Abstract
A viable cost-effective and isocratic approach employing C-18 column (250 mm × 4.6 mm, 5 μm) based HPLC has been utilized to separate and estimate the drug, tranexamic acid in pharmaceutical formulations. Tranexamic acid contains no π-electrons to act as fluorophore or chromophore hence pre-column derivatization was performed with benzene sulfonyl chloride in aqueous medium at room temperature. The derivatized drug was then estimated using C-18 column by exploiting a 25:75 (v/v) solvent mixture of acetonitrile and 0.1 M ammonium acetate (pH 5.0) as the mobile phase. The flow rate of mobile phase was 1 mL/min and detection was performed at a wavelength of 232 nm using UV detector. Retention time of tranexamic acid was 4.42 min. The method followed linear regression equation in the concentration range of 1–100 μg/mL with co-efficient of determination equal to 0.9994. The limit of detection and limit of quantitation were 0.3 and 1 μg/mL, respectively. The relative standard deviation and recovery ranges for tranexamic acid were found to be 0.11–2.47% and 97.60–103.25%, respectively. The suggested method is very sensitive and may have the potential to be used for tranexamic acid detection in medicinal formulations.
1 Introduction
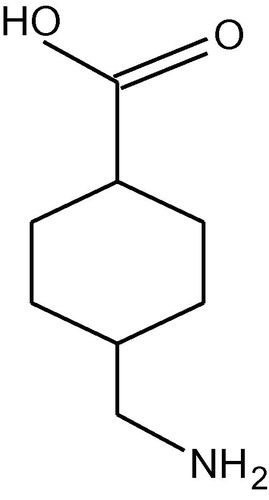
Tranexamic acid () having chemical name as trans-4-(aminomethyl) cyclohexanecarboxylic acid is a synthetic amino acid, similar to lysine, commonly used for curing abnormal bleeding in a variety of diseases (Boylan et al., Citation1996; Nilsson, Citation1980). The drug is hydrophilic in nature and has find application as antifibrinolytic as well as hemostatic drug (CitationFurtmuller et al., 2002). Since its introduction in the pharmaceutical market, it has been used to cure particularly heavy menstrual bleeding (CitationLukes et al., 2011) in addition to blood reduction in all types of surgeries (Dunn and Goa, Citation1999; Mayur et al., Citation2007; Caglar et al., Citation2008). Tranexamic acid performs its antifibrinolytic activity as it blocks competitively those sites of protein plasmin, plasminogen and plasminogen activator that bind lysine hence their interaction with fibrin is prevented. So plasminogen cannot be converted into plasmin and in this way proteolytic activity is prohibited (CitationHiippala et al., 1997).
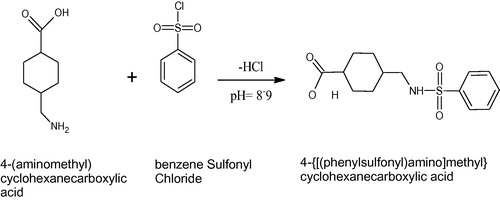
Tranexamic acid does not have any π-electrons so it cannot act as fluorophore or chromophore and hence cannot be measured through UV spectroscopy. It is therefore imperative to derivatize this compound so as to quantify its UV-active derivative through HPLC–UV. A review of the literature resulted in many papers describing derivatization of this drug with different reagents followed by their HPLC determinations. Some of these methods utilized methanolic ninhydrin (CitationNatesan et al., 2011), phenylisothiocynate (CitationHadad et al., 2007), 2-hydroxynaphthaldehyde in aqueous ethanol (CitationKhuhawar and Rind, 2001) and sodium picrylsulfonate (CitationNojiri et al., 1995) followed by their determination through HPLC–UV. In addition to these, LC-fluorescence method utilizing naphthalene-2,3-dicarboxaldehyde plus cyanide (CitationHuertas-Perez et al., 2007), o-phthalaldehyde (CitationElworthy et al., 1985), an electrochemical method (CitationShih et al., 2008), a LC–MS method (CitationChang et al., 2004) and UPLC–MS/MS (CitationAbou-Diwan et al., 2011) have also been established for the determination of tranexamic acid. An RP–HPLC method for the determination of tranexamic acid along with its related substances and a GC method are also reported in the literature (Du et al., Citation2010; Abbasi et al., Citation2009). All methods reported so far involve complexities for the accomplishment of derivatization and its quantification. In our method, a very simple reagent, benzene sulfonyl chloride has been used as derivatizing reagent and the reaction has been completed in simply distilled water at basic pH, which makes it a green approach. The derivatized product after completion of the reaction was quantified by using HPLC with UV detection with total run time of less than five minutes. The developed method can be successfully used for tranexamic acid determination in pharmaceutical formulations.
2 Experimental
2.1 Chemicals and reagents
Pure reference standard of tranexamic acid claiming purity of 99.65% was attained from Munawwar Pharma (Lahore, Pakistan). Acetonitrile is of HPLC grade, whereas other chemicals of analytical reagent grade were purchased from M.S. Traders (Agents of Fluka in Pakistan). Throughout the analysis, double distilled water was used. For mobile phase filtration, 0.45 μm nylon filters were used (Millipore, USA).
2.2 Equipment and chromatographic conditions
For the development of HPLC procedure, Shimadzu LC-20A (Kyoto, Japan) HPLC system, equipped with UV–Visible detector and auto sampler was used. 20 μL of analyte was injected in all experiments. Chromatographic conditions were optimized on a Merck C 18 column (250 × 4.6 mm, 5 μm) and the mobile phase was prepared by mixing acetonitrile and 0.1 M ammonium acetate (pH 5.0) in the ratio (25:75, v/v). The mobile phase flow rate was set as 1.0 mL/min and all the chromatographic work were performed at room temperature (25 ± 2 °C).
2.3 Derivatization of tranexamic acid
Tranexamic acid (2 g, 12.7 mmol) was taken in a beaker and dissolved in 15–20 mL of distilled water. Sodium carbonate solution (2–3%) was added drop wise until all the tranexamic acid get dissolves and pH reaches between 8 and 9. The pH of this solution was strictly maintained between 8and 9. Equimolar benzene sulfonyl chloride (1.904 mL) was then added to the solution. It was stirred on a magnetic stirrer at room temperature. The pH of the reaction mixture was kept at 8–9, by adding some drops of Na2CO3 solution, whenever the pH lowered. Stirring was continued till completion of the reaction. At this stage pH of the solution will stop to lower down and will remain constant. After that, 2–3 M HCl was added drop by drop into the solution until the formation of white precipitates’ stops. The precipitates formed were filtered, washed with distilled water and dried. This product was then further used for making standard solutions.
2.4 Preparation of stock and standard solutions
The stock solution of tranexamic acid (1000 μg/mL) was prepared by dissolving the product of tranexamic acid equivalent to 100 mg tranexamic acid in a 100 mL mobile phase. From this stock solution, several standard solutions of varying concentrations were prepared by making dilutions.
2.5 Preparation of sample solution
Twenty tablets were accurately weighed and finely ground with mortar and pestle. An accurately weighed portion of the grind equivalent to 200 mg of tranexamic acid was mixed with 0.19 mL of benzene sulfonyl chloride in 10–15 mL of the distilled water and proceeded as described in the derivatization procedure. The entire product obtained was dissolved in the mobile phase and then further diluted to obtain concentration equivalent to 10 μg/mL.
2.6 Linearity
To demonstrate linearity, five solutions 100, 50, 10, 5 and 1 μg/mL in the range 1–100 μg/mL were prepared and then analyzed. Each concentration was injected three times and peak areas were noted.
2.7 Accuracy
Accuracy is actually closeness of the experimental value to the true value. The most important technique to find accuracy is the “standard addition technique”. Three different concentrations of solutions were prepared according to this technique. All these solutions were prepared in 25 mL measuring flasks and the mobile phase was used to make the total volume up to the mark. The concentrations of three solutions were 10, 25 and 50 μg/mL, respectively. Their results were compared with those obtained with originally prepared standard solutions.
2.8 Precision
It represents the closeness of the individual measurements. It was stated in terms of relative standard deviation. The intra-day precision was performed by using 3 different concentrations of solutions. Each concentration was injected in the column, at an interval of one hour, in a span of three hours within a day. Inter-day precision was accessed by injecting three different concentrations of the solutions consecutively for three days.
2.9 Selectivity
To carry on selectivity, synthetic mixture of tranexamic acid with starch, lactose, magnesium stearate and micro crystalline cellulose which are generally used as excipients was prepared. This synthetic mixture then proceeded as described in sample preparation and then analyzed by the proposed method to check any kind of interference which might be caused by these alien species.
3 Results and discussions
3.1 Derivatization of tranexamic acid
Derivatization is actually considered to be such a process that can cause a chemical change in the analyte, which is going to be analyzed by a definite and particular analytical method (HPLC in this particular case). This chemical change provides suitable characteristics and properties to the analyte of interest. In the present study, derivatization of the tranexamic acid was carried out to impart such characteristics so as to enable it to be analyzed through UV detector.
Absorbance of derivatized product was checked by spectrophotometer after dissolving it in ethanol. The absorbance maximum was 232 nm which was used in HPLC system. The proposed schematic diagram of the reaction is given in .
3.2 Method development and its optimization
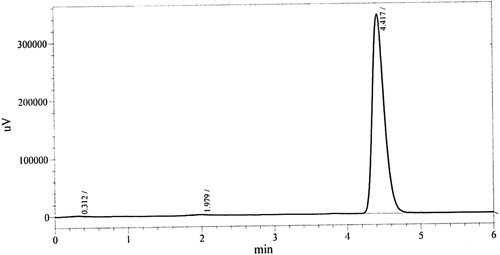
In this specified work the goal was to develop a sensitive, simple, accurate and isocratic HPLC method so as to determine tranexamic acid. In the beginning different mobile phases were tried in order to get best possible separation of tranexamic acid. The mobile phase containing 0.1 M ammonium acetate and acetonitrile having ratio 75:25 (v/v) and C-18 column as a stationary phase proved to be most suitable for separation purpose with a flow rate of 1 mL/min. By applying the explored chromatographic conditions, sharp peak of tranexamic acid was attained with a retention time of 4.42 ± 0.02 min. A chromatogram of tranexamic acid is shown in .
Figure 2 Representative chromatogram of tranexamic acid after derivatization. Chromatographic conditions: mobile phase ammonium acetate buffer pH 5.0 and acetonitrile 75:25 v/v, column C18 (250 mm × 4.6 mm, 5 μm), wavelength 232 nm, flow rate 1 mL/min.
The method development was initiated by using less polar mobile phase 50% acetonitrile but no peak appeared. Then the mobile phase polarity was enhanced by using 0.1 M ammonium acetate (pH 5.0). The selection of 0.1 M acetate buffer was based on our previous results where many of our compounds were eluted with well resolved sharp peaks (Ashfaq et al., Citation2007, Citation2008; Khan et al., Citation2010a,Citationb). Composition of the mobile phase was continuously changed to get the best possible separation. Increase of acetonitrile results in the elution of derivatized tranexamic acid with fronted peak whereas decrease in acetonitrile results in late elution. Ammonium acetate (pH 5.0) and acetonitrile in 75:25 (v/v) ratio brought about a sharp peak as well as good separation. Hence this composition was considered to be the best optimum composition of the mobile phase and further used in all experiments.
3.3 Method validation
The chromatographic method, which has been developed to determine tranexamic acid, was validated by using the guidelines of ICH (CitationICH, 1996). Various parameters of ICH guidelines involved during this study include precision, accuracy, linearity, limit of quantitation/detection, selectivity and robustness.
To demonstrate linearity, five solutions in the concentration range 1–100 μg/mL, 100, 50, 10, 5 and 1 μg/mL were prepared and then analyzed. Very good linearity was seen over the described concentration range for tranexamic acid. The calibration curve was drawn by taking different concentrations on x-axis and their mean areas on y-axis. Linear regression equation for tranexamic acid was Y = 17583x + 138159 with the value of co-efficient of determination as R2 = 0.9994. This shows that a good decent linear relationship exists between drug concentrations and their peak areas.
For all analytical procedures, the detection limit is considered to be a point at which our analysis is feasible and just appropriate. Statistical approach may be used to find this specific point. While LOQ value or limit of quantification is thought to be of that concentration we can report our quantitative results with very much confidence. This point can also be found out by using statistical approach. From all the prepared standard solutions with different concentrations of tranexamic acid limit of quantification (LOQ) and limit of detection (LOD) were found after the evaluation of signal to noise ratios which are 10:1 and 3:1 respectively. For tranexamic acid, the value of LOQ was 1 μg/mL and the value of LOD was 0.3 μg/mL.
The study of accuracy of the suggested method was completed by making three different concentration solutions using standard addition technique. The concentrations of solutions prepared for accuracy by addition technique were 10, 25 and 50 μg/mL. The solutions were analyzed by using the described method. Recovery experiments were used for calculations of % RSD values and percentage recoveries. The relative standard deviation and recovery ranges for tranexamic acid are given in .
Table 1 Accuracy study by the proposed HPLC method.
The three solutions which were used for studying accuracy of the suggested method were also used to study precision (inter and intra-day precision). These three different concentrations were 10, 25 and 50 μg/mL, respectively. The precision of the suggested method was defined in terms of % RSD by analyzing above three solutions. For intra-day precision, all the three concentrations were injected in the column, at an interval of one hour, three times in a single day. The inter-day precision was completed by injecting these concentrations of tranexamic acid consecutively for three days. Results of both intra and inter-day precision are given in .
Table 2 Study of precision by the proposed HPLC method.
For carrying out robustness, premeditate minor changes were made in the experimental conditions and then response against those changed parameters was measured. Results are given in , which reflect minor variation in chromatographic parameters against minor chromatographic changes.
Table 3 Robustness study.
To carry on selectivity, synthetic mixture of tranexamic acid with commonly occurring excipients in tablet formulations such as starch, lactose, magnesium stearate and micro crystalline cellulose was prepared. This synthetic mixture then proceeded as described in the sample preparation and then analyzed by the proposed method to check any kind of interference which might be caused by these alien particles. The results presented in , did not show any interference caused by these foreign particles.
Table 4 Selectivity of the proposed method.
The method was used for the determination of tranexamic acid in pharmaceutical formulations and the results are given in , which showed very good recovery.
Table 5 Assay results of tranexamic acid in tablets.
4 Conclusion
In this particular study an economic, simple, accurate, sensitive, selective and precise method was developed by using HPLC in order to determine tranexamic acid. This drug does not have any chromophore that is why derivatization was carried out so that it may be detected by UV detector of HPLC. The method was also validated according to guidelines provided by ICH. Method validation was completed by testing its precision, linearity, accuracy, values of LOD and LOQ. Compared to other methods, this specific HPLC method is quite feasible as well as cost-effective for the detection of tranexamic acid. This method is very suitable and appropriate because the peak of the drug appears in less than five minutes and run time is short. The suggested method is also quite suitable for routine determination of the drug. The method is also linear, precise and gives good accuracy as the data and calculations of results of different experiments indicate. The results obtained by accuracy and precision experiments conclude that this particular method is useful for quality control evaluation of tranexamic acid. From the values obtained by different experiments, it is proved that the new method of HPLC is suitable and appropriate for determination of the specific drug tranexamic acid.
Notes
Peer review under responsibility of University of Bahrain.
References
- K.U.AbbasiM.Y.KhuhawarM.I.BhangerDetermination of tranexamic acid using ethyl chloroformate as derivatizing reagent in pharmaceutical preparations and blood by GCChromatographia7011–12200917491754
- C.Abou-DiwanR.M.SniecinskiF.SzlamJ.C.RitchieJ.M.RheaK.A.TanakaR.J.MolinaroPlasma and cerebral spinal fluid tranexamic acid quantitation in cardiopulmonary bypass patientsJ. Chromatogr. B8792011553556
- M.AshfaqI.U.KhanS.S.QutabS.N.RazzaqHPLC determination of ezetimibe and simvastatin in pharmaceutical formulationsJ. Chil. Chem. Soc.52200712201223
- M.AshfaqI.U.KhanM.N.AsgharDevelopment and validation of liquid chromatographic method for gemfibrozil and simvastatin in binary combinationJ. Chil. Chem. Soc.53200816171619
- J.F.BoylanJ.R.KlinckA.N.SandlerR.ArellanoP.D.Greigg H.NierenberS.L.RogerM.F.GlynnTranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantationAnesthesiology85199610431048
- G.S.CaglarY.TasciF.KayikciogluA.HaberalIntravenous tranexamic acid use in myomectomy: a prospective randomized double-blind placebo controlled studyEur. J. Obstet. Gynecol. Reprod. Biol.1372008227231
- Q.ChangO.Q.P.YinM.S.S.ChowLiquid chromatography-tandem mass spectrometry method for the determination of tranexamic acid in human plasmaJ. Chromatogr. B8052004275280
- N.DuY.WangM.-M.CaiB.DiDetermination of tranexamic acid injection and its related substances by RP–HPLCChin. Pharm. J.452010144147
- C.J.DunnK.L.GoaTranexamic acid: a review of its use in surgery and other indicationsDrugs57199910051032
- P.M.ElworthyS.A.TsementzisD.WestheadE.R.HitchcockDetermination of plasma tranexamic acid using cation-exchange high-performance liquid chromatography with fluorescence detectionJ. Chromatogr. B3431985109117
- R.FurtmullerS.G.SchlagM.BergerR.HopfS.HughW.SieghartH.RedlTranexamic acid; a widely used antifibrinolytic agent causes convulsions by a γ-aminobutyric acid: a receptor antagonistic effectJ. Pharmacol. Exp. Ther.302002168173
- G.M.HadadA.El-GindyW.M.M.MahmoudOptimization and validation of an HPLC–UV method for determination of tranexamic acid in a dosage form and in human urineChromatographia662007311317
- S.T.HiippalaL.J.StridM.I.WennerstrandJ.V.V.ArvelaM.H.NiemelaS.K.MantylaR.P.KuismaJ.E.YlinenTranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplastyAnesth. Analg.841997839844
- J.F.Huertas-PerezM.HegerH.DekkerH.KrabbeJ.LankelmaF.ArieseSimple, rapid, and sensitive liquid chromatography-fluorescence method for the quantification of tranexamic acid in bloodJ. Chromatogr. A11572007142150
- ICH (Q2B)Note for guidance on validation of analytical procedures: methodology. International conference on Harmonisation1996IFPMAGeneva
- I.U.KhanS.M.S.JillaniM.AshfaqDetermination of atorvastatin and gemfibrozil in human plasma by reversed-phase liquid chromatographyLat. Am. J. Pharm.29201013831388
- I.U.KhanT.KausarM.AshfaqS.SharifDevelopment and validation of liquid chromatographic method for the simultaneous estimation of ezetimibe and lovastatin in human plasmaJ. Chil. Chem. Soc.552010461464
- M.Y.KhuhawarF.M.A.RindHPLC determination of tranexamic acid in pharmaceutical preparations and bloodChromatographia532001709711
- A.S.LukesP.A.KouidesK.A.MooreTranexamic acid: a novel oral formulation for the treatment of heavy menstrual bleeding womensHealth72011151158
- G.MayurP.PurviG.AshooD.PankajEfficacy of tranexamic acid in decreasing blood loss during and after caesarean section: a randomized case controlled prospective studyJ. Obstet. Gynecol. India572007227230
- S.NatesanD.ThanasekaranV.KrishnaswamiC.PonnusamyImproved RP-HPLC method for the simultaneous estimation of tranexamic acid and mefenamic acid in tablet dosage formPharm. Anal. Acta212011115
- I.M.NilssonClinical pharmacology of aminocaproic and tranexamic acidsJ. Clin. Pathol. Suppl.1419804147
- S.NojiriS.UeharaT.HagiwaraM.NishijimaHigh-performance liquid chromatographic determination of aspartic acid, taurine, and tranexamic acid by precolumn derivatization using sodium picrylsulfonateTokyo-toritsu Eisei Kenkyusho Kenkyu Nenpo4619955861
- Y.ShihK.WuJ.SueA.S.KumarJ.ZenDetermination of tranexamic acid in cosmetic products by high-performance liquid chromatography coupled with barrel plating nickel electrodeJ. Pharm. Biomed. Anal.485200814461450