Abstract
The fabrication of steel-matrix composites locally reinforced with TiC particulates by the self-propagating high-temperature synthesis (SHS) reactions in the 20 wt.% Cu–Ti–C system with various C particle sizes during casting has been investigated, respectively. The composites synthesized with ∼1 μm and ∼38 μm C particles consist of TiC, Cu and γ-Fe phases, while the one synthesized with ∼150 μm C particle mainly consists of TiC, Cu, γ-Fe and Fe2Ti phases. With the increase in the C particle size, the interface bonding between the reinforced region and matrix becomes poor, as well as the number of pores and blowholes in the locally reinforced region increases. The hardness and wear resistance of the composites are significantly higher than those of the matrix. With the decrease of the size of C particle, the hardness and wear resistance of the composites increase.
1 Introduction
It is well established that the steel matrix composites commonly have a good combination of hard ceramic (e.g. TiC, TiB2, WC and Al2O3) reinforcements and ductile metallic matrix, which make them become a promising candidate in wear resistance applications [Citation1–Citation4]. Generally, there are several methods for fabricating the particulate reinforced steel matrix composites, such as powder metallurgy [Citation5], conventional melting and casting [Citation6], reactive sintering [Citation7,Citation8], and self-propagating high-temperature synthesis (SHS) [Citation9,Citation10]. Among several fabrication techniques to synthesize in situ ceramic particulates locally reinforced steel matrix composites, SHS technology has attracted much attention because of its self-generation of energy required for the process, high-purity products as a result of the volatilization of some low boiling point impurities at elevated temperatures, high productivity and low energy consumption [Citation11,Citation12]. Therefore, the SHS and traditional casting routes provide an easy process to produce ceramic particulate locally reinforced steel matrix composites [Citation13,Citation14]. It combines the advantages of the SHS reaction with the easiness and the casting process with high efficiency.
Among various ceramic particulates, TiC is a potential material because of its outstanding properties, such as high hardness, low density, high melting temperature, high modulus, good wear and corrosion resistance as well as good wettability and stability in the steel melt compared to other ceramics [Citation15]. Therefore, the TiC is widely used as the reinforcement in steel matrix composites. Recently, TiC particulate locally reinforced steel matrix composites fabricated by SHS reaction from a Metal (Me)–Ti–C systems (Metal: Al, Cu, Ni, Fe and so on) have been the subject of intensive investigation [Citation16–Citation18]. Unfortunately, the very high reaction heat released during the formation of pure TiC could cause the appearance of large number of pores in the local reinforcing region. These pores can influence the wear resistance property of the steel matrix composites greatly. If the volume fraction of the TiC particulates in the local reinforcing region decreases, the porosity may decrease due to the decreasing combustion temperature in the SHS reaction. However, it may lower the wear resistance properties of the local reinforcing region.
Thus, in the present study, an attempt is carried out to decrease the porosity of the local reinforcing region and improve the resistance property by changing the sizes of C particle in the Cu–Ti–C system with no change of the volume fraction of the reinforcement in the local reinforcing region. The microstructures, porosity, hardness and wear resistance of the composites synthesized by using 20 wt.% Cu–Ti–C system with various sizes of C particle are investigated, respectively. It is expected that the preliminary results could be significant in promoting the development and practical application of the in situ particulate locally reinforced steel matrix composites.
2 Experimental details
The starting materials were made from commercial powders of Cu (99.5% purity, average particle size of ∼6 μm), Ti (99.5% purity, average particle size of ∼15 μm) and C (99.9% purity, average particle size of ∼1 μm, ∼38 μm, ∼75 μm and ∼150 μm, respectively). Ti and C powders with a ratio corresponding to that of the stoichiometric TiC mixed with 20 wt.% Cu content were used for the powder blends. After mixing for 6 h, the blends were uniaxially pressed into cylindrical compacts (about 22 mm in diameter and 14 ± 1 mm in length) to obtain green densities of 70 ± 2% theoretical density. After being dried in an oven at about 150 °C for 3 h to remove any trace of moisture, the compacts with various C sizes were placed at the bottom of a special sand mold, as shown in a. In addition, the combustion characteristics for the reactions with various C sizes were also studied by the differential thermal analysis (DTA, Rigaku-8150, Japan) experiments, which were conducted in a flowing argon gas (flowing rate: 60 ml/min) using a heating rate of 40 °C/min.
Fig. 1 Schematic diagram of the (a) compacts located in the sand mold and (b) composite material casting.
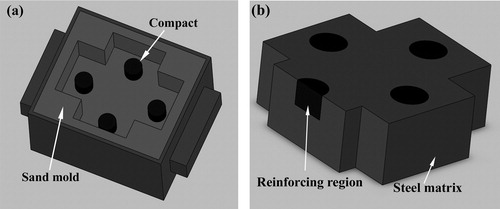
The medium manganese steel (0.72C–0.45Si–8.6Mn–Fe balance, all in wt.%) melt was prepared in a 5 kg medium-frequency induction furnace. Subsequently, the molten steel (about 1500 °C) was poured into the sand mold to ignite the SHS reactions of these compacts. After solidification, composite castings were removed and sectioned in the side position, as shown in b.
Because it was difficult to achieve the kinetics of SHS reaction of the Cu–Ti–C system with various sizes of C particle in the liquid steel, the SHS experiments were conducted first in a self-made vacuum vessel in order to offer some guidance to the fabrication and investigation of the locally reinforced steel matrix composites. The SHS experiments were conducted in a self-made vacuum vessel filled with Ar at 1 atm. The compacts were ignited on the graphite flat which was placed at the top of tungsten electrode and heated by the heat of arc. A small hole (2 mm in diameter and 2 mm in depth) was drilled at the top of the compact, and a thermocouple pair of W/Re5-W/Re26 (0.5 mm in diameter) was inserted into the hole and linked up with an temperature acquisition recorder by means of which a temperature–time curve could be recorded. More details about the experimental apparatus and procedure for the SHS reaction were given in a previous article [Citation19].
The wear tests were conducted under a load of 35 N using a pin-on-disk apparatus and the 600 mesh SiC abrasive papers were used as the counterface. The wear rate is defined as volume loss divided by sliding distance, and the volume loss is obtained from the ratio of weight loss to the density of the sample. The densities of the samples were measured by Archimedes’ water-immersion method. Microstructures were examined using scanning electron microscopy (SEM) (Model JSM-5310, Japan) together with energy-dispersive spectrometry (EDS) (Model Link-Isis, Britain). The phases were identified by X-ray diffraction (XRD) (Model D/Max 2500PC Rigaku, Japan).
3 Results and discussions
3.1 Effect of C particle size on the thermal analysis
In order to understand the ignition reaction of the 20 wt.% Cu–Ti–C system with ∼1 μm, ∼38 μm and ∼150 μm C particles, DTA experiments were conducted. The DTA curves and XRD patterns of the DTA products are shown in a and b, respectively. It was observed during the experiments that three strong exothermic peaks with the maximum at about 887 °C, 975 °C and 1021 °C as well as two endothermic peaks with the minimum at about 966 °C and 1006 °C appeared in the 20 wt.% Cu–Ti–C system with ∼38 μm C particle [Citation20], respectively. For the reactants with ∼150 μm C particle, the temperatures of the three exothermic peaks and two endothermic peaks are very similar to those in the reactants with ∼38 μm C particle. DTA curves indicate that two exothermic peaks with the maximum at about 975 °C and 1072 °C appeared in the reactants with ∼1 μm C particle. It is different from the systems with ∼38 μm and ∼150 μm C particles.
Fig. 2 (a) DTA curves and (b) XRD patterns of the DTA products synthesized with ∼1 μm, ∼38 μm and ∼150 μm C particles.
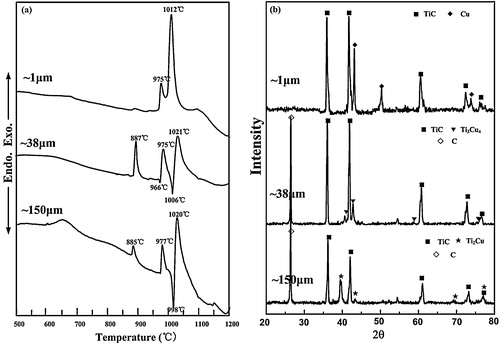
According to the previous studies [Citation20], when the 20 wt.% Cu–Ti–C system with ∼38 μm and ∼150 μm C particles was heated to 887 °C, a large number of TixCuy compounds were formed initially via solid-state diffusion reactions between Cu and Ti particles. The melting points and eutectic points of the TixCuy compounds are much lower than those of Cu and Ti. Once the temperatures reached the melting points or eutectic points of the TixCuy compounds, the Cu–Ti liquid formed quickly. Therefore, the endothermic peak at 966 °C corresponds to the formation of the Cu–Ti liquid phase. The formation of liquids and continuous heating significantly promote the diffusion of C and the formation of the Cu–Ti–C liquids; subsequently, TiC particulates were precipitated out of the liquids, which was an exothermic reaction, corresponding to the two exothermic peaks with the maximum at about 975 °C and 1021 °C in the DTA curves. According to these results, the ignition temperature of the SHS reaction could be prophesied at about 960 °C in the reactants with ∼38 μm and ∼150 μm C particles. According to the XRD patterns of the DTA products with ∼38 μm and ∼150 μm C particles quenched from 1200 °C, as shown in b, a large quantity of TiC are formed, and a small quantity of C and TixCuy compounds remain as well.
One question is how TiC is formed in the 20 wt.% Cu–Ti–C system with ∼1 μm C particle. It can be seen from a that the two exothermic peaks with the maximum at about 975 °C and 1012 °C appeared in the reactants with ∼1 μm C particle. It may be the reason that the ignition temperature of the SHS reaction could be prophesied at about 975 °C in the reactants with ∼1 μm C particle. Actually, it is well known that the finer C particle size leads to the decrease of contact area between Cu and Ti and the increase of contact area between Ti and C, which may delay the occurrence of reaction between Cu and Ti. However, once the Cu–Ti liquid is formed, C will quickly diffuse into the liquid to form TiC particulates. Because the fine C particle can enlarge the surface contact area between liquid and C particle, which in turn improves the rate of surface reaction between them, this results in a fact that the rate of heat dissipation is lower than that of heat release, and the reaction in the 20 wt.% Cu–Ti–C system with ∼1 μm C particle is complete without any intermediate phase, as shown in b.
DTA experimental results are valuable to help understanding the ignition behavior of Cu–Ti–C system with various C particle sizes. The results suggest that all the SHS reaction events of Cu–Ti–C systems with various C particle sizes start with the formation of TixCuy compounds and Cu–Ti liquids, respectively; subsequently, C diffuses into the liquids to form TiC, which is a highly exothermic reaction to ignite SHS reaction. Therefore, the rate of surface reaction between C particles and liquid in the local region is important to ignite SHS reaction. When coarse C particles are used, the rate of surface reaction is lowered, and it limits the formation of large quantities of TiC. And the heat which is released from the formation of a few TiC cannot make the reaction self-sustained. As a result, it requires a long heating time to ignite the SHS reaction of Cu–Ti–C system with the coarse C particles. Oppositely, the SHS reaction in the Cu–Ti–C system with the fine C particles can be ignited quickly.
The melting temperature of the steel matrix is about 1500 °C in this study, which is significantly higher than the formation temperature (960–975 °C, as mentioned above) of the TiC. When the molted steel was poured into the sand mold, the SHS reaction of the Cu–Ti–C system was ignited by the heat from the melted steel.
3.2 Effect of C particle size on the SHS reaction
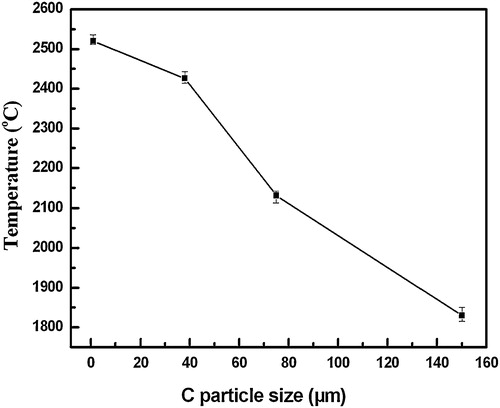
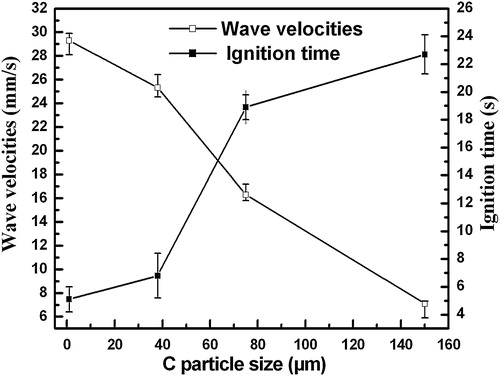
The variation of the maximum combustion temperature (Tc) with various sizes of C particle in the 20 wt.% Cu–Ti–C system was shown in . It can be seen that the Tc was approximately 2520 °C, 2426 °C, 2132 °C and 1830 °C for the C particle sizes of ∼1 μm, ∼38 μm, ∼75 μm and ∼150 μm, respectively. Clearly, the Tc decreases with the increase in the sizes of the C particle.
shows the variations of the ignition time and combustion wave velocity with various sizes of C particle. As indicated, the SHS reactions were ignited after heating for 5.1 s, 6.8 s, 18.9 s and 22.7 s for the ∼1 μm, ∼38 μm, ∼75 μm and ∼150 μm C particles, respectively. It is obvious that the ignition time shows a noticeable increase with the increase in the size of C particle, implying that the ignition of the SHS reaction becomes more and more difficult. According to the previous result [Citation10], the solidification time of the austenite manganese steel melt was estimated to be 62.7 s. Therefore, during the solidification process of the melted steel, it has sufficient time to ignite SHS reaction in the 20 wt.% Cu–Ti–C system with ∼1 μm, ∼38 μm, ∼75 μm and ∼150 μm C particles. The combustion wave velocity decreases monotonically with the increase in the size of the C particle as indicated in . The variations in the combustion temperature, ignition time and combustion wave velocities with various C particle sizes imply that the C particle size has a significant effect on the SHS behavior.
Fig. 4 Variation of the ignition time and combustion wave velocities in the 20 wt.% Cu–Ti–C system with various C particles.
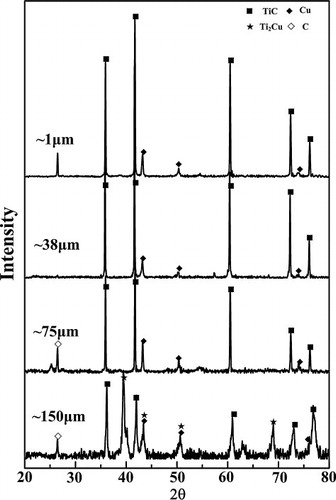
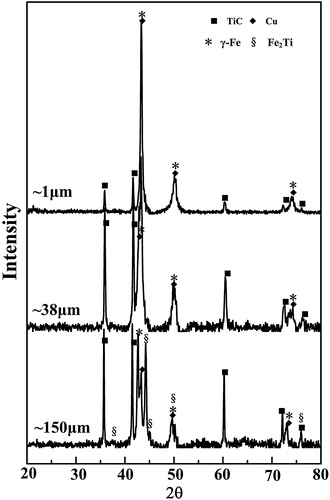
shows the XRD patterns of the SHS products for the samples with various C particle sizes. It can be seen that only TiC and Cu phases are detected in the products with ∼1 μm, ∼38 μm and ∼75 μm C particles, indicating that the reactions are complete. When the C particle size increases up to ∼150 μm, a small amount of Ti2Cu phase and unreacted C appeared besides the major phases of TiC and Cu, which suggests that the reaction products are dependent on the C particle size.
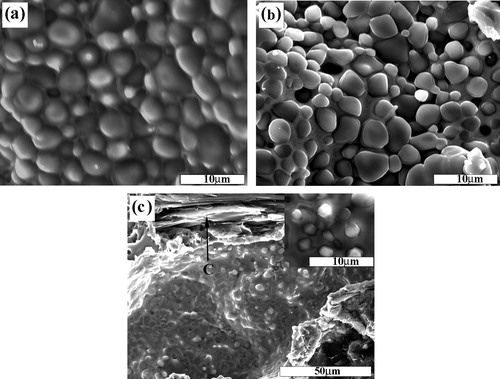
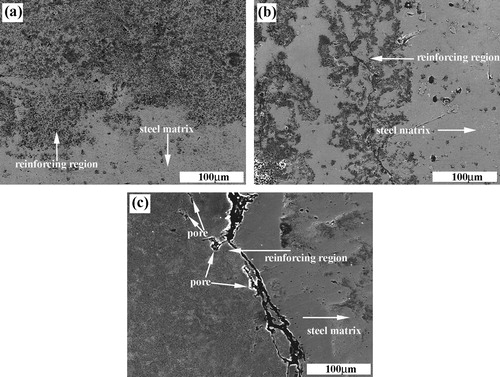
a–c shows the SEM images of the fracture surfaces of the SHS products with ∼1 μm, ∼38 μm and ∼150 μm C particle sizes, respectively. As shown in a and b, a large number of near-spherical TiC particulates are formed. While in c, large numbers of unreacted C particles are appeared. The fine TiC particulates exhibit near-spherical shape. The microstructure observation is consistent with the XRD results. With the increase in the size of C particle, the size of TiC particulate decreases considerably. The size reduction of the ceramic particulates may be caused by the lower combustion temperature of the reactants with coarse C particles, because crystal growth is an exponential function of the combustion temperature.
3.3 Effect of C particle size on the steel matrix composites
3.3.1 Phase components and microstructures
The austenite manganese steel-matrix composites locally reinforced with in situ TiC particulates were successfully fabricated using SHS reactions in the 20 wt.% Cu–Ti–C system with various C particle sizes during casting. XRD patterns of the locally reinforced austenite manganese steel-matrix composites are shown in . The XRD results reveal that the composites synthesized with ∼1 μm and ∼38 μm C particles consist of TiC, Cu and γ-Fe without any intermediate phases. While the composite synthesized with ∼150 μm C particle consists of TiC, Cu, γ-Fe and an intermediate Fe2Ti phase, which suggests that the reaction is not completed. Generally, the SHS reaction products are extremely porous, and therefore, the molten steel will spontaneously infiltrate into the pores of the reacted compacts driven by capillary forces during casting. As a result, a large quantity of γ-Fe is detected in the locally reinforcing region. Moreover, the TiC peaks in the composites fabricated by using ∼150 μm C particle are more intense than those in the compacts in the combustion chamber, suggesting the voluminous formation of TiC in the steel-matrix composite.
Fig. 7 XRD patterns of the local reinforcing region fabricated in the 20 wt.% Cu–Ti–C system with ∼1 μm, ∼38 μm and ∼150 μm C particles.
shows the SEM microstructures of the locally reinforced regions synthesized by using various sizes of C particles. It can be clearly seen that the TiC particulates exhibit the near-spherical shape and a relatively uniform distribution. These particulate morphologies are similar to that in the compacts in the combustion chamber. The sizes of TiC particulates in the composites synthesized by using ∼1 μm and ∼38 μm C particles are larger than those synthesized with the ∼150 μm C particle, which can be attributed to the increase of the combustion temperature (as shown in ). Moreover, during the SHS reaction, the temperature of the combustion zone is so high that the impurity diminishes, and thus, a large number of pores appear; simultaneously, steel melt infiltrates into the pores of the reacted perform and quickly transfers the heat emitted, which decreases the temperature of the combustion zone and shortens the growth time of crystals. Finally, some finer ceramic particulates are formed in the composites. Therefore, compared with the sizes of TiC particulates in the SHS products in the combustion chamber, the shape of TiC particulates in the composites becomes finer, as shown in .
3.3.2 Interface between reinforcing region and matrix
The interface micrographs of the composites synthesized by using 20 wt.% Cu–Ti–C system with ∼1 μm, ∼38 μm and ∼150 μm C particles are shown in , respectively. As shown in a and b, there developed a good metallurgical bonding between the reinforced region and matrix in the steel matrix composite synthesized with ∼1 μm and ∼38 μm C particles. For the composite with ∼150 μm C particle, the interface bonding becomes poor and there are some large pores (see c). It can be seen from that the SHS reactions in the 20 wt.% Cu–Ti–C system with ∼1 μm, ∼38 μm and ∼150 μm C particles are ignited after heating for 5.1 s, 6.8 s and 22.7 s, respectively, which are much shorter than the solidification time of the steel melt. Furthermore, the finer C particles lead to higher combustion temperature and faster combustion wave velocity, which makes sure that there is sufficient time for the steel melt to infiltrate into the reacted compact leading to the formation of a good interface bonding. Contrarily, the coarse C particles lead to lower combustion temperature and wave velocity. As a consequence, when the SHS reaction in the compact occurs, some steel melt may start to solidify and the fluidity of melt becomes poor, which constrain the sufficient infiltration of steel melt and lead to the poor interface bonding.
3.3.3 Porosity, hardness and wear resistance
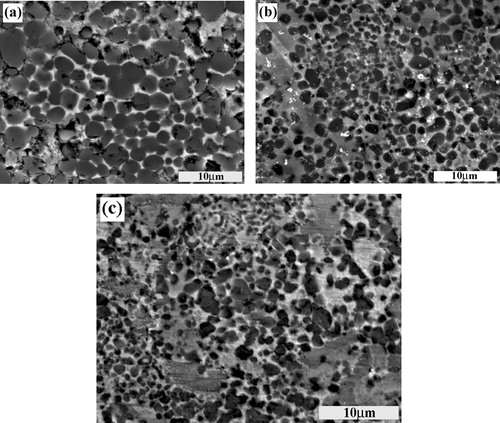
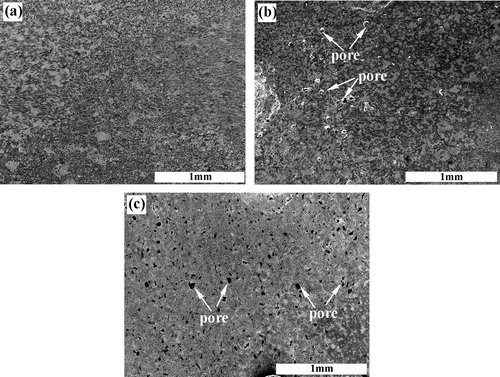
The distribution of pores and blowholes in the local reinforcing region of the steel matrix composites fabricated by using ∼1 μm, ∼38 μm and ∼150 μm C particles is shown in a–c, respectively. It can be seen that a large number of macro-pores and blowholes exist in the composite synthesized with ∼150 μm C particle. While few pores and blowholes can be found in the composites synthesized with ∼1 μm and ∼38 μm C particles, which reveals that near fully dense locally reinforced composites are fabricated successfully. Shorter ignition time, higher combustion temperature and faster wave velocity are in favor of the impurity evaporation from the melt and the sufficient infiltration of the steel melt, which can decrease the pores and increase the densities of the composites. Thus, the composites synthesized with ∼1 μm and ∼38 μm C particles have the fewest pores and the highest density.
Fig. 10 The distribution of pores in the local reinforcing region fabricated in the 20 wt.% Cu–Ti–C system with (a) ∼1 μm C, (b) ∼38 μm C and (c) ∼150 μm C particles.
The hardness values and wear rate of the steel matrix and the reinforced region are listed in . It can be seen from that the hardness and wear resistance of the reinforced region are significantly higher than those of the matrix. The improvement of the hardness and wear resistance is mainly attributed to the presence of high volume fraction of TiC ceramics. Furthermore, as the size of C particle decreases, the hardness and wear resistance of the composites increase, and the composite synthesized with ∼1 μm C particle has the highest hardness and develops the best wear resistance. It may be the reason that the composite synthesized with finer C particles has a near fully dense microstructure. Moreover, due to the shorter ignition time, higher combustion temperature and faster wave velocity of the compact synthesized with finer C particles, the molten steel could sufficiently infiltrate into the reacting compacts and surround the TiC ceramic particulates, which is also a benefit for the improvement of the hardness and wear resistance.
Table 1 The hardness values and wear rate of the steel matrix and the reinforcing regions.
4 Conclusions
In the SHS reaction of the 20 wt.% Cu–Ti–C system in the combustion chamber, the increase of the C particle size not only makes the ignition and self-sustainability of the SHS reaction difficult, but also decreases the combustion temperature and combustion wave velocity. The reaction products are dependent on the size of C particles. With the increase of the C particle size, the size of the TiC particulates decreases considerably.
DTA results show that the melting temperature of the steel matrix is higher than the formation temperature of the TiC particulates in the SHS reaction of the 20 wt.% Cu–Ti–C system with various C particle sizes. The SHS reaction of the 20 wt.% Cu–Ti–C system can be ignited by the heat from the melted steel.
Steel matrix composites synthesized with ∼1 μm and ∼38 μm C particles consist of TiC, Cu and γ-Fe without any intermediate phases. While the composite synthesized with ∼150 μm C particle consists of TiC, Cu, γ-Fe and an intermediate Fe2Ti phase. The size of the TiC particulates in the local reinforcing regions decreases with the increase of the C particle size. Moreover, with the increase in the C particle size, the interface bonding between the reinforced region and matrix becomes poor, the macro-pores and blowholes in the local reinforcing region become more. The hardness and wear resistance of the composites are significantly higher than those of the matrix. As the size of C particles decreases, the hardness and wear resistance of the composites increase, and the composite synthesized with ∼1 μm C particle has the highest hardness and best wear resistance.
Acknowledgements
This work is supported by ooperative Innovation Platform of National Oil Shale Exploration Development and Research, the National Natural Science Foundation of China (Nos. 51205160 and 51375006), the Science and Technology Development Project of Jilin Province (No. 201201025), and the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20120061120110).
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- B.L.ZouP.ShenX.Q.CaoQ.C.JiangMater. Chem. Phys.13220125162
- F.AkhtarJ. Alloys Compd.4592008491497
- A.M.D.NascimentoV.OcelíkM.C.F.IerardiJ.T.M.D.HossonSurf. Coat. Technol.202200821132120
- E.SchlentherC.G.AnezirisT.GrauleJ.KueblerMater. Sci. Eng. A5562012751757
- K.DasT.K.BandyopadhyayS.DasJ. Mater. Sci.37200238813892
- S.W.HuY.G.ZhaoZ.WangY.G.LiQ.C.JiangMater. Des.442013340345
- A.FaridH.FaizulMater. Lett.62200812421245
- Y.WangY.DingJ.WangF.ChengJ.ShiMater. Des.28200722022206
- G.H.LiuJ.T.LiJ. Asian Ceram. Soc.12013134142
- Y.F.YangH.Y.WangY.H.LiangR.Y.ZhaoQ.C.JiangMater. Sci. Eng. A4742008355362
- Z.A.MunirU.A.TamburiniMater. Sci. Rep.31989277365
- W.C.LeeS.L.ChungJ. Am. Ceram. Soc.8019975363
- S.C.TjongZ.Y.MaMater. Sci. Eng. Rep.2920004965
- Y.WangZ.Q.ZhangH.Y.WangB.X.MaQ.C.JiangMater. Sci. Eng. A4222006339345
- M.S.SongB.HuangM.X.ZhangJ.G.LiInt. J. Refract. Met. Hard Mater.272009584589
- Q.C.JiangB.X.MaH.Y.WangY.WangY.P.DongComposites Part A372006133136
- Y.H.LiangZ.W.HanZ.H.ZhangX.J.LiL.Q.RenMater. Des.4020126469
- M.S.SongM.X.ZhangS.G.ZhangB.HuangJ.G.LiMater. Sci. Eng. A4732008166171
- Y.H.LiangZ.W.HanX.J.LiZ.H.ZhangL.Q.RenMater. Chem. Phys.1372012200206
- Y.H.LiangH.Y.WangY.F.YangY.Y.WangQ.C.JiangJ. Alloys Compd.4522008298303