Abstract
Novel environmental-friendly inorganic red pigments, ((Bi0.72Er0.28−xYx)1−yFey)2O3 (0 < x ≤ 0.28, y = 0.20), were successfully synthesized using a conventional solid-state reaction method in order to further enhance the red hue of a ((Bi0.72Er0.28)0.80Fe0.20)2O3 pigment, which was previously reported by our group. The color of the samples depended on their composition and the most brilliant red hue was obtained for ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3. The a* value corresponding to red chromaticity was +33.1 for ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3, and it was greater than those of previously reported ((Bi0.72Er0.28)0.80Fe0.20)2O3 (a* = +30.9) and commercial Fe2O3 (a* = +28.9) pigments. Since the ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3 pigment is composed of nontoxic elements (Bi, Er, Y, Fe, and O), it should be an attractive alternative to the conventional Fe2O3 pigments.
1 Introduction
Inorganic pigments are applied in a wide variety of products such as paints, ceramics, plastics, enamels, and glasses, because they possess high thermal and UV stability [Citation1]. In particular, inorganic red pigments are in demand because of their wide applicability. Several inorganic red pigments such as lead oxides (red lead; Pb3O4 or 2PbO·PbO2), cadmium sulfoselenide red (CdSe·CdS), and mercuric sulfide red (HgS) were popularly used for many applications. However, the use of these compounds has been restricted because of the hazardous elements, such as lead (Pb), cadmium (Cd), and mercury (Hg), which are harmful not only to human health but also to the environment. On the other hand, iron oxide (Fe2O3) has been widely known as an environmentally friendly inorganic red pigment for many uses, but its color performance is unsatisfactory in comparison to those of the conventional toxic pigments. A number of studies have been done on new environmentally friendly inorganic red pigments [Citation2–Citation9], but the pigments cannot exceed the color of the nontoxic red iron oxide (Fe2O3). Although perovskite-type oxynitrides Ca(1−x)LaxTaO(2−x)N(1+x) have also been reported as nontoxic inorganic red pigments, toxic ammonia gas is required for the synthesis of these pigments [Citation10]. Furthermore, oxidation of these oxynitride compounds occurs at 420 °C or higher temperatures [Citation10], where they decompose and generate toxic nitrogen oxides.
Because of this situation, we focused on bismuth oxide (Bi2O3), which has already been reported as a nontoxic and insoluble compound [Citation11]. Bi2O3 exists in several polymorphic phases: a low-temperature stable α-phase (monoclinic structure), a high-temperature stable δ-phase (cubic fluorite structure), and a metastable β-phase (tetragonal structure) or γ-phase (body-centered cubic structure). At room temperature, Bi2O3 exists in a pale yellowish α-phase, which is stable up to 730 °C. At around 730 °C, a phase transition from the α-phase to the orange δ-phase occurs, and the δ-phase is stable up to the melting point (825 °C). The color of Bi2O3 originates in the charge-transfer transition from the valence band of a hybrid orbital of Bi6s and O2p to the conduction band of Bi6p [Citation12].
In our previous study, we elucidate that the high-temperature δ-Bi2O3 phase was stabilized at room temperature by introducing Er3+ ions into the Bi3+ site, and that the red chromaticity was significantly increased by further dissolution of Fe3+ into the lattice to insert the Fe3d level as a new energy level below the Bi6P conduction band. In particular, it was found that a ((Bi0.72Er0.28)0.80Fe0.20)2O3 pigment showed the highest redness value (a* = +30.9 in the CIE L*a*b* system) [Citation13]. However, the band gap energy of the ((Bi0.72Er0.28)0.80Fe0.20)2O3 pigment became too low because of significant lattice shrinkage, and the optical absorption extended into the red region. As a result, the red color was not so vivid, although it was higher than that of the commercial Fe2O3 pigment (a* = +28.9) [Citation13]. In this study, therefore, a part of the Er3+ (0.103 nm) [Citation14] site was substituted with larger Y3+ ions (0.104 nm) [Citation14] to relax the excessive lattice shrinkage. Therefore, ((Bi0.72Er0.28−xYx)1−yFey)2O3 (0 < x ≤ 0.28, y = 0.20) samples were synthesized as advanced environmentally friendly red pigments. The color properties were characterized and the composition was optimized to produce the most vivid red hue.
2 Experimental
The ((Bi0.72Er0.28−xYx)1−yFey)2O3 (0 < x ≤ 0.28, y = 0.20) pigments were synthesized using a conventional solid-state reaction method. As is the case with the ((Bi0.72Er0.28)0.80Fe0.20)2O3 pigment [Citation13], double calcination was adopted in the synthesis process to obtain the ((Bi0.72Er0.28−xYx)1−yFey)2O3 solid solutions certainly. In the first process, (Bi0.72Er0.28−xYx)2O3 solid solutions were prepared in a single-phase form of the cubic δ-Bi2O3 structure, and in the second one, Fe3+ ions were introduced into the (Bi0.72Er0.28−xYx)2O3 lattice maintaining the single-phase δ-Bi2O3 structure. Stoichiometric amounts of Bi2O3, Er2O3 and Y2O3 powders were mixed in an agate mortar, followed by mechanical mixing using a planetary-type ball-milling apparatus (Pulverisette 7, Fritsch GmbH) for 3 h. The homogenous mixed powder was calcined at 800 °C for 10 h under an air atmosphere. Finally, the powder obtained was mixed with Fe2O3 in an agate mortar and was calcined at 500 °C for 10 h under an air atmosphere.
All the samples were gently ground in an agate mortar before characterization. The sample compositions were analyzed by X-ray fluorescence spectroscopy (XRF, Rigaku Supermini200). The samples were characterized by X-ray powder diffraction (XRD; Rigaku, SmartLab) using Cu-Kα radiation (40 kV, 30 mA) to identify the crystal structure. Optical reflectance spectra were obtained using a UV–vis spectrometer (Shimadzu UV-2600) with barium sulfate as a reference. The band-gap energies of the samples were determined from the absorption edge of the absorbance spectra represented by the Kubelka–Munk function, f(R) = (1 − R)2/2R, where R is the reflectance [Citation15,Citation16]. The color properties of the samples were estimated in terms of the CIE L*a*b* system with a colorimeter (Konica-Minolta CR-300). The parameter L* represents the brightness or darkness of a color relative to a neutral gray scale, while the parameters a* (the red–green axis) and b* (the yellow–blue axis) qualitatively express the color. The particle morphology of the samples was observed using a scanning electron microscope (SEM; Shimadzu, SS-550). The size distribution and mean particle size were evaluated by measuring the diameters of 200 particles on the SEM micrographs.
3 Results and discussion
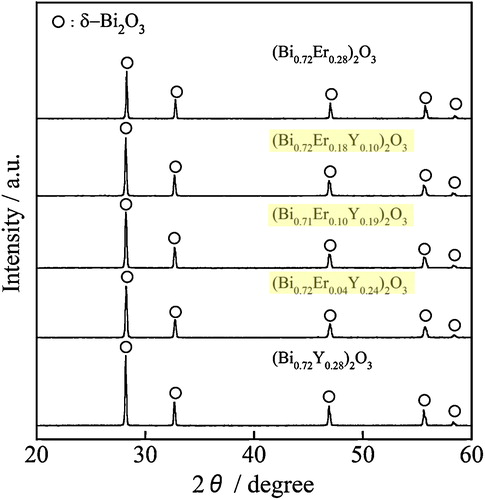
The compositions of all samples determined by the XRF analysis were in good agreement with the theoretical values. To confirm the formation of the cubic δ-Bi2O3 structure, the (Bi0.72Er0.28−xYx)2O3 (0 < x ≤ 0.28) samples, which were synthesized in the first step of the double calcination process, were measured using the XRD analysis. shows the XRD patterns of the (Bi0.72Er0.28−xYx)2O3 (0 ≤ x ≤ 0.28) samples. A single phase of the δ-Bi2O3-type structure was observed for all samples, and there were no extra lines due to other compounds or impurities. As summarized in , the cubic lattice parameter of the (Bi0.72Er0.28−xYx)2O3 (0 ≤ x ≤ 0.28) samples increased linearly with increasing the amount of Y3+, because the ionic radius of Y3+ (0.104 nm) [Citation14] is slightly larger than that of Er3+ (0.103 nm) [Citation14]. These results indicate that the solid solutions were formed successfully for all samples.
Table 1 Cubic lattice parameters of the (Bi0.72Er0.28−xYx)2O3 (0 ≤ x ≤ 0.28) samples with estimated standard deviation (esd).
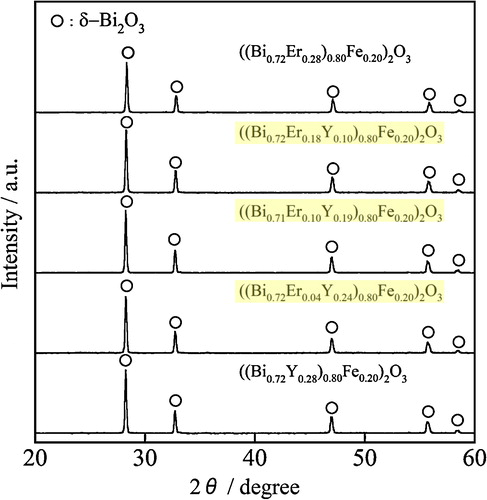
Subsequently, iron ion (Fe3+) was doped into the (Bi0.72Er0.28−xYx)2O3 lattice to introduce a red chromogenic species, and the XRD patterns of the ((Bi0.72Er0.28−xYx)1−yFey)2O3 (0 ≤ x ≤ 0.28, y = 0.20) samples were depicted in . Also in this case, a single-phase cubic δ-Bi2O3 structure was observed for all samples and no diffraction peaks of impurities were evident in the patterns. The cubic lattice parameters of ((Bi0.72Er0.28−xYx)1−yFey)2O3 are summarized in . It is confirmed that the lattice parameters of the ((Bi0.72Er0.28−xYx)1−yFey)2O3 samples were larger than that of ((Bi0.72Er0.28)0.80Fe0.20)2O3 [Citation13] and were increased with increasing Y3+ content. These results indicate that solid solutions were successfully formed for the ((Bi0.72Er0.28−xYx)1−yFey)2O3 (0 < x ≤ 0.28, y = 0.20) samples, as is the case with the (Bi0.72Er0.28−xYx)2O3 samples in .
Table 2 Cubic lattice parameters of the ((Bi0.72Er0.28−xYx)1−yFey)2O3 (0 ≤ x ≤ 0.28, y = 0.20) samples with estimated standard deviation (esd).
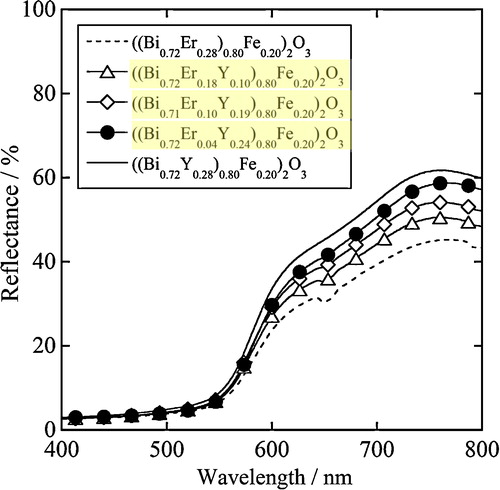
shows the UV–vis diffuse reflectance spectra for the ((Bi0.72Er0.28−xYx)1−yFey)2O3 (0 ≤ x ≤ 0.28, y = 0.20) samples. Strong optical absorption in the green light region at wavelengths shorter than 560 nm was observed for the samples. All samples are red, because green is the complementary color to red. Although the green light absorption was gradually decreased with increasing the amount of Y3+, the red light reflection between 605 and 750 nm increased.
Fig. 3 UV–vis diffuse reflectance spectra for the ((Bi0.72Er0.28−xYx)1−yFey)2O3 (0 ≤ x ≤ 0.28, y = 0.20) samples.
The L*a*b* color coordinate data and the apparent band-gap energies for the ((Bi0.72Er0.28−xYx)1−yFey)2O3 (0 < x ≤ 0.28, y = 0.20) samples are listed in with those of the previous ((Bi0.72Er0.28)0.80Fe0.20)2O3 and commercially available Fe2O3 (MR-320A, Morishita Bengara Kogyo Co. Ltd.) pigments for comparison. The a* values, which correspond to the red chromaticity in the positive direction, of the Y3+-doped samples were larger than that of the previous ((Bi0.72Er0.28)0.80Fe0.20)2O3 pigment (a* = +30.9). In addition, the a* value gradually increased with the Y3+ concentration in the ((Bi0.72Er0.28−xYx)1−yFey)2O3 (0 < x ≤ 0.24, y = 0.20) samples. Among these pigments, the ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3 pigment shows the highest redness value, a* = +33.1. On the other hand, the apparent band-gap energies of the samples increased with the amount of Y3+ as intended. This is caused by the decrease in the hybridization effect of Bi6s–O2p orbital by introducing Y3+ into the ((Bi0.72Er0.28)0.80Fe0.20)2O3 lattice () to relax the excessive lattice shrinkage. As a result, the red light reflection was increased by the Y3+ doping into the ((Bi0.72Er0.28)0.80Fe0.20)2O3 lattice ().
Table 3 L*a*b* color coordinate data and apparent band-gap energies for the samples.
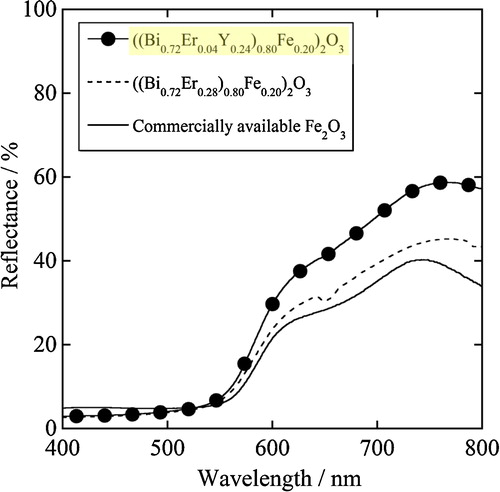
depicts the UV–vis diffuse reflectance spectra of ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3, ((Bi0.72Er0.28)0.80Fe0.20)2O3, and commercially available Fe2O3. Although the optical absorption in the green light region for these pigments was almost identical, the reflection in the red light region (605–750 nm) for ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3 was significantly higher than those for the others. Therefore, the redness value (a*) of the present ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3 pigment (a* = +33.1) was higher than those of previously reported ((Bi0.72Er0.28)0.80Fe0.20)2O3 (a* = +30.9) and also commercially available Fe2O3 (a* = +28.9) as listed in .
Fig. 4 UV–vis diffuse reflectance spectra of ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3, ((Bi0.72Er0.28)0.80Fe0.20)2O3, and commercially available Fe2O3.
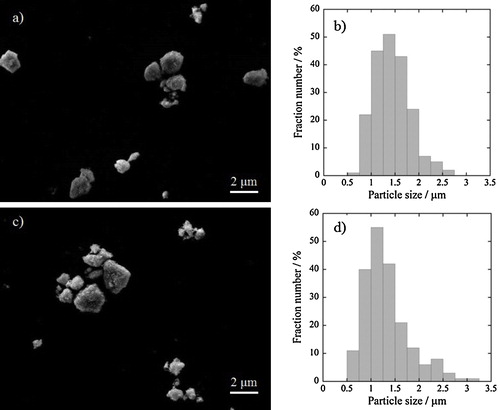
illustrates SEM images and size distribution profiles of the previous ((Bi0.72Er0.28)0.80Fe0.20)2O3 and the present ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3 pigments. Average particle size of the present ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3 pigment was about 1.32 μm, which was almost the same as that of the previous ((Bi0.72Er0.28)0.80Fe0.20)2O3 (1.43 μm). These results indicate that the enhancement in the red hue of the present ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3 pigment compared with that of the previous ((Bi0.72Er0.28)0.80Fe0.20)2O3 pigment was caused by the relaxation of the excessive lattice shrinkage by substitution of Y3+ for Er3+ as mentioned above, and the contributions from the particle size on the pigment properties can be eliminated.
4 Conclusions
((Bi0.72Er0.28−xYx)1−yFey)2O3 solid solutions were synthesized as environment-friendly inorganic red pigments to enhance the redness value of a ((Bi0.72Er0.28)0.80Fe0.20)2O3 pigment which was previously reported by our group. The redness value of the pigment was increased by substituting a part of Er3+ ions with larger Y3+ ions by the relaxation of the excessive lattice shrinkage. Among these pigments, the most brilliant red hue was obtained for the ((Bi0.72Er0.04Y0.24)0.80Fe0.20)2O3 pigment, and the red hue of this pigment (a* = +33.1) was more brilliant than that of the commercial Fe2O3 pigment (a* = +28.9) as well as that of ((Bi0.72Er0.28)0.80Fe0.20)2O3 pigment (a* = +30.9).
Acknowledgements
This work was supported by the Development of Alternative Technology for Hazardous Chemical Substances and Development of Novel Environment- and Human-friendly Inorganic Pigments for Three Primary Colors (FY2010-2014) programs of the New Energy and Industrial Technology Development Organization (NEDO) and the Ministry of Economy, Trade and Industry, Japan (METI).
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- E.B.FaulknerR.J.SchwartzHigh Performance Pigments2nd ed.2009Wiley-VCHWeinheim
- L.S.KumariP.P.RaoP.KoshyJ. Am. Ceram. Soc.93201014021408
- R.A.CandeiaM.I.B.BernardiE.LongoI.M.G.SantosA.G.SouzaMater. Lett.582004569572
- P.ŠulcováL.VitáskováM.TrojanJ. Therm. Anal. Calorim.992010409413
- M.LlusarL.VitáskováP.ŠulcováM.A.TenaJ.A.BadenesG.MonrósJ. Eur. Ceram. Soc.3020103752
- S.T.ArunaS.GhoshK.C.PatilInt. J. Inorg. Mater.32001387392
- G.GeorgeV.S.VishnuM.L.P.ReddyDyes Pigments882011109115
- V.S.VishnuG.GeorgeM.L.P.ReddyDyes Pigments852010117123
- V.S.VishnuM.L.P.ReddySol. Energy Mater. Sol. Cells95201126852692
- M.JansenH.P.LetschertNature4042000980982
- K.A.WinshipAdverse Drug React. Acute Poisoning Rev.21983103121
- A.KudoK.OmoriH.KatoJ. Am. Chem. Soc.12119991145911467
- WendusuT.MasuiN.ImanakaChem. Lett.41201216161618
- R.D.ShannonActa Crystallogr. Sect. A321976751767
- D.R.EpplerR.A.EpplerCeram. Eng. Sci. Proc.1719967787
- M.KatoM.TakahashiJ. Mater. Sci. Lett.202001413414