Abstract
The present research involves the study of the direct reaction of methanol with silicon in the presence of copper as a catalyst. This reaction was performed in a static form system and the reacting CH3OH vapor could be brought into contact with the Si-catalyst mixture fixed as a bed in the reaction tube at reaction temperatures 250, 300 and 350 °C. The catalysts surfaces were characterized by using a number of surface analysis techniques, such as XRD, SEM, and EDX. The products of the interaction were mainly Trimethoxysilane (TrMS) and Tetramethoxysilane (TMS) and the highest percentage were 28% for TrMS and 21% for TMS. The percentage of the methoxysilanes increases when the Cu content of the mixture and the temperature increased, while the percentage of non-silicon products HCOH and CO decreases when the Cu content and temperature increased. The rate of CH3OH reaction with Si directly depends on its pressure giving rise to an apparent reaction order of unity with respect to the CH3OH reactant. Activation energies, pre-exponential factors, and ∇S# of the reaction have been estimated. A compensation effect operated in the kinetics of the CH3OH interaction with silicon as indicated by the linear relationships which existed between the values of Ea and the corresponding values of log A. The introduction of additives into the catalyst caused either promotion or inhibition.
1 Introduction
In the series of aliphatic silicic acid esters, the methyl and ethyl esters are an important member and, in fact industrially perhaps the most important (Noll, Citation1968; Brinker and Scherer, Citation1990). They are prepared by the direct reaction of alcohols with silicon as well as the esterification of the corresponding chlorosilanes, but the process has difficulties in obtaining economic yields (Lewis and Rethwisch, Citation1993). As early as 1949, a patent was issued to E.G. Rochow describing a process for methyl silicate by reacting methanol with silicon (Rochow, Citation1949). Suzuki and Ono (Citation1990), have studied the direct synthesis published in a series of journal articles. They performed a mechanistic study of a direct synthesis of HSi(OCH3)3 and discussed the role of Cu3Si. In other works, they carried out the reaction in a fixed-bed flow reactor and introduced additives to increase the selectivity for HSi(OCH3)3 to almost 100% (Okamoto et al.Citation,1993, Citation1994a,Citationb). In addition, Suzuki and Ono obtained methyldimethoxysilanes with such reaction in the presence of a small amount of thiophene in the feeds (Okamoto et al., Citation2000).
Table 2 Gas phase analysis for the reaction of CH3OH with Si-catalyst mixtures.
Table 3 Gas phase analysis for the reaction of CH3OH with mixtures containing additives.
The interaction of organic molecules with semiconductor surface and in particular with silicon substrates has attracted a great deal of attention in the past few years, mainly due to its relevance in a number of technological applications. Shannon and Campion (Citation1990), investigated the chemistry of CH3OH on the silicon surface using Raman spectroscopy. Ehrely et al. (Citation1991), used FTIR in order to investigate the vibrational properties of the adsorbed system and concluded that the methoxy species is formed, i.e., methanol undergoes a dissociation adsorption forming Si–OCH3 and Si–H species. This is recently confirmed theoretically by Miotto et al. (Citation2005). The experimental results of Casaletto et al. (Citation2002) and Miriji et al. (Citation2007)", provide strong evidence that CH3OH is dissociative adsorbed on the surface through the O–H bond breakage and the Si–O bond formation. They have also found further fragmentation of methanol exposure with the formation of surface bonded CHx species.
In the present work, the interaction of methanol with silicon has been studied using static form fixed – bed reactor. The catalyst used in the experiments was Cu which was mixed with Si to the extent of 5, 10, and 15 wt.% of the total composition.
2 Experimental
2.1 The system
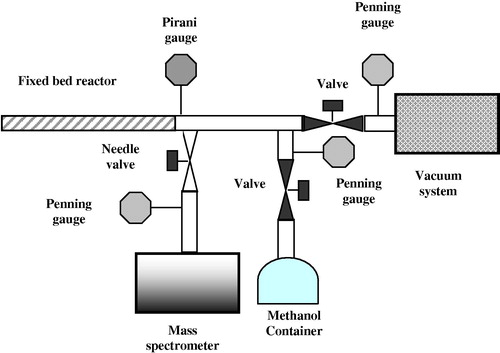
The vacuum system used in this work was constructed from stainless steel as shown in . It consisted of a number of specialized parts, involving vacuum apparatus, reactor, mass spectrometer, and temperature controller unit and connected as indicated in . The reactor tube was constructed of Pyrex glass tubing, 40 cm in length and 1 cm in internal diameter. An electrothermal heating tape (type Heat-by-the yard: Electrothermal engineering Limited, London) was used for heating the reaction system. The system was in a static form and the reacting CH3OH vapor could be brought into contact with Si-catalyst powder fixed as a bed in the reaction tube. A Quadravac (Q200) mass spectrometer-partial pressure gauge (obtained from Lybold) was used to analyze the gas phase of products and reactants.
2.2 Catalyst mixtures
The catalyst was freshly prepared CuCl and the procedure used was as described previously (Lowery and Cavell, Citation1949). The CuCl powders were sieved with 63 μm sieve and mixed well with silicon (and the fine powder of additive for catalysts containing additives experiments) (silicon grains <63 μm and purity 99.9%, was washed by a 40% HF aqueous solution for 1 h at room temperature) in a stopper flask. After a homogeneous mixture was obtained, the specimen was transferred into the reactor tube, and then dried under vacuum of 10−3 mbar for at least 10 h at 180 °C. The reaction system was disconnected from the other parts of the system, and heated to 350 °C, at a rate of 5 °C per min. At this temperature and after (2–2.5 h) the mixture became ready to be used in reaction.
2.3 Characterization methods
Physical adsorption of N2 gas at 77 K was used in this work for determining specific surface from the isotherm by adopting BET method using Gemini 2300 V5.01 from micrometrics Instrument Corporation. The catalysts surface was characterized by X-ray diffraction (XRD) using an Enraf Nonius FR590 X-ray generator with a CuK∞ source in the range 2θ = 4.4–124.6° and Carl Zeiss EVO-40 SEM with EDX at school of Chemistry – Cardiff University-UK.
2.4 Test catalytic
Two grams of the sample was first prepared as indicated in Section (2.2) and transferred into a reactor tube. The reactor was connected to the vacuum system and the reaction section was then opened to the pumps for at least 6 h. After establishing a vacuum of 10−5 mbar in the system, the reactor after outgassing for minimum of 10 h was cooled to the temperature at which the experiment started. The experiment was done by dosing the amount of CH3OH supply to the gas section in small doses (5 × 10−2–5 × 10−1) and allowed to react with powder sample. The products formed from the interaction were analyzed continuously by the Quadrupole mass spectrometer partial pressure gauge.
3 Results and discussion
3.1 Si-catalyst characterization
The SEM images show that the starting mixture (Si–CuCl mixture) contains two kinds of grains with different morphologies. Heating the mixture at 350 °C gave two striking changes in the grain morphologies: one is the disappearance of grains of CuCl. The second is an increase in the number of grains partly and completely covered with the deposits as shown in . Upon exposure to CH3OH, the reaction does not occur uniformly on the silicon surface areas and the pits are formed in these areas ().
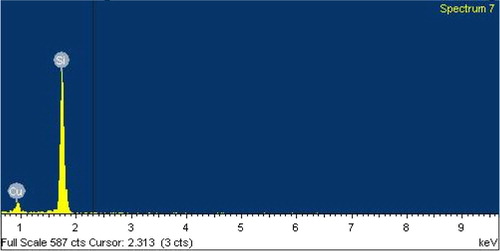
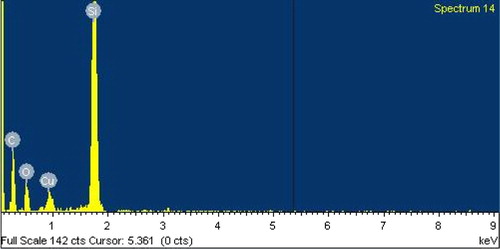
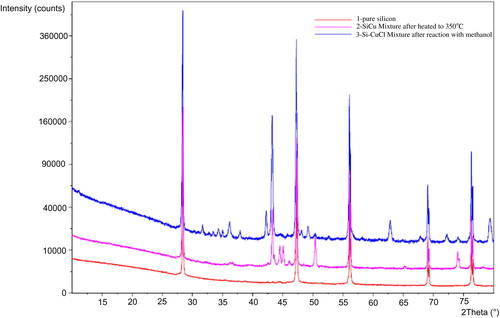
In , the EDX spectrum of Si after reaction with CuCl at 350 °C shows a signal of Si and Cu and no chlorine signal being observed which indicates that the reaction from Si–Cu alloy and SiCl4 desorbed to the gas phase. The spectrum of Si after reaction with CH3OH () shows signals belonging to C, Si, O, and Cu indicating that a chemical interaction exists between the silicon surface and CH3OH to produce methoxysilanes. On XRD analysis, a new diffraction peak at 2θ = 44.5 and 45.5 appears when Si–CuCl mixture is heated, and the intensity increases after methanol exposure () assignable to Cu3Si alloy (Clark, Citation1970).
3.2 The extent of adsorption
The extent (Θ) of CH3OH adsorption on Si-catalyst was expressed as: Θ = VMe/Vm where VMe represented the volume of CH3OH adsorbed and Vm represented the volume of N2 gas monolayer on each powder, the volumes were expressed in mm3 at S.T.P. shows the surface area (Ss), monolayer capacity (Vm), CH3OH uptake VMe and the corresponding values of Θ on each powder.
Table 1 The surface area and adsorption extent of CH3OH on the powders at 25 °C and 2.0 mbar.
CH3OH vapor, in the pressure range 10−2 to 2.0 mbar was admitted to the powder of silicon at room temperature and followed in successive stages to higher experimental temperatures, which were 25, 50, 100, 150, 200, 250, 300, 350, 400 °C. The uptake of CH3OH was followed at each temperature until its pressure remained virtually constant over a period of 15 min. Adsorption was found to be slight at temperatures ⩽250 °C, and the extent at 2.0 mbar pressure reached the value of 0.451 mm3 g−1. Adsorption was likely to be molecular at such temperatures since no products appeared in the gas phase. Therefore, the temperatures selected to perform the reaction are 300, 350, and 400 °C.
3.3 The interaction with Si-catalyst mixtures
The interaction of CH3OH vapor with Si powder without catalyst was followed with the formation of silicon free products such as H2, CH2O, and CO. Therefore, three mixtures of Si-catalysts powders have been used in a series of experiments containing 5%, 10%, and 15% by weight of copper. The surface area of these mixtures are given in .The adsorption was fast and at 250 °C and 2.0 mbar reached values of 24.55, 27.57, and 29.98 mm3 g−1 on 5%, 10%, and 15% Si-catalyst mixtures, respectively. On addition of 1.0% by weight of Al, Sn, ZnCl2, and CaCl2 to 10% Si–CuCl mixture, the adsorption reached values of 31.43, 28.99, 23.66, and 22.17 mm3 g−1at 250 °C and 2 mbar. The composition of products detected when the gas phase analysis and their variation with temperature for the three Si-catalysts and for mixtures containing additives are given in and . The peaks chosen for the quantitative analysis were of mass numbers 2, 28, 29, 31, 121, and 152 and a sensitivity factor values for each peak were 0.49, 0.78, 0.56, 0.69, 0.33 and 0.22, respectively.
The trend in the amounts of CH3OH adsorption on the various Si-catalyst mixtures follows the sequence 15% > 10% > 5%. This reflects the important role of copper, and the extent of close relationships between the extent of adsorption and the percentage of this metal in the mixture. This may be due to the high affinity of metals for the adsorption of gaseous species which may be present even in very low concentration, and the ability of d-electrons and their orbital for the bonding at the surface (Clark, Citation1970). This was also attributed to the extent and ease with which copper exists in the η-phase which is considered as a pre-requisite active phase for the direct reaction. Also, the extent of adsorption of CH3OH and for methoxysilanes formation on the catalysts containing additive mixtures may be put as: Al > Sn > ZnCl2 > CaCl2. This means that these additives affect the η-phase which is reflected in the increase of the amount of methoxysilanes, and to more extensive decomposition of CH3OH which leads to increases in the extent of adsorption.
It was reported by Voorhoeve, Citation1967, that promoter can exist in both the metallic Cu phase as well as in the η-phase. The results also indicate the following:
The percentage of TrMS and TMS in the gas phase increases when the Cu content of the mixture is increased from 5% to 15%. This may be resulted from the reaction of CuCl with Si which produces a mass consisting of Si particles irregularly covered by Cu. Most of the copper is likely to be present as η-phase. When the copper content of mixture increased, the amount of free copper increased. Such active Cu3Si complex (η-phase) is reported to facilitate the formation of TrMS, while the free Cu facilities of the formation of TMS according to mechanism involving a silylene intermediate (Okamoto et al., Citation1994a).
The percentage of non-silicon products HCOH and CO decreases when the Cu content is increased. This may be resulted as a consequence of a more extensive decomposition of CH3OH on the surface of free Cu which will be increased when the percentage of Cu in the mixture increased. This agrees with the mechanism illustrated by Saleh and Al-Mawlawi, Citation1981, for the dissociative adsorption of methanol on metal surfaces.
The composition of gas phase products is dependent on the temperature of the reaction. The percentage of TrMS and TMS in the products increased on raising the temperature of the reaction from 250 °C to 350 °C for all the mixtures used and the highest percentages (28% TrMS; 21% TMS)were obtained on Si-catalyst mixtures containing 15% Cu at 350 °C. The formation of free-silicon products on the 5% Cu mixture resulted in a sequence as: HCOH > CO > H2 at 250 °C, and the sequence was exchanged as H2 > HOCH > CO at 350 °C.
3.4 Kinetic investigation
The rate of CH3OH reaction was determined from the pressure variation in the system with time at a given constant temperature. By comparing the rate of CH3OH adsorption at two different temperatures, but essentially at the same surface coverage (Θ), Ea of adsorption was determined. From the initial rate (molecule per second per cm2 of the surface) and the appropriate value of Ea at a given temperature, the value of pre-exponential factor (A) in the rate equation: r = A exp Ea/RT was calculated. The values of A used to calculate the entropy of activation (∇S#) using the relation A = (kT/h) CgCs exp ∇S#/R, where Cg and Cs represented, respectively, the concentration of CH3OH per unit volume (cm3) and of the surface atoms per unit area (cm2) (Al-Hyderi et al., Citation1988). shows the kinetic data obtained for all mixtures investigated.
Table 4 Kinetic data for the reaction of CH3OH with Si-catalyst and mixtures containing additives.
The results obtained from the kinetic study indicate the following:
| 1- | The rate of reaction depended on CH3OH pressure and the reaction order (n) with respect to CH3OH was close to unity and independent on the value of Θ. | ||||
| 2- | The value of Ea for a given mixture, did not depend on the extent of adsorption (Θ), but depended on the type of mixture. If Ea is considered to be more effective in defining the rate of the reaction, at a constant temperature and over a narrow range of A values, than the pre-exponential factor, then the mixture might be arranged in a reactivity sequence toward CH3OH vapor as: 5% > 10% > 15%, While the additives could be arranged in a reactivity as: Al > Sn > ZnCl2 > CaCl2. | ||||
| 3- | The A values varied with the values of Ea which indicate the operation of a compensation effect throughout the interaction. Such a compensation effect is likely to arise from the presence of energetically heterogeneous reaction sites on the catalyst surface, and such sites increase by promoters and decrease by inhibitors. The ∇S# was less negative on moving from Al to CaCl2 (as sequence above). Thus, the activated complex of the CH3OH interaction with Si-catalyst gains more degrees of freedom and hence becomes more mobile as one moves from the left to the right of the sequence. | ||||
4 Conclusions
The direct reaction of methanol with silicon in the presence of copper as a catalyst depends on temperature as a major effecting parameter where adsorption extent was slight at temperatures ⩽250 °C, and was likely to be molecular at such temperatures since no products appeared in the gas phase. At temperatures higher than 250 °C, the percentage of TrMS and TMS in the gas phase increases when the Cu content of the mixture increased from 5% to 15%. On the other hand the extent of adsorption of CH3OH in methoxysilanes formation on the catalysts containing additive mixtures may be arranged in reactivity as:
The percentage of non-silicon products HCOH and CO decreases when the Cu content increased. The kinetic study indicates that the rate of reaction depends on CH3OH pressure and the reaction order (n) [with respect to CH3OH was close to unity], at the same time the value of Ea for a given mixture depends on the type of mixture and not on the extent of adsorption (Θ), accordingly, the mixtures may be arranged in a reactivity sequence toward CH3OH vapor as: 5% > 10% > 15%, While the additives can be arranged in a reactivity as: Al > Sn > ZnCl2 > CaCl2.
Acknowledgements
The authors acknowledge the Department of Chemistry/Baghdad university and IBN SINA STATE Company/Ministry of Industry/Iraq for their financial support of this research. A special thanks goes to Dr. Moayyad K. Jalhoom, the manager of the above company for his support.
References
- Y.K.Al-HyderiJ.M.SalehM.H.MatloobCatalytic decomposition of mercaptans on metal films of Fe, Pd, Ni, and AlJ. Chem. Soc.: Faraday Trans. I8419883207
- G.J.BrinkerG.W.SchererThe Physics and Chemistry of Sol–Gel Processing1990Academic PressNew York
- M.P.CasalettoR.ZanonM.CarboneK.AballeK.HornMethanol adsorption on Si(1 0 0)2 × 1 investigated by high-resolution photoemissionSurf. Sci.5052002251
- A.ClarkThe Theory of Adsorption and Catalysis1970Academic PressNew York
- W.EhrelyR.ButzS.MantlExternal infrared reflection absorption spectroscopy of methanol on an epitaxial grown Si(1 0 0)2 × 1 surfaceSurf. Sci.2481991193
- K.M.LewisD.G.RethwischCatalyzed Direct Reaction of Silicon1993ElsevierAmsterdam
- T.M.LoweryA.C.CavellIntermediate Chemistryfifth ed.1949Macmillan and Co. LimitedLondon
- R.MiottoG.P.StrivastavaFreeazMethanol adsorption on silicon (0 0 1)Surf. Sci.57532005287299
- S.A.MirijiS.B.HalliguedM.NevinE.NaliniK.R.PatilF.GaikwadAdsorption of methanol on mesoporous SBA-15Mater. Lett.61120078892
- W.NollChemistry and Technology of Silicones1968Academic PressNew York
- M.OkamotoM.OsakaK.YamamotoE.SuzukiY.OnoEffect of pretreatment conditions of Si–CuCl mixtures on the rate and selectivity in the reaction of silicon with methanol using copper(I) chloride catalystJ. Catal.14319936485
- M.OkamotoE.SuzukiY.OnoReaction pathway of formation of methoxysilanes in the reaction of silicon with methanol catalyzed by CuClJ. Catal.1451994537543
- M.OkamotoK.YamamotoE.SuzukiY.OnoSelective synthesis of trialkoxysilanes by reaction of Si with alcohols using CuCl as catalystJ. Catal.14719941523
- M.OkamotoH.AbeY.KusamaE.SuzukiY.OnoDirect synthesis of methyldimethoxysilane from metallic silicon and methanol using copper(I) chloride catalystJ. Organomet. Chem.61620007479
- E.G.RochowPreparation of tetramethyl silicateUS patent24731949260
- J.M.SalehD.Al-MawlawiInteraction of alcohol with evaporated metal filmsJ. Chem. Soc.: Faraday Trans. Part II7719812965
- C.ShannonA.CampionRaman spectroscopic investigation of the adsorption of acetonitrile and methanol on Si(1 0 0)-2 × 1Surf. Sci.2271990219
- E.SuzukiY.OnoMechanism of active-site formation in copper-catalyzed synthesis of trimethoxysilane by the reaction of silicon with methanolJ. Catal.1251990390400
- R.J.H.VoorhoeveOrganohalosilanes1967ElsevierAmsterdam p. 132