Abstract
This cross sectional survey was conducted from July to December 2015 in order to investigate the burden of gastrointestinal helminthes among guinea fowls in Gombe, Northeastern Nigeria. A total of six hundred fowls (viscera) were purchased from six randomly selected slaughter slabs. Out of the 600 birds examined, 479 (79.83%; 95% CI: 76.4, 82.9) were found harbouring one or more gastrointestinal helminthes. Of this, 238 birds (39.7%; 35.8, 43.6) were infected by nematode species and 392 birds (65.3%; 61.4, 69.0) by cestode species. A total of nine nematodes and seven cestodes species were recovered from these birds. There was no any trematode observed among the studied birds. The prevalences of the nematodes identified in descending order were: Ascaridia galli 56.7% (52.7, 60.6); Ascaridia numidae 38.0% (34.2, 42.0); Heterakis gallinarum 17.2% (14.4, 20.4); Heterakis meleagridis 8.3% (6.4, 10.8); Strongyloides avium 3.5% (2.3, 5.3); Subulura brumpti 3.2% (2.0, 5.0); Gongylonema ingluvicola 2.2% (1.3, 3.7) and both Dispharynx spiralis and Tetrameres numidae had 0.7% (0.3, 1.7). While for cestodes: Raillietina tetragona 72.8% (69.1, 76.2); Raillietina echinobothrida 67.3% (63.5, 71.0); Raillietina cesticillus 50% (46.0, 54.0); Raillietina magninumida 25.7% (22.3, 29.3); Hymenolopsis cantaniana 17.3% (14.5, 20.6); Davainea nana 4.2% (2.8, 6.1) and the lowest was observed in Choanotaenia infundibulum with 2% (1.2, 3.5). Infection rates did not differ significantly based on sex (P > 0.05). However, the occurrence of mixed infection as compared with single infection was statistically significant in both cestodes and nematodes (P < 0.001). The results obtained indicated high prevalence of gastrointestinal helminthes among guinea fowls. These birds may serve as important source of helminthes to other commercial birds in the study area.
1 Introduction
In recent years, the poultry industry has experienced an unprecedented growth and expansion especially in developing countries. In these countries, the poultry subsector plays an important role in that it provides immediate source of cheap protein in form of meat and eggs to the poultry farmers. Poultry and poultry products are considered important sources of cheap protein because when compared with the red meat (cow), the poultry meat (white meat) is frequently more affordable and cheaper. In Europe, it has been reported that in order to produce 1 kg of broiler meat, it takes 3.1 kg of dry matter feed, whereas non-dairy cattle and pigs require 6.2 kg and 24 kg respectively [Citation1]. Compared with red meat, the white meat has low content of saturated fats and is considered of high quality [Citation2]. Recently, there is an increase in the demand for cheap protein diets (poultry and poultry products) due to rapid growth of human population in the world. For instance, the consumption of poultry meat in developing countries from 1990s to 2005 has increased by 35 tonnes; almost doubling the increase that occurred during the same period in developed countries (http://www.fao.org). In developing countries, poultry production is mainly practiced in rural areas, because it requires less land, low capital and cheap labour easily provided by the family members [Citation3]. In most cases, the farming is targeted on one hand at providing meat and eggs for immediate family consumption during festivities and traditional ceremonies and as source of income and poverty alleviation on the other hand [Citation4]. Of the 15 billion chickens in the world, about 75% are found in developing countries (http://www.fao.org). In these countries, birds are managed under free range/backyard, semi intensive and intensive production systems. Under the backyard/free-range systems, the birds are provided with minimum shelter and housing facilities. They are allowed to freely roam in and around the house premises scavenging for food, water and other dietary requirements [Citation5]. In this way of their feeding habit, they eat grasses, insects, house refuse, flies, cockroaches, ants, beetles, earthworms etc. [Citation3,Citation6], many of which serve as intermediate hosts of helminth parasites. Therefore, this scavenging habit increases their risks of contracting gastrointestinal helminthes either by directly eating the worm larva or indirectly the intermediate hosts. In contrast, the intensive system is mainly practiced by commercial poultry farmers; where it is more organized in terms of adequate provision of good housing facilities, veterinary consultation services, welfare of the birds and disease treatment and control etc [Citation5]. In this system, the provision of effective biosecurity measures and adoption of modern technology has greatly lowered the negative impact posed by parasitic diseases among birds managed under the intensive cage systems [Citation6].
In Nigeria, the poultry subsector plays a vital role in the agriculture sector, in that it accounts for about 58% of the total livestock production in the country [Citation7]. For instance, the contribution of the poultry meat and egg production to the livestock share of the gross domestic product (GDP) increased from 26% in 1995 to 27% in 1999 [Citation8]. In the same study, the author also reported a steady increase of egg production during these years and this alone accounted for about 13% of the livestock share of the GDP. Within the livestock sector, the village poultry are often the most commonly owned and managed types of livestock. Guinea fowls are one of the several species of poultry along with indigenous chickens (Gallus gallus domesticus), turkeys (Meleagris gallopavo) and ducks (Cairina spp.) that are indigenous to sub-Saharan Africa [Citation4]. The population of guinea fowls has been estimated at 54.7 million and it compares favourably with the village chickens in terms of meat and egg production [Citation9]. In Nigeria, guinea fowls are mainly managed under the backyard system; where they provide meat and eggs to the smallholder poultry farmers. Guinea fowl production under the extensive system has seen little improvement over the years with little attention to welfare, health, housing facilities, vaccination programs and disease control [Citation10]. There is general acceptability of meat and eggs (source of protein) of guinea fowls by majority of the Nigerian populace, thus justifying the need for large-scale commercial production of these birds [Citation11]. However, under the free range/backyard system, the guinea fowls are allowed roaming freely; where they feed on a wide range of diets under poor husbandry which directly predisposes them to intermediate hosts of helminthes [Citation12]. This scavenging feeding habit allows the rapid buildup of high worm burden among the village chickens and guinea fowls.
Despite the increasing popularity of backyard/free-range production system in developing countries including Nigeria, the occurrence of parasitic diseases particularly coccidiosis, helminthiasis, bacterial and viral diseases resulting in losses due to mortality and morbidity [Citation12,Citation13] which have a negative impact on its ability to realizing its full production potential. Among the parasitic diseases, gastrointestinal helminthes were ranked high. Among these, nematodes and cestodes constitute the most important groups [Citation3,Citation14–Citation18]. Helminthes cause considerable economic losses to poultry farmers worldwide. They lower poultry productivity by competing for essential dietary nutrients with the infected hosts (resulting in lower weight gain), stunted growth and in severe cases of high worm burden, they can cause death by entirely blocking the gastrointestinal tract of the infected hosts [Citation19]. Multiple or mixed helminthiasis has also been reported to be prevalent among poultry managed extensively [Citation20]. While other authors reported the occurrence of drug resistant zoonotic pathogens among backyard chickens [Citation21,Citation22]. Various studies reported the prevalence of haemoparasites [Citation20], endoparasites [Citation20] and protozoan infection [Citation5,Citation17,Citation23] among village chickens. Helminthiasis among guinea fowls constitutes public health threat and may serve as a source of infection to commercial poultry and wild birds [Citation12]. In Nigeria, particularly in the rural areas, poultry production such as the backyard/free-range plays an important role by alleviating poverty and serving as a mean of income earner to the resource-poor households. It provides them with immediate petty cash from sales of live birds and eggs. Although, other studies of gastrointestinal helminthes of guinea fowls have been conducted in Northeastern Nigeria, the majority of these studies focussed on few slaughter slabs, while other studies utilized small sample sizes. Thus, there is a need for a comprehensive study utilizing large sample size from different slaughter slabs with the aim of exploring the endemic species of gastrointestinal helminthes among grey-breasted helmet guinea fowls. Therefore, the current study is designed to investigate the prevalence and burden of gastrointestinal helminthes among grey-breasted helmet guinea fowls (Numida meleagris galeata) slaughtered at six selected slaughter slabs in Gombe state of Northeastern Nigeria.
2 Materials and methods
2.1 Study area
The study was conducted in Gombe Metropolis, the capital of Gombe State. It lies in the guinea savannah zone of northeastern Nigeria at latitude 10°15′N and longitude 11°10′E. It has an area of 20,265 km2 and a population of about 2.36 million people (http://nigeria.opendataforafrica.org/xspplpb/nigeria-census). It shares common borders with Bauchi, Taraba, Yobe, Adamawa and Borno states all located in the northeastern region of Nigeria. It has eleven local government areas. Gombe state is a multi-ethnic society comprising different tribes with the Fulani tribes being the dominant tribe and inhabiting majority of the northern region, while other ethnicities of Tera (second dominant tribe), Waja, Tangale, Dadiya, Bolewa, hausa and Kanuri dominate the southern region of the state. The gombe metropolis is a typical heterogenous city comprising a mix of these tribes and other tribes from neighbouring states. The vegetation is generally guinea savannah grassland. During the hottest periods of the year, the average temperature could exceed 40 °C (March–May). The rainy season starts from April and ends in October with an average rainfall of 850 mm, whereas the dry season spans from November through March of every year (https://en.wikipedia.org/wiki/Gombe_State#cite_note-nn-2).
2.2 Study design and study population
This study involved purposive sampling from July 2015 to December 2015 of grey-breasted helmet guinea fowls. A total of six hundred fowls (visceral organs) comprising males (n = 317) and females (n = 283) were purchased from each slaughter slab (n = 100). The study population comprised guinea fowls sourced mainly from local birds’ markets located within the metropolis. These birds were managed under the backyard/free-range system and allowed to scavenge for food and water during the day and kept indoors at night. The visceral organs obtained for the present study were purchased from slaughter slabs, where the birds are slaughtered and sold for the public consumption.
2.3 Sample size
The sample size used in this study was calculated using Epi info® statistical software according to the methods described elsewhere [Citation3]. Based on literature review, the assumed prevalence of helminthes was considered as 35.5% [Citation20] for village chickens and the population size utilized was 100,000. Therefore, with a desired absolute precision of 3% and 95% level of confidence, sample size of at least 550 birds was required. Thus, we collected a total of six hundred samples (100 samples from each slaughter slabs) of fresh visceral organs of guinea fowls for the purpose of this study.
2.4 Sample collection
Fresh visceral organ from each of the sampled birds were collected on weekly basis for a period of six months from each slaughter slab comprising Gombe main market, Pantami market, Riyald/ Bagadaza market, Dukku park market, Tudun wada market and Shongo park market within Gombe metropolis between the months of July and December 2015. At the time of sample collection, data on sex and location were also recorded. The samples, when collected are transported ice cooled directly to the laboratory for onward parasitological processing. In order to avoid transfer of the parasites from one site of the alimentary tract to the other, the tracts were ligated using nylon ligatures as described elsewhere [Citation24]. Thereafter the ligated gastrointestinal tracts were cut opened longitudinally to collect adult parasite worms and ova as described by Nalubamba et al. [Citation24].
2.5 Collection of helminthes, parasitological identification and counting of parasites
Briefly, the ligated alimentary tracts were dissected longitudinally using sterile myoris scissors. The transection divides the tract into sections containing respective organs namely: esophagus, crop, proventriculus, gizzard, duodenum, small intestines, caeca and rectum. These were subsequently kept separately in petri dishes containing physiological saline. All worms visible to the naked eyes were removed using a pair of thumb forceps. The worms were grouped and counted from each tract in order to determine the worm burden. Recovered nematodes were preserved in 70% alcohol, while cestodes were fixed with acetic formalin alcohol, stained with haematoxylin and mounted in Canada balsam as previously described elsewhere. All the recovered worms and ova were identified according to the taxonomic keys previously described [Citation19]. The adult worms were mounted on glass slides using polyvinyl alcohol and identified directly under the stereomicroscope as described before [Citation19]. Faecal samples recovered from scrapping of the intestinal lumen were also examined by the flotation technique using saturated salt solution and examined for the presence of parasites ova under the microscope. The identification of the recovered worms and other samples collected for the present study was carried out by the microbiology and entomology diagnostic laboratory of the National Veterinary Research Institute (NVRI) located in Plateau state of North central Nigeria.
2.6 Statistical analysis
Raw data collected were initially managed in the Microsoft office excel® 2010, where simple frequencies and percentages of infection were determined before finally importing the data into Graphad Instat® software for other statistical analyses. The prevalence of the nematodes and cestodes was calculated using percentages. In order to assess the strength of association between helminthiasis and other independent variables such as slaughter slab market locations, sex and worm burden, chi-square test was employed. The observed prevalence of infection and its 95% Confidence interval were computed and the level of significance was set at P ≤ 0.05.
3 Results
Of the six hundred birds examined, 479 (79.83%; 95% CI: 76.4, 82.9) birds were found harbouring one or more gastrointestinal helminthes. Of this, 238 birds (39.7%; 35.8, 43.6) were infected by nematodes and 392 birds (65.3%; 61.4, 69.0) by cestodes (). Sixteen helminthes species comprising nine nematodes and seven cestodes species were recovered from the examined gastrointestinal tracts of the birds at different predilection sites (). There was no any trematode observed among the examined birds. Of the 317 male birds examined, 201 (63.4%; 58.0, 68.5) and 125 (39.4%; 34.2, 44.9) harboured various species of cestodes and nematodes respectively (). Similarly, out of the 283 females examined, 191 (67.5%; 61.8, 72.7) and 113 (39.9%; 34.4, 45.7) were infected with cestodes and nematodes respectively (). The occurrence of both cestodes (P = 0.2940) and nematodes (P = 0.9011) based on sex was not statistically significant (P > 0.05). However, the occurrence of cestodes and nematodes in either male or female birds was statistically significant (P < 0.001) (). The occurrence of cestodes as compared with nematodes was statistically significant (P < 0.001). The prevalences of the nematode species identified and their predilection sites in descending order were as follows, Ascaridia galli, Ascaridia numidae, Heterakis gallinarum and Heterakis meleagridis were observed in all parts of the gastrointestinal tracts of 340 (56.7%; 52.7, 60.6); 228 (38.0%; 34.2, 42.0); 103 (17.2%; 14.4, 20.4) and 50 (8.3%; 6.4, 10.8) birds respectively (, ). While, Strongyloides avium was found in the small intestines of 21 (3.5%; 2.3, 5.3) birds examined, Subulura brumpti in the caeca of 19 (3.2%; 2.0, 5.0) birds; Gongylonema ingluvicola observed in the crop of 13 (2.2%; 1.3, 3.7) birds and both Dispharynx spiralis and Tetrameres numidae were found in the gizzard of 4 (0.7%; 0.3, 1.7) birds each (). While among cestodes identified, majority of the birds were found heavily infected with Raillietina spp. The observed prevalences were, Raillietina tetragona found in the small intestines of 437 (72.8%; 69.1, 76.2) birds examined and Raillietina echinobothrida in the gizzard of 404 (67.3%; 63.5, 71.0) birds analysed (). While, Raillietina cesticillus, Raillietina magninumida, Raillietina magninumida, Hymenolopsis cantaniana, Davainea nana, and Choanotaenia infundibulum were all found in the small intestines of 300 (50%; 46.0, 54.0); 154 (25.7%; 22.3, 29.3); 104 (17.3%; 14.5, 20.6); 25 (4.2%; 2.8, 6.1) and 12 (2%; 1.2, 3.5) of the six hundred birds examined respectively (, ). Of the 238 birds infected with nematodes (39.7%; 35.8, 43.6), 61 (25.6%; 20.5, 31.5) and 177 (74.4; 68.5, 79.5) had single and mixed nematode infections respectively ( and ). Whereas, out of the 392 birds infected with cestodes (65.3%; 61.4, 69.0), 100 (25.5%; 21.5, 30.1) and 292 (74.5%; 70.0, 78.6) birds had single and mixed cestode infections respectively (). The occurrence of mixed as compared with single infection for both cestodes and nematodes encountered in the studied birds was statistically significant (P < 0.001) (). On the basis of market location where the birds were purchased, the prevalence of cestode species ranges from 59.0% (49.2, 68.1) to 72.0% (62.5, 79.9) respectively observed among the birds sourced from Shongo Park and Tudun wada market locations (). While for nematodes, it varied from 34% (25.5, 43.7) to 46% (36.6, 55.7) among the birds sampled from Riyald/Bagadaza market and Tudun wada markets respectively (). The occurrence of both cestodes (P= 0.7524) and nematodes (P = 0.7523) across the six different slaughter slabs was not statistically significant (P > 0.05, ).
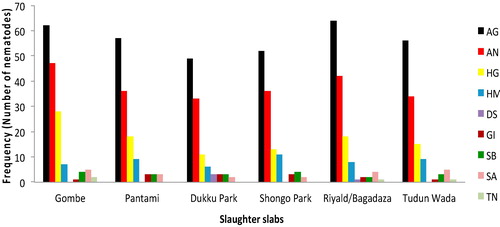
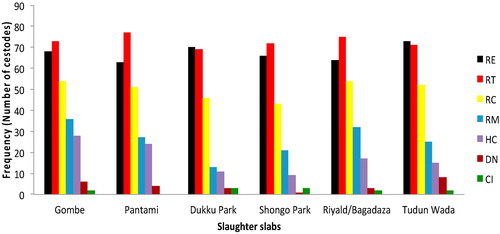
Table 1 Sex – wise prevalence of gastrointestinal helminthes among slaughtered guinea fowls in Gombe, Nigeria (n = 600).
Table 2 Occurrence and burden of gastrointestinal helminthes among slaughtered domesticated guinea fowls in Gombe, Northeastern Nigeria (n = 600).
Table 3 Worm burden among slaughtered guinea fowls in Gombe, Nigeria (n = 600).
Table 4 Frequency of single and mixed helminthes infection among slaughtered guinea fowls in Gombe state, Nigeria (n = 600).
Table 5 Prevalence of gastrointestinal helminthes according to market location among slaughtered guinea fowls in Gombe, Nigeria (n = 600).
4 Discussion
This survey of gastrointestinal helminthes among grey-breasted helmeted guinea fowls revealed high prevalence of helminthes (79.8%, 479/600) with significantly higher cestode (65.3%) infections compared with nematodes (39.7%) (P < 0.001). We found that grey-breasted helmeted guinea fowls were heavily infected with gastrointestinal helminthes and this might negatively affect their overall health status, efficiency and productivity performances. Previous studies documented the occurrence and economic significance of nematodes and cestodes among exotic, indigenous chickens and guinea fowls [Citation3,Citation14–Citation18,Citation20]. Helminthiasis has been described earlier as one of the prevalent diseases and a major constraint particularly among birds managed under the backyard/free-range smallholder poultry production systems; where it has been associated with significant economic losses to poultry farmers worldwide [Citation25].
The high prevalence of helminthiasis observed among the studied birds could be attributed to many factors. Most of the birds sampled were raised under free-range production systems; where they were allowed freely roaming around the environment and only to be sheltered at night. This feeding habit under poor husbandry (dirty and stagnant water with abundant snails) makes them vulnerable to intermediate hosts of helminthes such as ants, beetles, cockroaches, earthworms etc. Thus, these birds might have high risks of harbouring gastrointestinal helminthes compared with other birds managed under intensive system in the same study area [Citation12]. Another factor is the climatic condition of the study area especially the temperature and relative humidity. Variability of these parameters from one region to another might alter the population dynamics of the helminthes and this may lead to significant changes in the incidence, prevalence and worm burden (intensity) of the helminthes. Other factors such as the sample size, incidence of the infective stages and availability of the intermediate hosts in the study area and period during which the research is carried out [Citation26] could also play role in the variability of the prevalence observed in this study.
This study reported high prevalence of cestode species compared with nematodes. This is in congruent with reports by other researchers in Northeastern Nigeria [Citation27–Citation29]. In contrast, others reported high prevalence of nematodes compared with cestodes in both exotic, rural and guinea fowl chickens [Citation6]. These variations in occurrence of nematode and cestode species could be due to less accessibility to intermediate hosts of cestodes and availability of the infective stages of nematodes around the environment [Citation30]. The occurrence of cestodes and nematodes in both sexes of the studied birds was not statistically significant (P> 0.05). A similar finding was reported by earlier studies [Citation3,Citation31,Citation32]. This could be attributed to the fact that both male and female birds in the environment were allowed to scavange freely for their daily needs of feed and water to supplement their dietary requirements. Thus, they are equally exposed to high risks of acquiring the infective stages and intermediate hosts of gastrointestinal helminthes [Citation3]. In this study, there was no any trematode observed among all the studied birds. This could partly be due to non-accessibility or availability of infected snails in the environment. It may also be due to lack of favourable environmental conditions necessary for the growth and perpetuation of the vectors of trematodes in the study area [Citation30]. Similar finding was also reported in other parts of the world [Citation33–Citation35].
Of the nematodes reported in this study, the large chicken round worm Ascaridia galli had the highest prevalence among the examined birds followed respectively by A. numidae and H. gallinarum. A similar observation of A. galli among birds raised under the free-range systems has also been reported in many countries worldwide. da Silva et al. [Citation36] reported the occurrence of A. galli among chickens from Sao Paulo, Brazil. Similarly, Puttalakshmamma et al. [Citation35], reported a prevalence of 91.4% of A. galli among chickens sourced from Bangalore, India. Similarly, high prevalence of A. galli among free-range birds was also reported in Ethiopia (71.6%) and Germany (88%) [Citation37,Citation38]. Earlier studies reported A. galli as an important parasite with predilection in the small intestines of chickens. It has been frequently associated with significant economic losses (resulting from occlusion of the intestinal tracts particularly seen when there is heavy infection with adult worms), weight loss, lower or reduced egg production, decreased feed conversion rates and other associated costs of treatment. Other complications of this parasite included production of severe pathological conditions especially observed when there is concurrent infection (mixed infection) with other pathogens and may also transmit other pathogens like Salmonella in chickens [Citation39,Citation40]. The nematode, H. gallinarum, has been reported in chickens worldwide. Although, considered as non-pathogenic to chickens, it has been associated with reduced weight gain. Other pathological lesions ranging from congestion to hemorrhagic enteritis of the small intestines and caecum have also been reported [Citation3]. The H. gallinarum has been found to be associated with the transmission of the protozoan parasite Histomonas meleagridis, the aetiological factor of fatal (blackhead) disease in turkeys and chicks [Citation41].
Among the cestode species identified in this study, Raillietina spp. had the highest prevalence. Similar observations were reported by other researchers in Nigeria [Citation17,Citation20,Citation26,Citation27] and around the world [Citation3,Citation14,Citation30,Citation34–Citation36]; where it was reported to be associated with reduced weight gain among birds, resulting in significant economic losses to poultry farmers. The high prevalence observed could reflect the abundance of the intermediate hosts of Raillietina spp. around the environment of the study area. Chickens normally acquire the infection with Raillietina through ingestion of the intermediate hosts such as beetles, Musca domestica and ants of the genera Tetramorium pheidole, harbouring cysticercoids [Citation3,Citation30]. These intermediate hosts are available in abundance in and around the study area. It should be borne in mind that the guinea fowls sampled in the current study were managed under the backyard/free-range system; where they scavange freely in the environment during the day, foraging and feeding on variety of insects, grasses, stagnant water, beetles, houseflies and other related matters. This scavenging feeding nature predisposes them to high accessibility to the intermediate hosts of Raillietina [Citation3]. In this study, we reported a significantly higher occurrence of mixed infection for cestode and nematode helminthes among the studied birds. Among the nematodes identified, majority of the mixed infections observed, involved infection by Ascaridia spp. (A. galli and A. numidae) and Heterakis spp. (H. gallinarum and H. meleagridis). Whereas for cestodes, mixed infection was frequently seen in infections with Raillietina spp. and H. cantaniana. While other nematode and cestode species occurred less frequently in single infections. This finding is not surprising, because the free-range system allows birds to scavange and forage for their daily dietary requirements thereby increasing their risks of coming in contact with eggs and larval stages of helminthes. The finding of a significantly high mixed infection of helminthes among guinea fowls in the present study was also reported by other authors [Citation20], as being the most common infection among birds managed under the extensive system and poor husbandry.
5 Conclusions
In conclusion, heavy helminthiasis was observed among the grey-breasted helmeted guinea fowls in Gombe state, northeastern Nigeria. This study found high prevalence and burden of helminthes manifesting in both single and mixed infections among guinea fowls in Gombe state. Thus, it is imperative for the government to prevent and control the occurrence of helminthosis among grey-breasted guinea fowls, in order to boost its local production.
Acknowledgements
The authors wish to express their appreciation to the ministry of Animal husbandry and nomadic affairs, Gombe state. We are equally grateful to the entire technical staff of the microbiology and entomology diagnostic laboratory of the National Veterinary Research Institute (NVRI), Vom, Plateau state, North central Nigeria. This project was funded by the Gombe state Ministry of Animal and Nomadic Affairs, Nigeria through their efforts to boost local production of guinea fowls in the state.
Competing interests
The authors declare that they have no conflict of interest.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- J.AlcamoIMAGE 2.0: integrated modeling of global climate change1994Kluwer Academic PublishersDordrecht
- C.A.RamírezM.PatelK.BlokHow much energy to process one pound of meat? A comparison of energy use and specific energy consumption in the meat industry of four European countriesEnergy31200617111727
- T.FerdushyM.T.HasanA.K.M.Golam KadirCross sectional epidemiological investigation on the prevalence of gastrointestinal helminths in free range chickens in Narsingdi district, BangladeshJ Parasit Dis402016818822
- A.YakubuI.S.Musa-AzaraH.S.HarunaVillage guinea fowl (Numidia meleagris) production systems in Nasarawa State, north central Nigeria: flock characteristics, husbandry and productivityLivest Res Rural Dev262014
- J.R.LawalS.M.JajereU.I.IbrahimY.A.GeidamI.A.GulaniG.MusaPrevalence of coccidiosis among village and exotic breed of chickens in Maiduguri, NigeriaVet World92016653659
- A.K.JavaregowdaB.Kavitha RaniS.P.RevannaG.UdupaPrevalence of gastro-intestinal parasites of backyard chickens (Gallus domesticus) in and around ShimogaJ Parasit Dis402016986990
- T.T.AmosAnalysis of backyard poultry production in Ondo State, NigeriaInt J Poult Sci52006247250
- S.O.OjoProductivity and technical efficiency of poultry egg production in NigeriaInt J Poult Sci22003459464
- B.NwaguB.FulayiF.NwaguHatchability of Guinea fowl eggs in NigeriaTrop Anim Health Prod2919976364
- A.MohammedE.OtchereL.SaiduP.AbduVaccination of Nigeria indigenous chickens, Guinea fowls and Turkey against Newcastle disease: an assessment of haemagglutination inhibitionBull Anim Health Prod Africa461998157160
- K.L.AyorindeGuinea fowl (Numida meleags) as a protein supplement in NigeriaWorlds Poult Sci J47199116
- S.A.LukaI.S.NdamsGastrointestinal parasites of domestic chicken Gallus gallus domesticus Linnaeus 1758 in Samaru, Zaria, NigeriaSci World J220072729
- J.NwantaP.AbduW.EzemaEpidemiology, challenges and prospects for control of Newcastle disease in village poultry in NigeriaWorlds Poult Sci J642008119127
- R.KatochA.YadavR.GodaraJ.K.KhajuriaS.BorkatakiS.S.SodhiPrevalence and impact of gastrointestinal helminths on body weight gain in backyard chickens in subtropical and humid zone of Jammu, IndiaJ Parasit Dis3620124952
- W.MollaH.HaileG.AlmawW.TemesgenGastrointestinal helminths of local backyard chickens in North Gondar Administrative Zone, EthiopiaRev Med Vet1632012362367
- T.W.SchouA.PerminH.R.Juul-MadsenP.SørensenR.LabouriauT.L.H.NguyênGastrointestinal helminths in indigenous and exotic chickens in Vietnam: association of the intensity of infection with the major histocompatibility complexParasitology1342007561573
- J.S.O.AyeniO.O.DipeoluA.N.OkaemeParasitic infections of the grey-breasted helmet guinea-fowl (Numida meleagris galeata) in NigeriaVet Parasitol1219835963
- M.D.RuffImportant parasites in poultry production systemsVet Parasitol841999337347
- E.SoulsbyHelminths, arthropods and protozoa of domesticated animals1982Baillière TindallLondon
- P.A.NnadiS.O.GeorgeA cross-sectional survey on parasites of chickens in selected villages in the subhumid zones of south-eastern NigeriaJ Parasitol Res20102010141824 10.1155/2010/141824
- L.PohjolaS.NykäsenojaR.KivistöT.SoveriA.HuovilainenM.L.HänninenZoonotic public health hazards in backyard chickensZoonoses Public Health632016420430
- J.ManningV.GoleK.ChousalkarScreening for Salmonella in backyard chickensPrev Vet Med1202015241245
- S.SharmaA.IqbalS.AzmiI.MushtaqZ.A.WaniS.AhmadPrevalence of poultry coccidiosis in Jammu region of Jammu & Kashmir StateJ Parasit Dis3920138589
- K.S.NalubambaE.C.BwalyaN.B.MudendaH.M.MunanganduM.MunyemeD.SquarrePrevalence and burden of gastrointestinal helminths in wild and domestic guineafowls (Numida meleagris) in the Southern Province of ZambiaAsian Pac J Trop Biomed52015663670
- J.P.FabiyiStudies on the parasites of grey breasted helmet guinea fowl (Numidae meleagridis) of Vom area of Plateau state, NigeriaBull Epizoot Dis Africa201972235238
- N.N.AtsandaS.M.JajereN.B.AdamuJ.R.LawalM.K.ZangoM.B.ChindoPrevalence of helminth parasites of helmeted guinea fowlNew York Sci J820159397
- M.I.AhmedP.K.SinhaPrevalence of poultry helminthiasis in an arid zone in NigeriaIndian Vet J701993703704
- E.N.GadzamaG.C.SrivastavaPrevalence of gastrointestinal parasites of market chicken in Borno State NigeriaZariya Vet11986126128
- K.P.YoriyoJ.P.FabiyiS.U.AdamsS.M.PandaIntensities of helminth parasites of free-ranging chickens in Bauchi and its environsYankari J22005135137
- C.SreedeviCh.JyothisreeV.Rama DeviP.AnnapurnaL.JeyabalSeasonal prevalence of gastrointestinal parasites in desi fowl (Gallus gallus domesticus) in and around Gannavaram, Andhra PradeshJ Parasit Dis402016656661
- A.HembramM.R.PandaB.N.MohantyC.R.PradhanM.DehuriA.SahuPrevalence of gastrointestinal helminths in Banaraja fowls reared in semi-intensive system of management in Mayurbhanj district of OdishaVet World82015723726
- K.JunkerL.DebushoJ.BoomkerThe helminth community of Helmeted Guineafowls, Numida meleagris (Linnaeus, 1758), in the north of Limpopo Province, South AfricaOnderstepoort J Vet Res752008225235
- H.B.MagwishaA.A.KassukuN.C.KyvsgaardA.PerminA comparison of the prevalence and burdens of helminth infections in growers and adult free-range chickensTrop Anim Health Prod342002205214
- E.O.MungubeS.M.BauniB.A.TenhagenL.W.WamaeS.M.NziokaL.MuhammedPrevalence of parasites of the local scavenging chickens in a selected semi-arid zone of Eastern KenyaTrop Anim Health Prod402008101109
- G.C.PuttalakshmammaK.J.AnandaP.R.PrathiushG.S.MamathaS.RaoPrevalence of gastrointestinal parasites of poultry in and around BangaloreVet World12008201202
- G.S.da SilvaD.M.RomeraL.E.C.FonsecaM.V.MeirelesHelminthic parasites of chickens (Gallus domesticus) in different regions of São Paulo State, BrazilBraz J Poult Sci182016163168
- W.AbedeT.AsfawB.GeneteB.KassaP.DorchiesComparative studies of external parasites and gastro-intestinal helminths of chickens kept under different management system in and around Addis Ababa (Ethiopia)Rev Med Vet1481997497500
- F.KaufmannG.DaşB.SohnreyM.GaulyHelminth infections in laying hens kept in organic free range systems in GermanyLivest Sci1412011182187
- C.DahlA.PerminJ.P.ChristensenM.BisgaardA.P.MuhairwaK.M.D.PetersenThe effect of concurrent infections with Pasteurella multocida and Ascaridia galli on free range chickensVet Microbiol862002313324
- N.M.EigaardT.W.SchouA.PerminJ.P.ChristensenC.T.EkstrømF.AmbrosiniInfection and excretion of Salmonella enteritidis in two different chicken lines with concurrent Ascardia galli infectionAvian Pathol352006487493
- Russel LK, Springer WT. Histomoniasis. In Hofstad MS, Calnek BW, Reid WM, Hemlbolt CF, Yoder HW, editors. Diseases of poultry. 7th ed. Iowa, Iowa, USA: Iowa State University; 1978. p 832–40.
