages along the whole intestine, paintbrush haemorrhages in the intestinal and gastric serosal surfaces, congestion of the stomach, especially at the fundic region. The liver was pale with multifocal areas of necrosis, congestion and haemorrhages in the lungs, especially at the cranioventral part of the middle lobe. Slight congestion of the brain meninges, embolic showers on the kidneys, extending deep into the cortex with multifocal areas of necrosis, and congestive splenomegaly. Another died animals also had severely congested and yellow liver as well as intestinal beading.
3.5 Histopathological findings
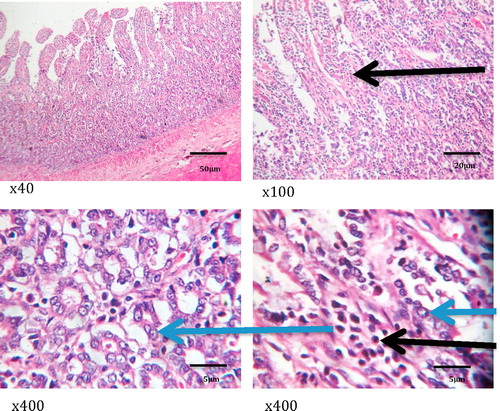
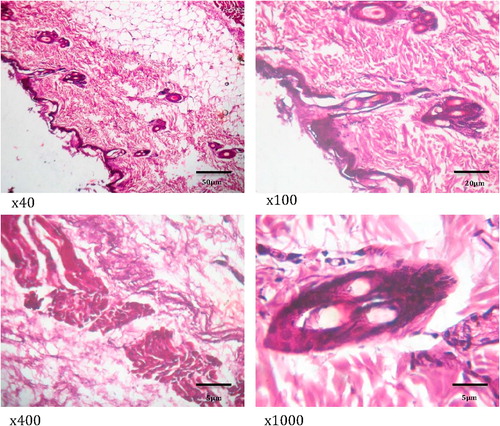
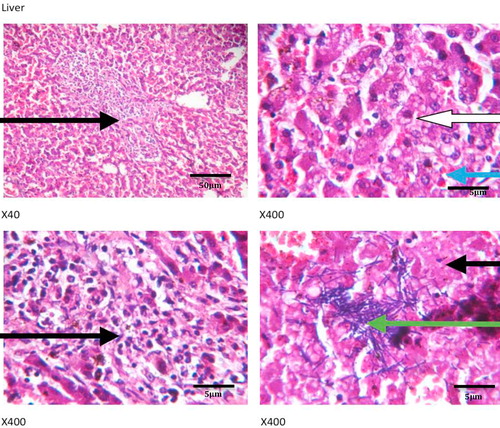
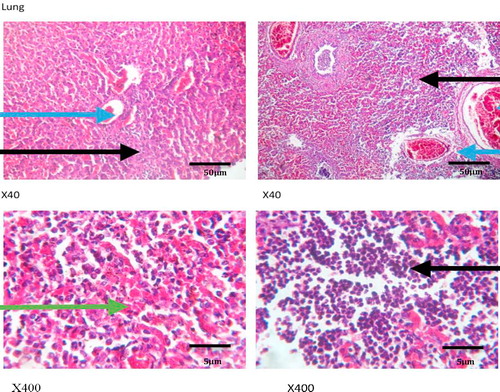
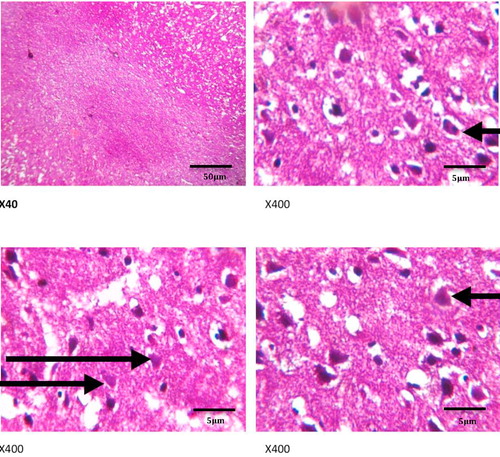
show the histology of the liver, lungs, brain and intestines of those dogs that died as a result of the acute coumaphos toxicity. In the liver, focal areas of necrosis of the hepatocytes, inflammatory cells infiltration of the hepatic sinusoids; congestion of vessels and sinusoids as well as disseminated micro-vesicular steatosis were observed (). Inflammatory cell infiltration of the interstitial spaces, haemorrhage into the alveoli and congestion of blood vessels were observed in the lungs (). Toxic changes in the form of red neurons were also observed in the brain () while the affected intestines show disseminated infiltration of inflammatory cells in the sub mucosa and vesicular nuclei in the enterocytes lining the crypts (). No visible lesion was however observed in the skin ().
4 Discussion
It is a generally accepted fact that organophosphate poisoning constitutes an important health risk to humans and animals due to contamination of food, water, air and the environment in general [Citation17,Citation18]. This has led to the establishment of regulatory bodies and modalities for the monitoring of acceptable exposure of humans to organophosphates poisoning in the developed countries including the United States [Citation19]. These compounds and the mechanism of toxicity, which is its anticholinesterase activities have been documented by several authors [Citation1,Citation5,Citation10]. However, majority of evaluation studies have centered on toxicity via the oral route and some via inhalation of organophosphates in flame retardants [Citation20]. Lettle studies on dermal exposure were found in the literature, yet dermal route is an important route through which organophosphate absorption could result in poisoning [Citation21].
In the present study of the effects of organophosphate – coumaphos acaricides bath in the dog, we observed significant level of monocytopenia, eosinopenia and reduction in basophil counts in the exposed animals as early as 8 h after exposure. Anaemia and leukocytosis were marginal, at 8 hrs after exposure. Anaemia and leukocytosis were less pronounced at 8 hrs after exposure probably because of the shortness of time at which the samples were taken. At 24 and 36 hrs however, pronounced normocytic normochromic anaemia and pan leucopenia was observed as PCV and RBC counts as well as the monocytes, eosinophil and basophil counts were lower in the Cx and Dx groups. This was in conjunction with clinical manifestation of excessive effects of cholinergic stimulations in the form of retching, vomiting and bloody diarrhoea. Anaemia is a common manifestation of organophosphate poisoning in human and animals, coupled with fasciculation of muscles [Citation22] and other cholinergic signs including muscular weakness and abdominal pain [Citation23]. The anaemia usually develops as a result of both intestinal haemorrhage and destruction of erythrocyte membrane leading to haemolysis [Citation24].
The PCV in the group Dx (24 hrs post exposure) was higher than that of the control group; this can only be an aftermath of dehydration as a result of loss of fluid through diarrhoea and vomiting. These observations are consistent with previous findings in organophosphate poisoning in animals and humans. Organophosphates cause acute elevated cholinergic activities by blocking acetyl cholinesterase, which degrade acetylcholine at the nicotinic and muscarinic cholinergic receptors in the parasympathetic and pre ganglionic sympathetic pathway as well as the neuromuscular junctions [Citation5,Citation8].
One of the hallmarks of the toxic effects of Coumaphos in this study was the significantly elevated urea value in the affected animals, at 8, 24 and 36 hrs post exposure. Elevated plasma urea and creatinine levels in the blood have been used as diagnostic and clinical indicators of kidney damage and in acute renal failure [Citation25–Citation27]. This is also corroborated by the presence of multifocal areas of necrosis on the kidneys in all the animals that died as a result of the organophosphate toxicity in this study. Increased plasma urea levels which may also due to increased tubular reabsorption of urea in this study may also be an indicator of low blood pressure associated with dehydration, as a result of diarrhoea and vomiting in the affected animals, since creatinine levels were not affected, thus indicating adequate glomerular filtration in the kidneys [Citation28]. Furthermore, significantly lower systolic blood pressure was observed in the dogs of group C that were exposed to 10x the recommended concentration of Coumaphos, but not in other groups (). The lack of consistency in the fall in blood pressure across the exposed group might be a result of compensatory activities of the sympathetic nervous system, antidiuretic, atrial natriuretic peptide and aldosterone activities and other mechanisms, which are activated after hypovolaemia (considerable reduction in blood volume) [Citation29]. These assist the system in pooling of fluid from the interstitial spaces and reduce diuresis. The heart rate and the force of contraction increase as well as peripheral vascular resistance, with the aim of increasing venous return, thereby restoring the blood pressure and tissue perfusion, despite the reduction in the blood volume [Citation30].
The manifestation of acute Coumaphos toxicity was also seen in the plasma level of low-density lipoprotein (LDL) in the dog. Dogs exposed to 20× the recommended concentration of Coumaphos had significantly lower LDL values. High-density lipoprotein (HDL) on the other hand only showed marginal elevation in this group (D) of animals. Low density lipoprotein acts as functional ferry that transports cholesterol in the blood into cells and facilitates its uptake [Citation31]. It is considered as the “bad” cholesterol because it facilitates accumulation of cholesterol in the blood vessels thereby contributing to the formation of plagues and atherosclerosis [Citation32]. LDL values have been reported to increase with oxidative stress especially in obese individuals as a result of increased peroxidation of lipids [Citation33].
Although, hepatic congestion and hepatocellular necrosis were seen at post mortem examination in the affected animals, liver enzymes – ALT, AST, GGT and ALP values only showed marginal increases which were not statistically significant, but may require further appraisal in clinical diagnosis. This may not be unconnected with acute nature of the toxicity, because elevated plasma enzymes are common indicators of hepatitis, hepatocellular injuries and biliary obstruction, especially with ALT, AST and GGT [Citation34,Citation35], while ALP, though not specific, is more associated with skeletal muscle activities, bone resorption and remodeling. Acute Plasma ALP values could also increase in cases of intestinal mucosal damage and renal diseases such as nephrosis (as reported in the present study) and renal tubular acidosis [Citation36,Citation37]. Other signs of acute toxicity including inflammatory cell infiltration, congestion and haemorrhages in the lungs and intestine, while toxic red neurons were seen in the brain, thus corroborating the nervous signs that were observed in the animals clinically.
In a recent study by García-García et al. [Citation38], the authors reported that exposure of intensive agricultural workers to pesticide was associated with adverse health effects, as pesticides were absorbed via ocular and dermal routes. It Leads to significant clinical, haematological and biochemical changes with concomitant fall in erythrocytes acetylcholinesterase levels in the exposed individuals.
Constant use of excessively high concentrations of organophosphate acaricides, especially among livestock farmers stems out from reports of resistance of ectoparasites to organophosphates insecticides [Citation11,Citation12,Citation39]. But the effect on the animals and humans that are so exposed must also be considered. It could be recommended therefore, that the use of excess concentration of organophosphate must be avoided while regular sero-monitoring of exposed individuals evaluated on a regular basis, irrespective of the route of exposure.
5 Conclusions
From the current study, indiscriminate use of high concentrations of coumaphos as organophosphate acaricides bath has toxic implication as a result of dermal absorption in the dog, despite the fact that the organophosphate was not ingested. Organophosphate toxicity led to normocytic normochromic anaemia and monocytopenia, eosinopenia and reduced basophil count. It also resulted in elevated plasma urea levels, which was in tandem with post mortem findings associated with acetylcholinesterase inhibitor poisoning. Using of Coumaphos at higher concentrations than the recommended dose should be considered in order to avoid its toxic effects.
Competing interest
The authors hereby declare that there are no conflicting interests.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- M.EddlestonN.A.BuckleyP.EyerA.H.DawsonManagement of acute organophosphorus pesticide poisoningLancet3712008597607
- M.ChiariC.CortinovisN.VitaleM.ZanoniE.FaggionatoA.BiancardiPesticide incidence in poisoned baits: a 10-year reportSci Total Environ6012017285292
- M.A.KhairyJ.L.LuekR.DickhutR.LohmannLevels, sources and chemical fate of persistent organic pollutants in the atmosphere and snow along the western Antarctic PeninsulaEnviron Pollut2162016304313
- D.OtrantoR.WallNew strategies for the control of arthropod vectors of disease in dogs and catsMed Vet Entomol222008291302
- M.EddlestonThe pathophysiology of organophosphorus pesticide self-poisoning is not so simpleNeth J Med662008146148
- M.MeijerT.HamersR.H.S.WesterinkAcute disturbance of calcium homeostasis in PC12 cells as a novel mechanism of action for (sub)micromolar concentrations of organophosphate insecticidesNeurotoxicology432014110116
- S.C.JoshiR.MathurN.GulatiTesticular toxicity of chlorpyrifos (an organophosphate pesticide) in albino ratToxicol Ind Health232007439444
- H.AardemaJ.MeertensJ.LigtenbergO.M.Peters-PolmanJ.TullekenJ.ZijlstraOrganophosphorus pesticide poisoning: cases and developmentsNeth J Med662008149153
- D.M.ShihL.GuX.Yu-RongM.NavabMice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosisNature3941998284287
- A.HazarikaS.N.SarkarS.HajareM.KatariaJ.K.MalikInfluence of malathion pretreatment on the toxicity of anilofos in male rats: a biochemical interaction studyToxicology185200318
- N.K.SinghH.SinghN.K.SinghS.S.RathMultiple mutations in the acetylcholinesterase 3 gene associated with organophosphate resistance in Rhipicephalus (Boophilus) microplus ticks from Punjab, IndiaVet Parasitol2162016108117
- K.J.BandaraS.P.KarunaratneMechanisms of acaricide resistance in the Cattle tick Rhipicephalus (Boophilus) microplus in Sri LankaPestic Biochem Physiol13920176872
- K.A.StaffordG.C.C.ColesDrug resistance in ectoparasites of medical and veterinary importanceAntimicrob drug resist2017springer735744
- R.B.DaveyJ.E.GeorgeEfficacy of coumaphos applied as a dip for control of an organophosphorus-resistant strain of Boophilus microplus (Acari: Ixodidae) on cattleJ Econ Entomol92199913841391
- B.OkutucuA.DınçerÖ.HabibF.ZıhnıogluComparison of five methods for determination of total plasma protein concentrationJ Biochem Biophys Methods702007709711
- A.A.MegahedM.W.H.HiewW.GrünbergP.D.ConstableEvaluation of 2 portable ion-selective electrode meters for determining whole blood, plasma, urine, milk, and abomasal fluid potassium concentrations in dairy cattleJ Dairy Sci99201673307343
- Á.Rodríguez-HernándezM.CamachoL.A.Henríquez-HernándezL.D.BoadaN.Ruiz-SuárezP.F.ValerónAssessment of human health hazards associated with the dietary exposure to organic and inorganic contaminants through the consumption of fishery products in SpainSci Total Environ5572016808818
- J.E.CasidaOrganophosphorus xenobiotic toxicologyAnnu Rev Pharmcol Toxicol572017309327
- B.E.MilesonJ.E.ChambersW.ChenW.DettbarnM.EhrichA.T.EldefrawiCommon mechanism of toxicity: a case study of organophosphorus pesticidesToxicol Sci411998820
- F.XuG.GiovanoulisS.van WaesJ.A.Padilla-SanchezE.PapadopoulouJ.MagnérComprehensive study of human external exposure to organophosphate flame retardants via air, dust, and hand wipes: the importance of sampling and assessment strategyEnviron Sci Technol50201677527760
- A.CicesS.BayersA.E.VerzìL.A.SchachnerD.P.WestG.MicaliPoisoning through pediatric skin: cases from the literatureAm J Clin Dermatol182017391403
- B.A.ChhabriaA.BhallaTongue fasciculations in organophosphate poisoningN Engl J Med3752016e47
- F.R.ChowdhuryM.S.BariM.J.AlamM.M.RahmanB.BhattacharjeeJ.A.QayyumOrganophosphate poisoning presenting with muscular weakness and abdominal pain-a case reportBMC Res Notes72014140
- M.J.CoyeP.G.BarnettJ.E.MidtlingA.R.VelascoP.RomeroC.L.ClementsClinical confirmation of organophosphate poisoning by serial cholinesterase analysesArch Intern Med1471987438442
- A.S.LeveyJ.P.BoschJ.B.LewisT.GreeneN.RogersD.RothA more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equationAnn Intern Med1301999461470
- S.UchinoJ.A.KellumR.BellomoG.S.DoigH.MorimatsuS.MorgeraAcute renal failure in critically ill patients: a multinational, multicenter studyJAMA2942005813818
- B.S.BagaladK.P.MohankumarG.S.MadhushankariM.DonoghueP.H.KuberappaDiagnostic accuracy of salivary creatinine, urea, and potassium levels to assess dialysis need in renal failure patientsDent Res J1420171318
- H.RaynerM.ThomasD.MilfordUnderstanding kidney diseases2016Springer
- U.N.DasRenin–angiotensin–aldosterone system in insulin resistance and metabolic syndromeJ Transl Intern Med420166672
- R.CarterC.Hinojosa-LabordeV.A.ConvertinoVariability in integration of mechanisms associated with high tolerance to progressive reductions in central blood volume: the compensatory reservePhysiol Rep42016e12705
- R.K.WadheraD.L.SteenI.KhanR.P.GiuglianoJ.M.FoodyA review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortalityJ Clin Lipidol102016472489
- D.M.Lloyd-JonesP.B.MorrisC.M.BallantyneK.K.BirtcherD.D.DalyS.M.DePalmaACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease riskJ Am Coll Cardiol68201692125
- H.K.VincentA.G.TaylorBiomarkers and potential mechanisms of obesity-induced oxidant stress in humansInt J Obes302006400418
- M.UzunhisarcikliA.AslanturkS.KalenderF.G.ApaydinH.BasMercuric chloride induced hepatotoxic and hematologic changes in ratsToxicol Ind Health32201516511662
- M.StepienV.FedirkoT.Duarte-SallesP.FerrariH.FreislingE.TrepoProspective association of liver function biomarkers with development of hepatobiliary cancersCancer Epidemiol402016179187
- J.C.JansenS.TimalM.van ScherpenzeelH.MichelakakisD.VicogneA.AshikovTMEM199 deficiency is a disorder of Golgi homeostasis characterized by elevated aminotransferases, alkaline phosphatase, and cholesterol and abnormal glycosylationAm J Hum Genet982016322330
- H.DasM.AliL.DeviP.KirthikaJ.GaliP.BeheraStudies on some important enzymes of non-descript sheep of AssamJ Livest Sci820175962
- C.R.García-GarcíaT.ParrónM.RequenaR.AlarcónA.M.TsatsakisA.F.HernándezOccupational pesticide exposure and adverse health effects at the clinical, hematological and biochemical levelLife Sci1452016274283
- P.VudrikoJ.Okwee-AcaiD.S.TayebwaJ.ByaruhangaS.KakoozaE.WampandeEmergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in UgandaParasit Vectors920164