Abstract
All solid-state Li-ion batteries offer unprecedented improvements in energy density and safety compared to contemporary Li-ion batteries. As one of the most common oxide cathode materials for traditional Li-ion batteries, LiCoO2 (LCO) is also under consideration for use in all solid-state batteries. However, differences in the coefficients of thermal expansion (CTE) between LCO and the solid electrolyte during composite electrode fabrication, and the differential expansion and contraction during electrochemical cycling resulting from Li insertion and de-insertion, will cause stresses resulting in battery capacity fade. To characterize the thermo-mechanical properties, this study utilized hot pressing to prepare high relative density (95%) LCO polycrystalline pellets. The elastic modulus (E), shear modulus (G), hardness (H), and Poisson’s ratio (υ) of LCO were determined to be ∼191 GPa, ∼80 GPa, ∼8.2 GPa (at peak indentation loads ≤5 mN), and 0.24, respectively. The CTE was determined to be ∼1.3 × 10−5/°C over the temperature range 50–400 °C. We believe these data are important to predict or increase the cycle life of commercially available LCO as a cathode material for state-of-the-art Li-ion and advanced solid-state batteries.
1 Introduction
Ceramic all solid-state batteries are garnering interest to enable safe, high energy density and large-format energy storage technology because of their intrinsic stability [Citation1,Citation2]. Though there are numerous bulk-scale solid-state cell configurations, the composite oxide electrode is one of the most chemically stable and is non-flammable. The composite oxide configuration consists of a fast oxide-ion conductor, an oxide electrode, and a conductive additive, which are blended/randomized and densified at elevated temperature [Citation2]. Compared to contemporary liquid electrolyte containing Li-ion batteries in which individual cathode particles are free to expand and contract during cycling, the proposed composite oxide configurations will generate stresses at the solid electrolyte/cathode interface. However, because the cathode particles are chemically bound to the solid electrolyte and are relatively hard, the expansion and contraction of LCO during cycling will generate stresses at the electrode-electrolyte interface. Furthermore, any difference in CTEs between the cathode and solid electrolyte will cause stresses upon cooling after densification at elevated temperature. These stresses within electrodes can lead to their fracture, resulting in battery capacity loss and power fade. Thus characterization of the mechanical properties of solid electrodes and electrolytes is important in evaluating the mechanical integrity of the interface between the solid electrode and electrolyte. Previous work characterized the mechanical properties of oxide electrolytes using acoustic analysis, Vickers hardness and nanoindentation tests [Citation3 Citation2013;Citation5]. However, there are very few reports that describe the mechanical properties (e.g., elastic modulus and hardness) of one of the most common oxide cathodes, LCO [Citation6], which is also of interest in all-solid-state composite cathodes. In addition, no experimental data are available in the literature on Poisson’s ratio, shear modulus and CTE of LCO. The purpose of this work is to report salient elastic and mechanical properties of commercially available LCO to facilitate the development of solid-state composite cathodes. Since the mechanical properties of single-crystalline cathodes are critical for predicting their cycle life using computational models [Citation7], elastic modulus and hardness of LCO single-crystals were measured by nanoindentation. To directly measure the Poisson’s ratio and shear modulus, the pulse-echo acoustic method was used. In addition, Poisson’s ratio is necessary to determine the elastic modulus using nanoindentation. The CTE of bulk LCO was measured using dilatometry from room temperature to 400 °C. These mechanical and physical properties can help better understand the micromechanical environment and to predict the compatibility between LCO and solid-state electrolytes, e.g., Li7La3Zr2O12 (LLZO), during densification and electrochemical cycling, respectively.
2 Experimental procedure
2.1 Densification
As-received LCO powders (EQ-Lib-LCO, MTI, California, USA) were cold-pressed into billets (12.7 mm diameter 4.0 mm thick) at room temperature in a stainless steel die at ∼385 MPa to obtain a green relative density ∼60%. The cold-pressed billets were then densified using a rapid induction hot-pressing technique at 900–1000 °C and 60 MPa for 60 min under Ar. The bulk density was determined using Archimedes’ principle, where cyclohexane was used as the immersion liquid. The hot-pressed LCO specimens were polished using standard metallographic techniques. The final finish used a 1 μm diamond polishing compound (Diamond Paste, South Bay Technology, California, USA).
2.2 Characterization
X-ray diffraction was performed using a diffractometer (Rigaku 600 Miniflex XRD, Tokyo, Japan) with CuKα radiation in a 2θ range of 10–70° at 15 mA and 40 kV. The XRD patterns were analyzed using a whole pattern fitting (WPF) routine with Jade 2010 software (MDI, Livermore, California, USA). Microstructures of the LCO powers and hot-pressed specimens were analyzed using a Dualbeam SEM/FIB microscope (accelerating voltage: 50 V–30 kV,beam current: 0.8 pA–26 nA, Helios Nanolab 650, FEI, Oregon, USA). The average particle or grain size was estimated from SEM micrographs using ImageJ software [Citation8]. Acoustic properties were analyzed using a pulse-echo technique, where longitudinal and shear transducers (M110 and V156, Olympus NDT Inc., Massachusetts, USA) were secured onto the polished surfaces of LCO specimens, respectively, to measure the acoustic wave velocities. The longitudinal and shear wave velocities were determined using Eq. Equation(1)(1) :
(1) Where V is the wave velocity (VS and VL: shear and longitudinal wave velocities, respectively), h is the thickness of the specimen, and t is the wave travel time through the measured thickness and is defined as the transit time between subsequent echoes. Since the specimen thickness was ∼2.13 mm (the measured mass density ρ was ∼4.79 g/cm3), the longitudinal and the shear wave velocities were calculated to be ∼6961 m/s and 4088 m/s, correspondingly. Nanoindentation was carried out on a Hysitron Ti 950 Triboindenter (Minneapolis, Minnesota, USA) loaded with a standard diamond Berkovich indentation tip (highest indentation load: 10 mN). Hardness was determined from at least 10 indentation measurements at each indentation load. The Elastic modulus was calculated from the load-displacement curve during unloading using the Oliver–Pharr method [Citation9]. The elastic modulus and Poisson’s ratio of the Berkovich diamond tip are 1140 GPa and 0.07, respectively [Citation9]. The CTE was measured using an industry standard thermomechanical analyzer (Q400, TMA, Delaware, USA) in five cycles over the temperature range 50–400 °C.
3 Results and discussion
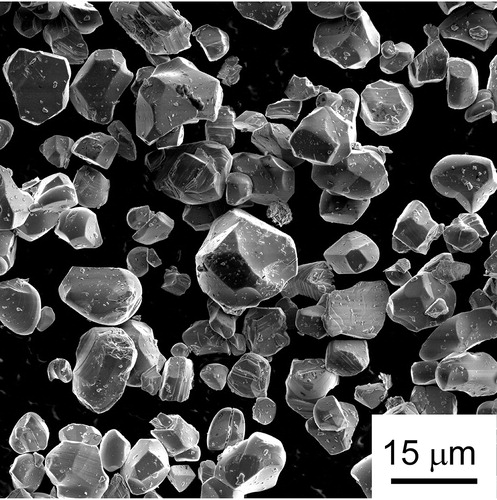
shows an SEM micrograph of the as-received LCO powder. The particle size ranges from ∼2.5 to 17.5 μm and the average particle size is estimated to be ∼8.5 ± 3.5 μm. XRD analysis () of the 900 °C hot-pressed LCO confirmed that the bulk LCO maintained the same crystal structure compared to the as-received powder (PDF No.: 98-000-0283) [Citation10]. In addition, the increased intensity of (003) reflection indicates that the bulk LCO is (003) textured. However, LCO started to decompose at sintering temperatures higher than 900 °C. As shown in (c), peaks of a cubic Li0.14Co0.86O phase (PDF No.: 98-000-0283) appeared in the XRD pattern of the 1000 °C hot-pressed LCO. The mass ratio between LCO and Li0.14Co0.86O was quantified (Jade profile fitting) to be ∼3:1.
Fig. 2 XRD patterns of hot-pressed LCO. (a) a reference power XRD pattern, (b) 900 °C hot-pressed LCO, (c) 1000 °C hot-pressed LCO.
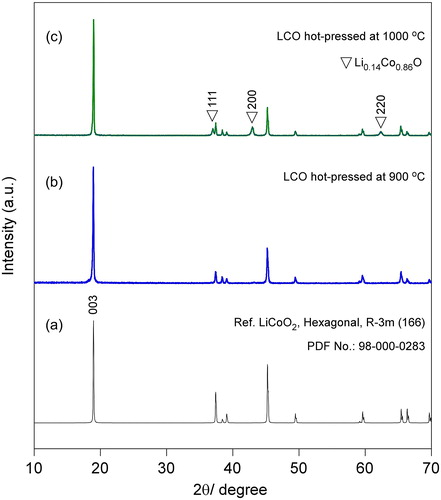
An SEM micrograph of a polished surface of the 900 °C hot-pressed LCO is shown in (a), where no obvious pores are apparent (the features that appear as pores are more likely to be evidence of particle pullout during polishing and not porosity). The density of the specimen is 4.79 g/cm3, corresponding to a high relative density ∼95% (theoretical density: 5.05 g/cm3) [Citation10]. An SEM micrograph of a freshly fractured surface is presented in (b), from which a mixed fracture mode (transgranular and intergranular) is observed with the intergranular mode being dominant. The specimen is dense and no obvious grain growth is observed.
Fig. 3 SEM micrographs of the 900 °C hot-pressed LCO. (a) Polished surface and (b) freshly fractured surface.
To directly measure the Poisson’s ratio and elastic modulus of the polycrystalline LCO, pulse-echo measurement was conducted assuming that the specimen is isotropic. The elastic modulus (E), shear modulus (G) and Poisson’s ratio (υ) are calculated using the following Eqs. Equation(2)(2) –Equation(4)
(4) [Citation11 Citation2013;Citation13]:
(2)
(3)
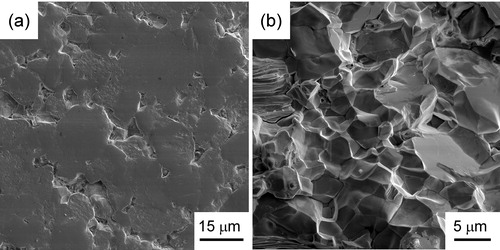
(4) where ρ is the mass density of the specimen. illustrates the representative pulse-echo spectra, where the period t (time gap between subsequent echoes) for the longitudinal and shear waves are ∼0.612 and 1.042 μs, respectively. With VL and VS being calculated to be 6961 m/s and 4088 m/s, respectively, the E, G and υ are then determined to be ∼198 GPa, 80 GPa and 0.24, respectively, from Eqs. (2)–(4).
To measure the hardness and elastic properties of LCO single crystals and cross-check the pulse-echo results, nanoindentation was conducted and compared (). A load-displacement curve is shown in (a), where the peak load is 2 mN and the maximum indentation depth is ∼75 nm; the measured elastic modulus and indentation hardness were ∼205 GPa and 9.9 GPa, respectively.
Fig. 4 Representative pulse-echo spectra of the bulk LCO (Relative density: 95%): (a) Longitudinal and (b) shear waves. Transducer excitation voltage is on the vertical axis and the acoustic wave propagation time on the horizontal axis. The time between reflections is indicated in μs.
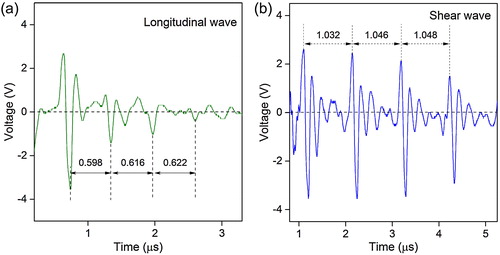
Fig. 5 Nanoindentation results of the bulk LCO (Relative density: 95%): (a) a load-displacement curve with a peak load of 2 mN, (b) elastic modulus and hardness as a function of indentation load, (c) In-situ atomic force microscopy (AFM) image showing indents at a peak load of 2 mN, and (d) AFM image at a peak load of 5 mN. The inset is a corresponding reverse gradient AFM image.
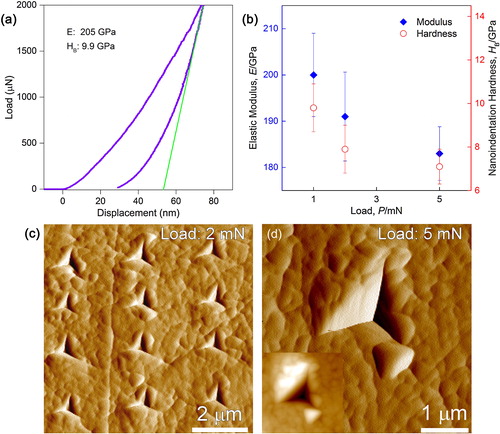
(b) shows the load dependence of E and H of LCO grains, varying in the range of 183–200 GPa and 7.1–9.8 GPa, respectively. The average E and H obtained at peak loads ≤5 mN were ∼191 and 8.2 GPa, respectively. In addition, both E and H varied in a similar manner: they decreased with increasing indentation load simultaneously. This indentation size effect is widely reported in ceramic materials, which may have been due to cracking or dislocation motion caused by deeper penetration of the indenter [Citation14 Citation2013;Citation16]. (c) shows an in-situ AFM image of indents under a peak load of 2 mN. Pile-up around the indents was occasionally observed and the average indent size was ∼1.2 μm. A representative AFM image of a 5 mN indent is shown in (d), where an uplifted fragment at the lower right side of the indentation indicates cracking. The discrepancy between the elastic moduli measured by nanoindentation (average ∼191 GPa at indentation loads ≤5 mN) and pulse-echo (∼198 GPa) is only ∼3.5%, indicating that dense polycrystalline LCO exhibits almost the same elastic properties as single-crystalline LCO.
The average CTE of the bulk LCO was measured to be ∼1.3 (10−5/°C) between 50 and 400° C, consistent with the reported theoretical volumetric CTE value of LCO, ∼1.2–1.5 (10−5/°C), in the temperature range ∼75–325 °C [Citation17]. A summary of the mechanical properties of LCO is listed in and compared to the reported data of LCO, LiNi0.33Mn0.33Co0.33O (NMC), and LLZO. It should be noted that Qu et al. fired hot-pressed bulk LCO to 1100 °C for 12 h to increase the grain size for nanoindentation [Citation6], while LCO was found to decompose above 900 °C in our study. Thus the relatively large discrepancy of the reported mechanical properties of LCO by Qu et al. from our results could be caused by the presence of phases from LCO decomposition.
Table 1 Mechanical and physical properties of bulk LCO (compared to the reported data of LCO NMC and LLZO).
Upon cooling, the stress generated between LCO and solid electrolyte materials, e.g. LLZO, can be roughly evaluated by Eq. Equation(5)(5) [Citation18], assuming isotropic elastic behavior of LCO and LLZO,
(5) where Δα is the CTE mismatch between LCO and LLZO, ∼2.0 × (10−6/°C); ΔT is the difference between sintering temperature and room temperature, e.g., 850 °C; ELCO and ELLZO are elastic moduli of LCO (∼190 GPa) and LLZO (∼150 GPa), respectively. The calculated stress between the LCO/LLZO interface is ∼140 MPa. However, when considering the Poisson’ ratios of LCO and LLZO, the internal stress developed during cooling between the two oxides can be calculated by Eq. Equation(6)
(6) (assuming spherical LLZO crystals embedded in an infinite isotropic LCO phase) [Citation19]:
(6) where υLCO and υLLZO are the Poisson’s ratios of LCO and LLZO, respectively. Eq. Equation(6)
(6) yields a calucated value of the stress ∼260 MPa, almost twice as high as that obtained from Eq. Equation(5)
(5) . At present, no fracture stress data is available for LCO and LLZO. However, fracture stress data is available for Y3Al5O12 (a similar cubic garnet to LLZO) ranged from 200–300 MPa [Citation20]. Thus the composite oxide electrodes, e.g., LCO/LLZO configuration, could be at risk of fracturing because of internal stresses developed during cooling. To more accurately predict interface stresses between LCO and solid electrolytes during densification and electrochemical cycling, Finite Element Analysis (FEA) can be used. In conclusion, here we present the results on the mechanical and physical properties of LCO (Shear modulus, CTE and Poisson’s ratio υ of LCO are reported for the first time) with the intent that this information can be used to predict the cycle life of LCO as a cathode material and to better design the solid electrolyte/cathode interface to avoid stress-related failures of all-solid-state Li-ion batteries.
4 Conclusions
Bulk LCO cathode materials (relative density: ∼95%) were synthesized using hot-pressing at 900 °C and 60 MPa for 60 min in Ar. The elastic modulus E, shear modulus G, hardness H, and Poisson’s ratio υ of the bulk LCO determined by nanoindentation and pulse-echo were ∼191 GPa, ∼80 GPa, ∼8.2 GPa, and ∼0.24, respectively. Additionally, the CTE of the polycrystalline LCO was measured to be ∼1.3 × 10−5/°C over the temperature range 50–400 °C. These experimentally measured mechanical and physical properties of LCO provide valuable information for a better design of Li-ion and advanced solid-state batteries using LCO as a cathode material.
Acknowledgment
EC, NJT, JW, and JS would like to acknowledge support from the Advanced Research Projects Agency-Energy (DE-AR0000653) and the University of Michigan; JW would also like to acknowledge support from the U. S. Army Research Laboratory.
References
- P.KnauthH.L.TullerJ. Am. Ceram. Soc.85200216541680
- J.SakamotoN.DudneyW.WestJ.NandaSuper-ionic Conducting Oxide Electrolytes2016World ScientificSingapore391414
- S.YuR.D.SchmidtR.Garcia-MendezE.HerbertN.J.DudneyJ.B.WolfenstineJ.SakamotoD.J.SiegelChem. Mater.282015197206
- Y.KimH.JoJ.L.AllenH.ChoeJ.WolfenstineJ.SakamotoJ. Am. Ceram. Soc.136201671374
- J.E.NiE.D.CaseJ.S.SakamotoE.RangasamyJ.B.WolfenstineJ. Mater. Sci.47201279787985
- M.QuW.H.WoodfordJ.M.MaloneyW.C.CarterY.M.ChiangK.J.Van VlietAdv. Energy Mater.22012940944
- W.H.WoodfordY.-M.ChiangW.C.CarterJ. Electrochem. Soc.1572010A1052A1059
- M.D.AbràmoffP.J.MagalhãesS.J.RamBiophotonics Intern.1120043642
- W.C.OliverG.M.PharrJ. Mater. Res.7199215641583
- J.AkimotoY.GotohY.OosawaJ. Solid State Chem.1411998298302
- R.D.SchmidtJ.SakamotoJ. Power Sources3242016126133
- A.SakudaA.HayashiY.TakigawaK.HigashiM.TatsumisagoJ. Ceram. Soc. Jpn.1212013946949
- R.ChaimM.HefetzJ. Mater. Sci.39200430573061
- A.C.Fischer-CrippsA.C.Fischer-CrippsFactors Affecting Nanoindentation Test Data2002SpringerNew York6182
- E.J.ChengH.KatsuiT.GotoJ. Am. Ceram. Soc.201524232436
- J.QuinnG.QuinnJ. Mater. Sci.32199743314346
- S.O.DangModelling Thermodynamic Properties of Intercalation Compounds for Lithium Ion Batteries, RWTH Aachen University. PhD Thesis2015
- W.BoasR.W.K.HoneycombeProc. R. Soc. A1861946577110.1098/rspa.1946.0035
- J.SelsingJ. Am. Ceram. Soc.441961 419–419
- J.WolfenstineJ.AllenJ.ReadJ.SakamotoJ. Mater. Sci.48201358465851
- E.J.ChengK.HongN.J.TaylorH.ChoeJ.WolfenstineJ.SakamotoJ. Eur. Ceram. Soc.2017 submitted for publication
- A.A.HubaudD.J.SchroederB.J.IngramJ.S.OkasinskiJ.T.VaugheyJ. Alloys Compd.6442015804807