?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Epoxy/porous SiO2 composites were prepared with the pore surface modified using various silane coupling agents. The N2 adsorption and desorption isotherm shows that the porous SiO2 used for raw materials has sufficiently high pore volume. Their pore sizes, calculated using Barrett–Joyner–Halenda method as less than 20 nm was markedly smaller than the mean free path of the gases used for this study. The respective degrees of gas selectivity CO2/N2, CH4/N2, and O2/N2 were measured. Results show that the epoxy/porous SiO2 composite surface-modified by APTES only exhibits CO2/N2 gas selectivity at a lower pressure drop. It originates in the affinity between amino group of the APTES and CO2 gas. The epoxy/porous SiO2 composite treated by APTES also shows gas separation capability. The 80% N2/20% CO2 mix gas was converted into 68.2% N2/31.8% CO2 gas after gas separation tests at 25 °C. The gas separation capability was maintained at high temperatures. The 80% N2/20% CO2 mix gas was converted into 70.8% N2/29.2% CO2 gas at 100 °C.
1 Introduction
Porous materials are widely used as structural and environmental materials, and as biomaterials, among other uses. Gas separation using porous materials has lately attracted attention from the perspective of low running costs. Therefore, zeolite [Citation1–Citation7], carbon [Citation8–Citation14], and silica membranes [Citation15–Citation19] have been actively investigated. Mesoporous silica membranes are now being examined with interest because the pore wall surface is easily modified by silane coupling agents [Citation17–Citation19]. However, mesoporous silica presents some difficulties for use in research. First, its extremely thin pore walls are easily damaged while handling. Secondly, when MCM-41 is used as mesoporous silica, which has straight pores, the pore direction of MCM-41 should orient parallel to the gas permeation direction without pinholes. In light of these circumstances, we previously proposed porous composites having randomly oriented mesopores and pore walls consisting of SiO2 [Citation20]. These porous composites were prepared simply by mixing epoxy and porous SiO2. The SiO2 pores had been previously filled with the appropriate liquid (10% EtOH/90% H2O in mass). Furthermore, the present porous SiO2 was mixed with epoxy resin and a hardener. The obtained samples mainly have approximately 20 nm pores in porous SiO2. Changing the porous SiO2 loading can change the porosity. The composite containing 40–70% porous SiO2 showed gas permeability. The obtained porous composites showed Knudsen diffusion, which indicates that the gas molecules go through the pores originating to the porous SiO2 because the pore size (around 20 nm) is smaller than the mean free path of N2 and CO2 gas (70–80 nm). These results suggest that the obtained composites are available for evaluation of the relation between properties of the pore wall and the gas separation capability. Moreover, composites of this type are regarded useful for new gas separation filters.
For this study, epoxy/porous SiO2 composites were prepared using modification of pore surfaces with silane coupling agents. Furthermore, the gas selectivity was calculated from the gas permeability coefficient of the provided composites. The relations between the chemical composition of the pore wall and the gas selectivity were considered. Moreover, the temperature dependence of the gas separation capability of the sample showing the highest gas selectivity was investigated.
2 Experimental
2.1 Sample preparation
The preparation concept was presented in an earlier report [Citation20]. For the present study, two preparation methods were used.
One is a method to prepare composites from the epoxy and the surface-modified SiO2 in advance. The 10% EtOH liquid mixture (H2O:EtOH = 9:1 in mass) was mixed with the porous SiO2 presented in using a propeller-less planetary-type mixer (AR-250; Thinky Corp., Tokyo, Japan) rotated at 800 rpm and revolved at 2000 rpm for 2 min. The liquid mixture loading was about 2.5 mL for 1 g of porous SiO2. The mixture was mixed with epoxy resin (EpoFix Resin, Ballerup, Denmark) and hardener (EpoFix Hardeners, Ballerup, Denmark) rotated at 800 rpm and revolved at 2000 rpm for 2 min. The epoxy/porous SiO2 ratio is 50/50 in volume. The obtained mixture was cast into about 20 mm Ø and heated at 60 °C for 48 h.
Table 1 Porous using SiO2 in this study.
The other is a method to prepare composites from epoxy and pure SiO2. Then, the obtained composites were treated using silane coupling agents. The untreated composite was soaked into silane coupling agents in vacuum for 12 h. The sample was dried at 60 °C for 48 h. For gas separation capability tests, the tube-shaped composite (28 mm Ø outside diameter, 19 mm Ø inside diameter, 40 mm long) was also prepared.
All composites were polished with #100 and #2000 emery diamond disks (Maruto Instrument Co. Ltd., Tokyo, Japan). The composite thickness was adjusted to 3.0 mm.
2.2 Characterization
The porous properties were investigated by determining their N2 adsorption and desorption isotherms at 77 K using a high-precision surface area and pore size analyzer (Belsorp mini II; Bel Japan Inc., Osaka, Japan). The sample was preheated at 110 °C for 24 h under vacuum conditions before the measurement. The pore size distributions were calculated from the desorption isotherms using the Barrett–Joyner–Halenda (BJH) method [Citation21]. The surface chemical compositions of the impregnated samples were analyzed using X-ray photoelectron spectroscopy (XPS, model 5500 MT; PerkinElmer Inc.) with monochromated Al Kα radiation having a pass energy of 23.5 eV. To evaluate CO2 affinity, CO2 adsorption and desorption tests using that instrument were also conducted. The CO2 adsorption was evaluated using temperature programmed desorption (TPD; TPD-65, Bel Japan Inc., Osaka, Japan). The sample was preheated in vacuum at 200 °C for 1 h, and was then cooled to room temperature before measurements. CO2 gas was then introduced to the sample and adsorbed at 30 °C for 1 h. After vacuum evacuation, the sample was heated at 10 °C/min in flowing He carrier gas. Then the amount of desorbed gas was detected using a mass spectrometer from 30 to 150 °C. The spectrum was also measured under the same conditions without adsorbing CO2 gas to distinguish desorbed gas and evolved gas during sample decomposition. The spectra were measured for mass quantities of 44 (corresponding to CO2).
The microstructures of the obtained epoxy/porous SiO2 composites were observed using scanning electron microscopy (SEM; JSM-5310, JEOL, Tokyo, Japan).
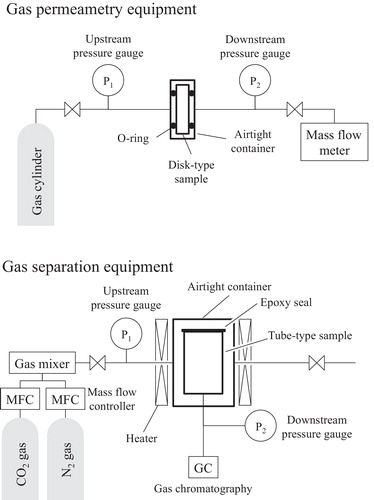
shows the N2 and CO2 gas permeability coefficients, μ, as measured at room temperature in a room with controlled temperature using Eq. Equation(1)(1)
(1) and a gas permeameter. The filter was sealed in an airtight container using Viton® O-rings.
(1)
(1) In Eq. Equation(1)
(1)
(1) , ΔP = (P1 − P2) stands for the pressure drop from the entrance to the exit of the filter (GC61; Nagano Keiki Co., Ltd., Tokyo, Japan); Q denotes the flow rate measured using a mass flow meter (Model 8500; Kojima Instruments Inc., Kyoto, Japan). Here, A and L respectively denote the cross sectional area and the filter thickness. The gas selectivity α is the ratio of the obtained gas permeability coefficient of two gases. The gas separation apparatus is also presented in . shows that the tube-typed sample was set. The mixed gas was prepared from pure N2 and CO2. The CO2 loading of mix gas was adjusted to 20.0%. The constant pressure was controlled using a backpressure valve. The mixed gas flow was more than 12 h. Then, the CO2 loading of mixed gas was measured using micro gas chromatography (CP-4900; Agilent Technologies Inc., CA, United States).
3 Results and discussion
3.1 Characterization of SiO2
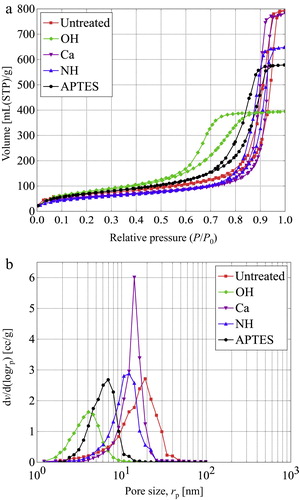
presents results of N2 adsorption and desorption test and the pore size distributions calculated from desorption isotherms using the BJH method. The isotherms of the samples except for those of the OH sample show similarly low adsorption at less than about 0.7 of P/P0. However, they increase very steeply from higher pressure. The maximum adsorption values are, respectively, about 795, 785, 648, and 579 ml (STP)/g for the untreated, Ca, NH, and APTES samples. The pore volume of the samples is the lowest value (about 395 mL/g), corresponding to half the value of the untreated sample. The desorption isotherms show desorption at 0.7–0.8 of P/P0. The isotherm of OH sample shows that the adsorption is observed at 0.6–0.9 of P/P0, which is slightly lower than the others. Furthermore, desorption is suppressed at P/P0 more than 0.7–0.8. The characteristics of these adsorption–desorption isotherms correspond to type IV behavior according to the IUPAC classification [Citation22], typically given by mesoporous silica gel [Citation23–Citation26]. The change of isotherms is thought to originate in surface modification. The pore size distribution calculated from the results above illustrates slightly different curves for each sample ((b)). Apart from slight differences, the average pore size was 5–20 nm, which was sufficiently smaller than the size of mean free path of the gases.
3.2 Preparation of epoxy/porous SiO2 composites
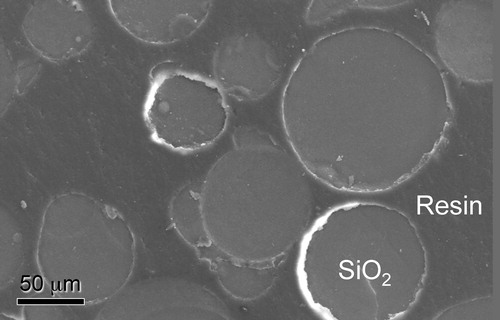
The epoxy/porous SiO2 composites were prepared from these porous SiO2 and epoxy resin. shows a typical SEM micrograph of the obtained epoxy/porous SiO2 composites. The spherical porous SiO2 was buried in epoxy resin, and partially contacting with each other. This microstructure was same as the previous report [Citation20]. For the present study, the epoxy/porous SiO2 composites were prepared by two methods. Both samples showed mostly the same microstructure. There may be no difference in this study; however, it is expected that one of the two methods provided in this study is available in the future work, e.g., preparation of samples having special microstructures or sample for other applications.
3.3 Gas selectivity
shows the gas selectivity of the obtained epoxy/porous SiO2 composites as a function of the pressure drop. Pellet-shaped composites were used in this section. The O2/N2 gas selectivity was about 0.9 at all pressure drops measured in this study. It originates from the fact that the gas permeability of O2 was slightly lower than that of N2. Theoretical values of the gas selectivity, , of Knudsen diffusion were calculated as follows using the respective molecular weights M of two molecules [Citation27].
(2)
(2) The gas selectivity of Knudsen diffusion is about 0.94, corresponding to the experimentally obtained results. It also suggests that O2 and N2 gas have no affinity with all modified pore walls because the gas selectivity of all composites shows the same value. Moreover, as described in Section 3.1, the pore size and total pore volume of the porous SiO2 changed. Nevertheless, these changes only slightly affected the gas selectivity.
Fig. 4 (a) O2/N2, (b) CH4/N2, and (c) CO2/N2 gas selectivity of the obtained epoxy/porous SiO2 composites as a function of the pressure drop.
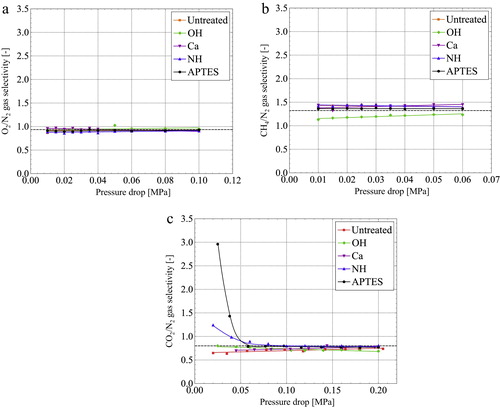
Similarly, the CH4/N2 gas selectivity was approximately 1.32 because the CH4 gas permeability coefficient was slightly high. This value also shows fair agreement with the theoretical value. Therefore, it is inferred that O2, N2 and CH4 gas mainly diffuses Knudsen model. Different from the results presented above, the CO2/N2 gas selectivity shows a different behavior. The gas selectivity of untreated, OH and Ca samples was constant at about 0.8 in the pressure drop measured in this study. It corresponded to the theoretical value calculated using Eq. Equation(2)(2)
(2) . The gas selectivity of the NH and APTES samples was also about 0.8 at about 0.08 MPa or more. In this pressure range, Knudsen diffusion is regarded as dominant. However, the gas selectivity of the NH sample increased concomitantly with decreasing pressure drop, and achieved about 1.23. The APTES sample showed the same tendency. The maximum gas selectivity was improved to 2.96. This result also suggests that the NH and APTES samples have CH4/CO2 gas selectivity.
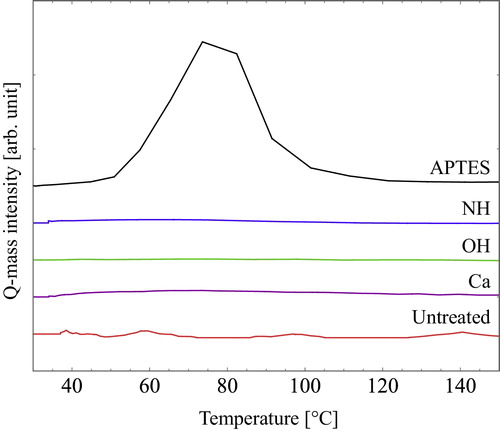
To evaluate the gas selectivity enhancement, the affinity between CO2 gas and the pore wall was measured. shows CO2-TPD spectra of the porous SiO2. Untreated, Ca and OH samples show no peak, which indicates that the CO2 only slightly gets adsorbed on the pore walls of these samples. In contrast, the APTES sample shows a peak at around 75 °C. This peak is believed to be attributable to desorption of CO2 from the pore walls. The NH2 group of APTES and CO2 gas formation of carbamate were reported [Citation28,Citation29]. Knowles et al. also described that CO2, which chemically adsorbed onto APTES at 20 °C, desorbed at 75 °C [Citation30]. Our results corresponded to their reports. They indicated that CO2 chemically adsorbed onto the pore walls. A peak attributable to the carbonates was expected to be observed for NH samples, but no such a peak was observed, which suggests that the amount of amino group of APTES is higher than that of NH samples. The amount of amino group was estimated using XPS. The N/Si ratios of APTES and NH were, respectively, about 0.25 and 0.09. The CO2 adsorption amount of NH was lower than the detection limit of the TPD apparatus because the N/Si ratio of the NH sample was about one-third that of APTES. The CO2/N2 gas selectivity of NH was not improved as that of APTES: the TPD results were supported.
3.4 Gas separation
shows the gas separation capability of the APTES sample with the highest gas selectivity. The composites were formed in the shape of tubes for this measurement. Additionally, it was confirmed that these samples have CO2/N2 gas selectivity, which was the same as that of the pellet-shaped samples. The CO2 loading after operation at 25 °C and at 0.05 MPa was 16.7%. It was lower than that of the supplied gas. (c) shows that the CO2/N2 gas selectivity is about 0.8 under this condition. However, the CO2 loading after operation at 25 °C and at 0.02 MPa concentrated to 31.8%. Under this condition, the CO2/N2 gas selectivity is about 3, indicating that the CO2 passes easily through the composites.
Table 2 Chemical composition of the mixed gas after going through the porous filter.
Surface diffusion is known to have temperature dependence. Gas separation is difficult at high temperatures [Citation31]. Therefore, the temperature effect for gas separation capability was finally evaluated. The obtained results are also presented in . The CO2 loading slightly decreased concomitantly with increasing operation temperature. The CO2-TPD results show that the CO2 gas desorbed gradually from 50 °C. Furthermore, desorption of CO2 was completed at 120 °C. Therefore, a concern arose that the composites cannot be used at temperatures higher than 50 °C. Nevertheless, the composites maintained their performance at 100 °C.
4 Summary
For this study, surface-modified epoxy/porous SiO2 composites were prepared. Their gas separation capability was measured. The following results were obtained.
| (1) | The N2 adsorption–desorption isotherm shows that the porous SiO2 modified by various silane coupling agents has sufficient high pore volume. Their pore size calculated using the BJH method was less than 20 nm, which was sufficiently smaller than the mean free path of the gases used for this study. | ||||
| (2) | The epoxy/porous SiO2 composites were prepared from these porous SiO2 and surface-modified porous SiO2. It is confirmed that the microstructure was same as the previous report. | ||||
| (3) | The respective degrees of gas selectivity of CO2/N2, CH4/N2, and O2/N2 were measured. The epoxy/porous SiO2 composite treated by APTES alone shows CO2/N2 gas selectivity at a lower pressure drop, which means that this sample also has CO2/CH4 gas selectivity. | ||||
| (4) | CO2/N2 gas selectivity originates in the affinity between the amino group of the APTES and CO2 gas because the samples modified by other silane coupling agents showed no gas selectivity. | ||||
| (5) | The epoxy/porous SiO2 composite treated by APTES also shows gas separation capability. The 80% N2/20% CO2 mix gas was converted into 68.2% N2/31.8% CO2 gas at 25 °C. | ||||
| (6) | The gas separation capability decreased slightly. However, the 80% N2/20% CO2 mix gas was converted into 70.8% N2/29.2% CO2 gas at 100 °C. | ||||
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- P.BernardoE.DrioliG.GolemmeInd. Eng. Chem. Res.48200946384663
- J.C.WhiteP.K.DuttaK.ShqauH.VerweijLangmuir2620101028710293
- K.KusakabeT.KurodaA.MurataS.MorookaInd. Eng. Chem. Res.361997649655
- K.KusakabeT.KurodaS.MorookaJ. Membr. Sci.14819981323
- K.AokiK.KusakabeS.MorookaJ. Membr. Sci.1411998197205
- T.TomitaK.NakayamaH.SakaiMicroporous Mesoporous Mater.6820047175
- D.W.ShinS.H.HyunC.H.ChoM.H.HanMicroporous Mesoporous Mater.852005313323
- R.M.BarrerE.StrachanProc. R. Soc. A23119555274
- R.AshR.M.BarrerC.G.PopeProc. R. Soc. A2711963118
- R.AshR.M.BarrerC.G.PopeProc. R. Soc. A27119631933
- R.AshR.M.BarrerP.SharmaJ. Membr. Sci.119761732
- M.YoshimuneI.FujiwaraK.HarayaCarbon452007553560
- E.P.FavvasE.P.KouvelosG.E.RomanosG.I.PilatosA.Ch.MitropoulosN.K.KanellopoulosJ. Porous Mater.152008625633
- M.N.IslamK.TanakaH.KitaK.OkamotoJ. Chem. Eng. Japan392006131136
- G.XomeritakisN.G.LiuZ.ChenY.-B.JiangR.KohnP.E.JohnsonC.-Y.TsaiP.B.ShahS.KhalilS.SinghC.J.BrinkerJ. Membr. Sci.2872007157161
- Y.SakamotoK.NagataK.YogoK.YamadaMicroporous Mesoporous Mater.1012007303309
- D.LuebkeC.MyersH.PennlineEnergy Fuels20200619061913
- G.D.BothunK.PeayS.IliasJ. Membr. Sci.3012007162170
- N.AbidiA.SivadeD.BourretA.LarbotB.BoutevinF.Guida-PietrasantaA.RatsimihetyJ. Membr. Sci.2702006101107
- T.IsobeM.NishimuraS.MatsushitaA.NakajimaMicroporous Mesoporous Mater.1832014201206
- E.P.BarrettL.G.JoynerP.P.HalendaJ. Am. Chem. Soc.731951373380
- K.S.W.SingD.H.EverettR.HaulL.MoscouR.A.PierottiJ.RouquerolT.SiemieniewskaPure Appl. Chem.571985603619
- D.ZhaoJ.FengQ.HuoN.MeloshG.H.FredricksonB.F.ChmelkaG.D.StuckyScience2791998548552
- D.C.M.DutoitM.SchneiderA.BaikerJ. Catal.1531995165176
- H.NaonoR.FujiwaraM.YagiJ. Colloid Interface Sci.7619807482
- S.DaiY.H.JuH.J.GaoJ.S.LinS.J.PennycookC.E.BarnesChem. Commun.20002000243244
- R.J.R.UhlhornK.KeizerA.J.BurggraafJ. Membr. Sci.461989225241
- M.OstwalR.P.SinghS.F.DecM.T.LuskJ.D.WayaJ. Membr. Sci.3692011139147
- A.L.ChaffeeG.P.KnowlesZ.LiangJ.ZhangP.XiaoP.A.WebleyInt. J. Greenhouse Gas Control120071118
- G.P.KnowlesS.W.DelaneyA.L.ChaffeeInd. Eng. Chem. Res.45200626262633
- K.KeizerR.J.R.UhlhornR.J.Van VurenA.J.BurggraafJ. Membr. Sci.391988285300