Abstract
Studies on the dispersion of carbon nanotubes (CNTs) in silicon nitride (Si3N4) ceramics to provide the latter with electrical conductivity have been carried out in recent years. The density and the strength of Si3N4 ceramics were degraded, however, because the CNTs prevented Si3N4 from densifying. The CNTs disappeared after firing at high temperatures owing to the reaction between CNTs and Si3N4 or SiO2, or both Si3N4 and SiO2. In order to improve the density and suppress the reaction, sintering aids for lower-temperature densification of Si3N4 are needed. In this study, we added HfO2 as a sintering aid to a Si3N4–Y2O3–Al2O3–AlN–TiO2 system to fabricate CNT-dispersed Si3N4 ceramics at lower temperatures. Furthermore, bead milling was applied to disperse the CNTs homogeneously. Agglomerates of CNTs were pulverized by bead milling without obvious changes in morphology to eliminate larger fracture origins in CNT-dispersed ceramics. As a result of both the addition of HfO2 and bead milling, we successfully fabricated dense CNT-dispersed Si3N4 ceramics with high strength and electrical conductivity.
1 Introduction
Silicon nitride (Si3N4) ceramics have excellent mechanical, chemical, and thermal properties, which include high strength, high toughness, high hardness, high corrosion resistance, high heat resistance, high heat conductivity, and electrical insulation. They have been applied to structural components such as bearings, engine parts, gas turbines, and cutting tools [Citation1–Citation3]. As Si3N4 itself is an insulator, conventional Si3N4 ceramics are also insulators. In some cases, electrical insulation of the Si3N4 ceramics attracts dust easily owing to static electricity, which then reduces the life time of bearings, particularly in tribological applications. One approach to solve this problem is to make the Si3N4 ceramics electrically conductive. Some of the studies on electrically conductive Si3N4 ceramics used the ionic-conductive glassy phase or added conductive particles such as TiN or SiC [Citation4,Citation5]. The mechanical properties of these Si3N4 ceramics are insufficient for structural applications, however.
Carbon nanotubes (CNTs) have high electrical conductivity, high strength, high elastic modulus, high thermal conductivity, and high aspect ratio [Citation6–Citation8] and they are used as fillers for ceramic composites [Citation9–Citation11]. When CNTs are dispersed in the sintered body, electrically conductive paths are formed by the small amount of CNTs [Citation12,Citation13]. Studies to give Si3N4 ceramics electrical conductivity by dispersing CNTs into their structures have been carried out in recent years [Citation14–Citation16]. A common approach uses direct mixing by ball milling ceramic powders with carbon nanotubes and sintering the powders using hot-pressing [Citation17] or spark-plasma sintering (SPS) [Citation18–Citation21]. In our previous studies, the electrically conductive Si3N4 ceramics were fabricated using gas-pressure sintering (GPS [Citation22]) as a conventional sintering method [Citation23,Citation24]. The CNTs inhibited densification in the sintering process, and they reacted with Si3N4 and SiO2 at higher temperatures [Citation12]. The gases generated from the reaction also suppressed densification of the Si3N4 ceramics, and the added CNTs disappeared during firing [Citation24]. In order to suppress the reaction and the disappearance of CNTs, a densification process at a lower temperature was necessary. We focused on HfO2 as a sintering aid because it promotes the densification of Si3N4 on the account of the reaction between HfO2, Y2O3, and SiO2 at around 1600 °C to generate a liquid phase [Citation25,Citation26]. Compared to other sintering aids, HfO2 makes it more likely for the Si3N4 ceramics to be densified at lower temperatures. As a result, by firing at lower temperatures, suppression of the disappearance of CNTs resulted in the dense CNT-dispersed Si3N4 ceramics with high electrical conductivity [Citation27]. The bending strength of CNT-dispersed Si3N4 ceramics was degraded, however, because of the presence of larger fracture origins resulting from the agglomerations of CNTs. It is well known that CNTs easily form strong agglomerates owing to van der Waals forces serving as the attractive intermolecular forces. The agglomerates of CNTs induced large defects, which degraded their mechanical properties. For this reason, homogeneous dispersion of CNTs is needed to improve the strength of CNT-dispersed ceramics. Among several types of mechanical processes for the dispersion of nanoparticles, bead milling is well-known as an effective technique to disperse nanoparticles using a fine grinding medium (<1 mm). Yoshio et al. reported that bead milling led to well pulverized agglomerates of CNTs, which were uniformly dispersed in ethanol [Citation28]. In this case, the bending strength of CNT-dispersed Si3N4 ceramics was improved; however, the electrical conductivity decreased. The objective of this study is to fabricate CNT-dispersed Si3N4 ceramics with high strength and electrical conductivity at the same time by adding HfO2 as a sintering aid for lower-temperature densification and by bead milling to disperse the CNTs homogeneously.
2 Experimental procedure
Multiwalled carbon nanotubes (VGCFs™, Showa Denko Co., Japan) with an average diameter of 60 nm and an average length of 6 μm, were used in this study. These as-received CNTs formed strong agglomerates or bundles through attractive intermolecular forces. The CNTs were dispersed in ethanol by bead milling. CNTs (0.2 wt.%) were added to ethanol with polyethylenimine (EPOMIN SP-103, M.W. = 250, Nippon Shokubai Co., Japan) as a dispersant. The amount of the added dispersant was 0.5% of the weight of ethanol (CNTs). After premixing the CNTs and ethanol by ultrasonic irradiation for 20 min, bead milling (Minicer, Ashizawa Finetech Ltd., Japan) was applied to the premixed CNT suspension for 2 h at 3000 rpm. The medium used in this study was Al2O3 beads measuring 300 μm in diameter.
CNT-dispersed Si3N4 ceramics were fabricated using the CNT suspension prepared by bead milling. The basic chemical composition of the sintering aid was 5 wt.% Y2O3, 3 wt.% Al2O3, and 5 wt.% AlN. Either 5 wt.% HfO2 or 5 wt.% TiO2 was also added to this batch composition, and the resulting sample was labeled sample H or sample T accordingly. The CNTs were then added to the ceramics powder in concentrations of 0, 0.5, and 1.0 wt.%. The powder was mixed with the CNT suspension by ball milling at 110 rpm for 24 h, using Si3N4 balls measuring 5 mm in diameter. After eliminating the ethanol, these powders were passed through a 250-μm mesh sieve to obtain the granules, which were molded into green bodies. After dewaxing, they were fired at a temperature from 1600 to 1750 °C in a 0.9-MPa N2 atmosphere for 2 h. After gas-pressure sintering, hot-isostatic pressing (HIP) was carried out at 1700 °C in 100-MPa N2 for 1 h. For comparison, CNT-dispersed Si3N4 ceramics were also fabricated using ball milling with the same firing condition.
After machining, the density was measured by Archimedes’ method, the phases present were estimated by X-ray diffractometry (Multiflex, Rigaku Corp., Japan), the microstructure was observed by scanning electron microscopy (JSM-6390LA, JEOL Ltd., Japan), the inner structure was observed by infrared microscopy (BX-51IR, Olympus Corp., Japan), the electrical conductivity was measured by 4-terminal method. Bending strength was measured by 3-point bending test having 30 mm of lower span at 0.5 mm/min of loading rate using mechanical testing machine (Autograph AG-X, Shimadzu Corp., Japan), with the reference to JISR1601. Hardness was evaluated using Vickers hardness tester (AVK-C1, Akashi, Japan) by loading at 198 N with the reference to JISR1610. Fracture toughness was measured by IF method at the same time as measurement of the hardness and it was calculated using the equation proposed by Niihara [Citation29].
3 Results and discussion
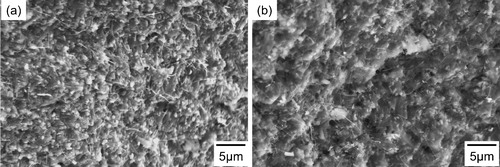
shows the SEM images of the fracture surfaces of two sample H's with 1.0 wt.% CNTs mixed by bead milling and ball milling and then fired at 1600 °C. The white tuberous particles that appeared to protrude from the fracture surfaces were CNTs in both of the samples. It was found that many CNTs remained in the sintered body, which means that even in the bead-milling process, the reaction between CNTs and Si3N4 or SiO2, or both Si3N4 and SiO2, was suppressed by the addition of HfO2 as a sintering aid. Yoshio et al. reported that the morphology of the CNTs remained unchanged after bead milling [Citation28]. Although bead milling effectively pulverized the CNTs because of the high shear stress, the mechanical damage incurred by bead milling was found to be as minor as that by ball milling because the reaction should have occurred at the defect sites on the CNT surfaces. A comparison of the fracture surfaces of CNT-dispersed Si3N4 ceramics showed the random presence of CNTs in both samples prepared by ball milling and bead milling; in other words, the microstructure in a local area was independent of the milling process.
Fig. 1 SEM images of the fracture surfaces of sample H's with 1.0 wt.% CNTs prepared by (a) bead milling and (b) ball milling and then fired at 1600 °C.
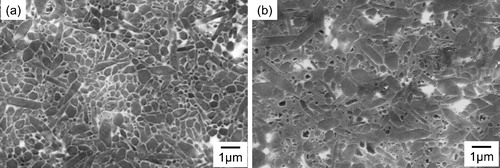
shows SEM images of etched surfaces of samples with 1.0 wt.% CNTs mixed by (a) bead milling and (b) ball milling, followed by firing at 1600 °C. Elongated β-Si3N4 grains were found in both samples. The size of Si3N4 in the sample prepared by bead milling was smaller than that prepared by ball milling. Yoshio et al. reported that CNTs exist at the grain boundaries in Si3N4 ceramics and limit grain-boundary migration in these ceramics [Citation24]. The CNTs were better dispersed in the bead-milled sample than in the ball-milled sample. The larger grain boundaries in the bead-milled sample further limited grain-boundary movement during sintering owing to the homogeneous dispersion of CNTs. As a result, a microstructure with finer β-Si3N4 grains was obtained by bead milling.
Fig. 2 SEM images of the microstructures of the sample H's with 1.0 wt.% CNTs prepared by (a) bead milling and (b) ball milling and then fired at 1600 °C.
lists the density, bending strength, electrical conductivity, fracture toughness, and Vickers hardness of sample H's fired at (a) 1600, (b) 1650, (c) 1700, and (d) 1750 °C. The relative densities of all the specimens were very high: over 94% after GPS and over 99% after HIP. In particular, the relative density after GPS was higher in the bead-milled sample than the ball-milled sample. Inhomogeneous sintering and grain growth resulting from the inhomogeneous dispersion of CNTs should have degraded the density of the sintered body. As a result, by adding HfO2 as a lower-temperature sintering aid and employing bead milling, dense CNT-dispersed Si3N4 ceramics were obtained after firing at 1600 °C.
Table 1 Mechanical and electrical properties of sample H's prepared by bead milling or ball milling and then fired at (a) 1600, (b) 1650, (c) 1700, and (d) 1750 °C.
Bead milling also improved the bending strength of CNT-dispersed Si3N4 ceramics compared with ball-milled samples at any firing temperature. The bending strength of a bead-milled sample was as high as those of Si3N4 ceramics without CNTs. The electrical conductivities of CNT-dispersed Si3N4 ceramics with 0.5 and 1.0 wt.% CNTs fired at 1750 °C were 1.5 × 10−4 and 11 S/m, respectively. By using bead milling, the electrical conductivity of the CNT-dispersed Si3N4 ceramics was developed while maintaining the high bending strength. In this study, the bending strength and electrical conductivity of the CNT-dispersed Si3N4 ceramics prepared by adding 1.0 wt.% CNTs fired at 1600 °C were 980 MPa and 5.6 S/m respectively, which were higher than the values of CNT-dispersed Si3N4 ceramics fired at 1600 °C that Yoshio et al. obtained by bead milling [Citation28]. These improvements resulted from the addition of HfO2 as a sintering aid. The fracture toughness and Vickers hardness of the bead-milled CNT-dispersed Si3N4 ceramics were as high as those of the ball-milled CNT-dispersed Si3N4 ceramics.
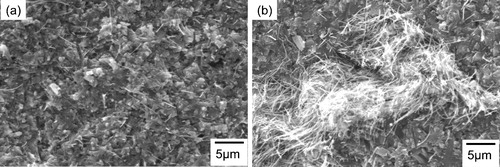
In order to understand how bead milling improved the strength of the CNT-dispersed Si3N4 ceramics, the origins of fractures in the ceramic samples were observed. shows the SEM images of the fracture origins of the samples with 1.0 wt.% CNTs prepared by (a) bead milling and (b) ball milling and fired at 1600 °C. A fracture origin in the sample prepared by ball milling was a large agglomerate of CNTs with a diameter of over 30 μm, which resulted from insufficient dispersion of the CNTs. Consequently, the strength of the CNT-dispersed Si3N4 ceramics was degraded in the ball-milled sample. On the other hand, a fracture origin of the CNT-dispersed Si3N4 ceramics prepared by bead milling was a pore or a region of insufficient densification, which was smaller than the agglomerate of CNTs in the ball-milled sample. The decrease in the size of the fracture origin resulted in a bending strength that was as high that of the Si3N4 ceramics without CNTs (as shown in a).
Fig. 3 SEM images of the origins of fractures in sample H's with 1.0 wt.% CNTs prepared by (a) bead milling and (b) ball milling and then fired at 1600 °C.
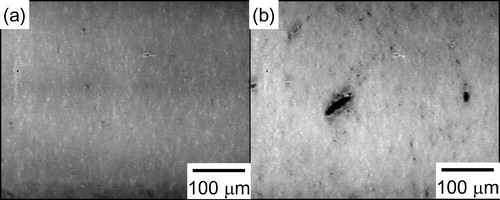
Uematsu et al. reported that observation of the internal structure by transparent optical microscopy was very effective in the detection of all but a few large defects that controlled the strength in the ceramics [Citation30,Citation31]. However, such an observation under visible light was not suitable for the CNT-dispersed Si3N4 ceramics because Si3N4 has a high reflective index and CNTs easily absorb visible light. In this study, the transmission mode of infrared microscopy [Citation32] was applied to observe the internal structure of the CNT-dispersed Si3N4 ceramics. shows the infrared microscope images of the internal structure of the samples with 1.0 wt.% CNTs fabricated by (a) bead milling and (b) ball milling and then fired at 1600 °C. The darker regions in the photographs should correspond to the regions with higher concentrations of CNTs. In the CNT-dispersed Si3N4 ceramics prepared by ball milling, there was a large darker region measuring over 30 μm, which was similar to the size of a fracture origin on the fracture surface. This means that there were many large agglomerates of CNTs in the sample prepared by ball milling. On the other hand, there was no such large darker region in the CNT-dispersed Si3N4 ceramics prepared by bead milling, i.e., the ceramics had a more homogeneous inner structure. It was found that the higher strength of the bead-milled sample resulted from the homogeneous structure.
4 Conclusions
In this study, CNT-dispersed Si3N4 ceramics were fabricated by adding HfO2 for lower-temperature densification and using bead milling for homogeneous dispersion of CNTs. The relative densities of the samples fabricated by bead milling were higher than those of the samples fabricated by ball milling. The bending strength and electrical conductivity of the CNT-dispersed Si3N4 ceramics with 1.0 wt.% CNTs fired at 1600 °C were 980 MPa and 5.6 S/m, which were higher than those of the samples prepared in a previous study. The bending strength of the CNT-dispersed Si3N4 ceramics fabricated by bead milling was improved from those of samples fabricated by ball milling because of pulverization of the CNT agglomerates. Bead milling together with the addition of HfO2 is an effective process to improve the strength of CNT-dispersed Si3N4 ceramics.
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- K.KomeyaH.KotaniJSAE Rev.719867279
- Y.TajimaMater. Res. Soc. Symp. Proc.2871993198201
- M.HottaN.KondoH.KitaT.OhjiY.IzutsuT.ArimaJ. Asian Ceram. Soc.132013308313
- H.KawaokaT.SekinoT.KusunoseK.NiiharaJ. Mater. Res.18200327522755
- L.GaoJ.G.LiT.KusunoseK.NiiharaJ. Eur. Ceram. Soc.242004381386
- S.IijimaNature354199156
- S.RochieAnn. Chim. Sci. Mater.252000529532
- H.J.DaiSurf. Sci.5002002218241
- G.D.ZhanJ.D.KuntzJ.WanA.K.MukherjeeNat. Mater.220033842
- J.H.LehmanK.E.HurstG.SinghE.MansfieldJ.D.PerkinsC.L.CromerJ. Mater. Sci.45201042514254
- R.BhandavatW.KuhnE.MansfieldJ.LehmanG.SinghACS: Appl. Mater. Interfaces4120121116
- J.TatamiT.KatashimaK.KomeyaT.MeguroT.WakiharaJ. Am. Ceram. Soc.88200528892893
- S.YoshioJ.TatamiT.WakiharaK.KomeyaT.MeguroKey Eng. Mater.40320091922
- E.L.CorralJ.CesaranoIIIA.ShyamE.Lara-CurzioN.BellJ.StueckerN.PerryM.Di PrimaZ.MunirJ.GarayE.V.BarreraJ. Am. Ceram. Soc.9110200831293137
- C.BalázsiZ.KónyaF.WéberL.P.BiróP.AratóCompos.: Pt. B32006418424
- A.KovalčíkováCs.BalázsiJ.DuszaO.TapasztóCeram. Int.382012527533
- J.DuszaG.BluganJ.MorgielJ.KueblerF.InamT.PeijsM.J.ReeceV.PuchyJ. Eur. Ceram. Soc.29200931773184
- M.BelmonteJ.González-JuliánP.MiranzoM.I.OsendiJ. Eur. Ceram. Soc.30201029372946
- C.BalázsiZ.ShenZ.KónyaZ.KasztovszkyF.WéberZ.VértesyL.P.BiróI.KiricsiP.AratóCompos. Sci. Technol.652005727733
- E.L.CorralH.WangJ.GarayZ.MunirE.V.BarreraJ. Eur. Ceram. Soc.312011391400
- F.InamH.YanD.D.JayaseelanT.PeijsM.J.ReeceJ. Eur. Ceram. Soc.302010153157
- M.MitommoJ. Mater. Sci.116197611031107
- M.MatsuokaS.YoshioJ.TatamiT.WakiharaK.KomeyaT.MeguroJ. Korean Ceram. Soc.4942012333336
- S.YoshioJ.TatamiT.WakiharaT.YamakawaH.NakanoK.KomeyaT.MeguroJ. Ceram. Soc. Jpn.119120117075
- H.LiK.KomeyaJ.TatamiT.MeguroY.ChibaM.KomatsuJ. Ceram. Soc. Jpn.10942001342346
- D.HorikawaJ.TatamiT.WakiharaK.KomeyaT.MeguroKey Eng. Mater.40320093538
- M.MatsuokaS.YoshioT.YamakawaJ.TatamiT.WakiharaK.KomeyaT.MeguroTrans. MRS J.37120121114
- S.YoshioJ.TatamiT.YamakawaT.WakiharaK.KomeyaT.MeguroK.AramakiK.YasudaCarbon26201141314137
- K.NiiharaR.MorenaD.P.H.HasselmanJ. Mater. Sci. Lett.119821316
- N.ShinoharaM.OkumiyaT.HottaK.NakahiraM.NaitoK.UematsuJ. Am. Ceram. Soc.837200016331640
- S.NakamuraS.TanakaR.FurushimaK.SatoK.UematsuJ. Ceram. Soc. Jpn.11762009742747
- K.UematsuN.UchidaZ.KatoS.TanakaT.HottaM.NaitoJ. Am. Ceram. Soc.8412001254256