Abstract
This study investigated the effects of green, white and black tea (Camellia sinensis) on lactic acid production and the viability of Streptococcus thermophilus and Lactobacillus spp. in yogurt during 3 weeks of refrigerated storage. Three types of tea water extracts were added into a milk-starter culture mixture and incubated at 42 °C until the pH was reduced to 4.5. All yogurts were then refrigerated (4 °C) for up to 21 days and samples were analyzed for pH, titratable acid and viable counts of yogurt bacteria. Higher pH values (p < 0.05) were shown in tea yogurts than plain yogurt (PY). Green tea yogurt (GTY) showed the highest pH followed by black tea yogurt (BTY) and white tea yogurt (WTY), respectively for the overall storage period. However, higher acid production was observed in all tea yogurts (0.78–0.99% lactic acid equivalent; LAE) than plain yogurt (0.70–0.91% LAE). Inclusion of three types of tea extracts did not affect significantly (p > 0.05) the viability of Lactobacillus spp. and S. thermophilus compared to PY during storage. All the three types of tea yogurt maintained a high level of S. thermophilus and Lactobacilllus spp. counts through refrigerated storage and this can ensure health benefits to be delivered to consumers on daily consumption.
Keywords:
1 Introduction
Probiotics are live microorganisms that when present in sufficient amounts in the digestive tract may confer health benefits on the host (CitationLourens-Hattingh and Viljoen, 2001). Thus, it is necessary for most of these live cultures to survive during their shelf life prior being consumed. Combination of lactic acid starter bacteria with probiotic (Bifidobacterium, Lactobacillus) is widely used in yogurt manufacture (Vinderola et al., Citation2000, Citation2011"). Lactic acid starter bacteria are acid sensitive (CitationMarteau et al., 1997) and are unable to resist bile salts. Thus, these bacteria show very poor ability to survive the passage through the stomach and the intestinal tract. For this reason, lactic acid starter bacteria were not originally considered to be probiotics (CitationAnalie and Bennie, 2001). A previous study proposed that yogurt containing Streptococcus thermophilus and Lactobacillus delbrueckii spp. bulgaricus could be regarded as beneficial because both bacteria provide health benefits to the host (CitationGuarner et al., 2005). These bacteria are able to release β-galactosidase enzymes that improve the digestion of nutrients in the intestine and modulate immune responses for human health (CitationLee et al., 2001). To be effective as probiotics, the viable cell counts (VCC) of the final yogurt product should be in the range of 108–109 cfu/g right before ingestion to ensure a sufficient therapeutic minimum of 106–107 cfu/g could reach the colon. This equates to a daily consumption of about 100–200 g of yogurt.
Several novel approaches have been considered to increase the survival of probiotic bacteria such as plant extracts, phenolic compounds and antioxidative substances (CitationSaxelin, 2008). Tea (Camellia sinensis) is a common beverage being consumed worldwide. It is known for a wide range of health promoting properties that may exert antimicrobial action but without changing lactic acid bacteria (CitationNajgebauer-Lejko, 2014). Tea can be divided into three types based on the method of processing the leaves, namely the non-fermented green and white tea, partially fermented oolong tea and fermented black tea (CitationHorzic et al., 2009). In the manufacture of green tea, the plucked tea leaves are immediately withered and subjected to steaming (Japan) or pan-frying (China) prior to rolling and drying to inactivate the endogenous polyphenol oxidase (PPO) enzyme as well as native microflora in the leaves (Gondoin et al., Citation2010; Kim et al., Citation2011"). This prevents the polyphenols mainly catechins in tea from oxidation thus retaining a large amount of polyphenols in green tea leaves. In the processing of black tea, steaming or pan-frying steps are omitted but the leaves are crushed or bruised to disrupt cellular compartmentalization and bring the phenolic compounds in the leaves into close approximation with the enzyme PPO, thus allowing the rolled leaves to be fully fermented by the active PPO enzyme (CitationRusak et al., 2008). As a result of the fermentation a large portion of the catechins are condensed into larger polyphenols known as theaflavins and thearubigins which contribute to the characteristic reddish-black color, reduced astringency and bitterness as well as elimination of the leafy and grassy flavor in black tea (CitationKim et al., 2011). White tea is defined by plucking standards, comprising only the bud or first leaves that are plucked and dried with minimal processing. Thus, the delicate white leaf hairs are left unbroken giving the appearance of white tea (CitationHilal and Engelhardt, 2007).
Since yogurt bacteria can be affected by the inclusion of green tea (Jaziri et al., Citation2009; Najgebauer-Lejko et al., Citation2011; Gaudreau et al., Citation2013"), it is important to establish the differences in the types of tea used on viability of yogurt bacteria. Therefore, the objective of this study was to evaluate the changes in acid production and viability of S. thermophilus and Lactobacillus spp. due to the addition of three types of tea (green, black and white tea) and the stability during 21 days of refrigerated storage.
2 Materials and methods
2.1 Materials
Pasteurized full cream milk (Dutch Lady, Malaysia) was used for making yogurt. The three types of ground tea leaves used in this study were Long Jing green tea, Shou Mei white tea (China origin; Purple Cane Enterprise, Malaysia) and black tea (Malaysia origin; Lipton, Malaysia) purchased from a local hypermarket. Commercially available starter culture powder (Chris-Hansen, Denmark) used in yogurt preparation consists of a mixture of Lactobacillus acidophilus LA-5, Bifidobacterium Bb-12, Lactobacillus casei LC-01, S. thermophilus Th-4 and L. delbrueckii ssp. bulgaricus in the ratio of 4:4:1:1:1. Buffered peptone water, De Man Rogosa and Sharpe (MRS) agar and M17 agar were purchased from Oxoid, UK.
2.2 Preparation of yogurt
Plain yogurt and three types of tea-yogurt were prepared according to the method described by CitationJaziri et al. (2009) with slight modifications. Pasteurized full cream milk (100 ml) was warmed to 85 °C for 30 min. Then, treated milk was mixed with green, white or black teas (2 g/100 ml) corresponding to the strength of a “normal cup of tea” (CitationYam et al., 1997). The teas were allowed to infuse into the milk for 10 min, followed by filtration through a fine tea strainer to remove visible particles. The resulting tea-milk infusions (90 ml) were aliquoted into disposable plastic containers placed in an incubator (45 °C), followed by the addition of 10 ml (10% w/v; CitationShori and Baba, 2012) starter culture (1 L of full cream milk incubated with a starter culture powder at 41 °C for 12 h, initial counts of S. thermophilus and Lactobacillus spp. were 7.6 ± 2.3 and 4.9 ± 0.2 log CFU/ml, respectively) into the milk-tea infusion. This was followed by the addition of 2 g full cream milk powder to correct the milk solid content. Plain yogurt was prepared in the same manner as previously described without tea infusate. All inoculated milk and milk-tea infusates were incubated in a water-bath at 42 °C until the pH values reached 4.5. The yogurts were then refrigerated (4 °C) up to 21 days. Samples of each yogurt type were removed from the fridge, the following day (day 1) and on days 7, 14 and 21 of storage for further analysis.
2.3 Determination of pH and titratable acid (TA)
The pH and TA measurements were determined as described by CitationShori and Baba (2012).
2.4 Determination of bacteria viable cell counts
Yogurt samples (1 ml) were decimally diluted using 9 ml sterile 0.1% buffered peptone water prepared in tubes and autoclaved at 121 °C for 15 min (CitationShori and Baba, 2012). The mixture was thoroughly shaken for uniform distribution, and several other diluents (10−1–10−6) were prepared using the serial dilution method. The enumeration of S. thermophilus and Lactobacillus spp. were carried out using M17 and MRS agars, respectively as described by CitationShori and Baba (2012).
2.5 Statistical analysis
A Completely Randomized Design (CRD) experiment was applied in three different batches of yogurt (n = 3), each batch consisting of green, white or black tea extracts in yogurt besides plain-yogurt as control. All experiments were performed in duplicate. Results of each analysis were expressed as mean ± standard deviation. All data were subjected to a two way analysis of variance (ANOVA), and the significance of differences between means was determined on the basis of Duncan test at a significance level of p < 0.05. The statistical analysis was carried out using IBM SPSS Statistics version 20.0 software.
3 Results and discussion
3.1 Effects of tea on S. thermophilus viability in yogurt
The changes in S. thermophilus counts in all tea yogurts during storage are shown in . It was found that inclusion of three types of tea extracts in yogurt did not affect the viability of S. thermophilus compared to PY (p > 0.05), during the entire storage period.
Figure 1 Changes in viable cell counts of Streptococcus thermophilus in yogurts in the presence and absence of tea during 21 days of refrigerated storage at 4 °C. Error bars represent a pooled standard deviation of the mean (n = 3). The level of significance was preset at p < 0.05. Plain yogurt (control) refers to yogurt without the incorporation of tea (milk + starter culture only).
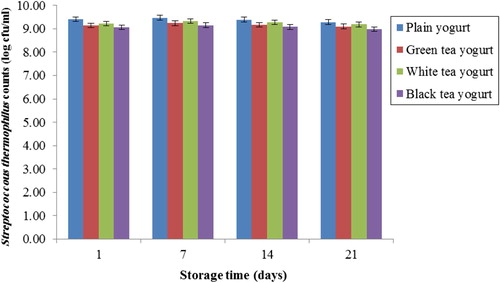
One of the strategies applied to improve the growth of probiotic bacteria is the addition of prebiotic substances (El-Dieb et al., Citation2012; Oliveira et al., Citation2011"). The present study indicated that the addition of three types of tea (green, white and black) in probiotic yogurt had a negative effect on viability of S. thermophilus during 21 days of storage. This is in disagreement with CitationJaziri et al. (2009) who reported that the chromatographic profiles of six phenolic compounds present in green and black tea (Catechin gallate, epigallocatechin, catechin, epigallocatechin gallate, gallocatechin gallate and epicatechin gallate) after treatment with S. thermophilus yogurt bacteria showed no significant (p < 0.05) alteration of these phenolic compounds compared to before treatment. Similarly, CitationNajgebauer-Lejko (2014) found the concentrations of green tea infusion (10% and 15%) did not influence the viability of S. thermophilus. However, CitationKailasapathy et al. (2008) found that the addition of 10 g/100 g passion fruit or mixed berry reduced the survival (p < 0.01) of probiotic bacteria such as L. acidophilus LAFTI L10 in yogurt. The bacterial species and strain in addition to chemical structure and concentration of the polyphenols play a significant role in the sensitivity of probiotics to phenolic compounds (CitationTabasco et al., 2011). The present study indicated that the buffering action of tea extracts sustained the viability of S. thermophilus in yogurt compared to PY during refrigerated storage (). The reduction of S. thermophilus during the last 2 weeks of storage could be attributed to the increase in organic acid production (), causing inhibition of S. thermophilus growth (Shah, Citation2000; Mishra and Prasad, Citation2005; Madureira et al., Citation2011").
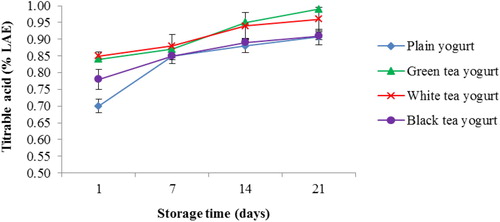
Figure 4 Changes in titratable acidity (TA) of yogurts during 21 days of refrigerated storage at 4 °C. Error bars represent a pooled standard deviation of the mean (n = 3). The level of significance was preset at p < 0.05. Plain yogurt (control) refers to yogurt without the incorporation of tea (milk + starter culture only).
3.2 Effects of tea on Lactobacillus spp. viability in yogurt
The viability of Lactobacillus spp. in tea yogurts during storage is shown in . Inclusion of three types of tea extracts did not affect significantly (p > 0.05) the average viable cell counts of Lactobacillus spp. compared to PY.
Figure 2 Changes in viable cell counts of Lactobacillus spp. in yogurts in the presence and absence of tea during 21 days of refrigerated storage at 4 °C. Error bars represent a pooled standard deviation of the mean (n = 3). The level of significance was preset at p < 0.05. Plain yogurt (control) refers to yogurt without the incorporation of tea (milk + starter culture only).
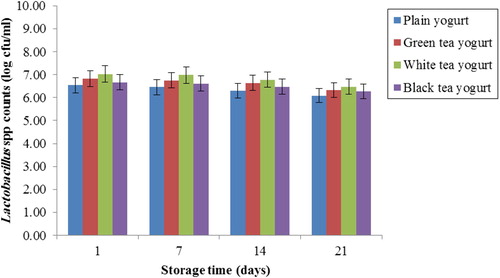
Yogurt containing live cultures confers health benefits to the host when they are consumed in appropriate quantities (CitationSaxelin et al., 2003). Thus, it is necessary for most of these live cultures to survive during their shelf life prior to being consumed. Plant ingredients were found to enhance the viability of probiotics in dairy products (Shori, Citation2013a,Citationb; Shori and Baba, Citation2013; Ranadheera et al., Citation2012; Baba et al., Citation2014"). Polyphenols such as catechin, epicatechin and gallic acid are found to be metabolized by the gut microflora and had positive effects toward Lactobacillus spp. viability (CitationLee et al., 2006). CitationNajgebauer-Lejko et al. (2011) have shown that 5–15% of green tea added to yogurt resulted in higher L. delbrueckii subsp. bulgaricus counts when compared with plain yogurt. A recent study has found that some Lactobacillus spp. maintained a high level of viable cells in the green tea extract (CitationLópez de Lacey et al., 2014), probably due to glucosidase activity of bacteria that may consumed in sugars from glycosylated flavonoids as a source of energy (CitationSchneider et al., 1999). The flavonoids in green tea such as rutin and kaempferol-3-O-rutinoside are known to contain rhamnose in their structures (CitationLópez de Lacey, 2012). Another study verified the improvement of probiotic bacterial stability in fruit juices combined with the green tea extract which was related to oxygen-scavenging and antioxidants of green tea that generate a good anaerobic condition for probiotic bacterial growth (CitationShah et al., 2010). The idea of tea polyphenol as a source of probiotics to stimulate the growth of yogurt bacteria could also be justified by the phenolic composition in different types of tea extracts (CitationJaziri et al., 2009). The decrease in viable counts of the Lactobacillus spp. through 21 days of storage may be attributed to post-acidification (CitationKneifel et al., 1993).
3.3 Post-acidification of tea yogurt
Changes in pH and TA values of tea yogurt during storage are presented in Figs. and . All tea yogurts showed higher pH values than PY over 21 days of storage (). The pH values in GTY and BTY were higher (4.5; p < 0.05) than WTY (4.43) on day 1 of storage. pH values of all yogurts significantly (p < 0.05) decreased after 7 days of storage, followed by a non-significant increase to the initial values on the last 2 weeks of storage (). The total acidity in the three types of tea yogurt was higher (p < 0.05) than PY on day 1 of storage, GTY and WTY had the highest TA values (). Prolonged refrigerated storage for 2 weeks significantly (p < 0.05) increased the rate of TA to about 0.18% LAE for PY and 0.1% LAE for all tea yogurts. TA value of WTY and BTY showed no significant differences compared to PY on day 21 of storage (). However, the TA rate for GTY increased (p < 0.05) with a prolonged storage period of 21 days.
Figure 3 Changes in pH values of yogurts during 21 days of refrigerated storage at 4 °C. Error bars represent a pooled standard deviation of the mean (n = 3). The level of significance was preset at p < 0.05. Plain yogurt (control) refers to yogurt without the incorporation of tea (milk + starter culture only).
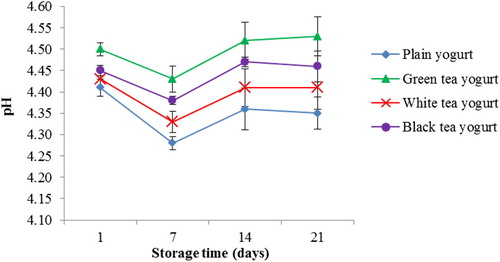
The preferred pH value in commercial yogurt is 4.5, this value helps to maintain the yogurt throughout shelf life, maintain a mild flavor and a pleasant product appearance (CitationHui et al., 2007). However, undesirable pH (<4.0) can cause L. bulgaricus to produce extreme lactic acid, acetaldehyde and proteolytic by-products (CitationStephaine et al., 2009). Higher pH values in tea yogurts than PY during 21 days of storage suggested that some components present in tea leaves may have inhibitory effect toward the growth and metabolism of yogurt bacteria. This finding is in agreement with CitationNajgebauer-Lejko (2014) who found higher pH values in yogurt supplemented with 5 and 10% green tea than plain yogurt during 21 days of refrigerated storage. In addition, a similar observation was also seen during fermentation which the earliest time for yogurt to reach pH 4.5 was 3 h for PY, followed by 3.5, 4 and 4.5 h for WTY, BTY and GTY, respectively (data not shown). The slow reduction in the pH of yogurt upon supplementation of a plant extract rich in phenolic compounds suggests an increase in the buffering capacity of yogurt, which resisted pH changes despite the accumulation of organic acid (CitationMichael et al., 2010). However, this pH buffering effect may be advantageous to the proliferation of yogurt bacteria. The significant (p < 0.05) reduction in pH observed during the first week of storage, indicating post-acidification attributed to the metabolic activity of viable yogurt bacteria (Kneifel et al., Citation1993; Beal et al., Citation1999"). The non-significant (p > 0.05) increase in the pH of tea yogurts during the last 2 weeks of storage, could be the result of accumulation of proteolytic by-products with an alkaline nature during bacteria proteolytic activity in yogurt (CitationKelly et al., 2006).
Lactobacillus spp. are associated with post acidification during cold storage, via accumulation of lactic acid and other organic acids such as butyric acid, citric acid, acetic acid, acetaldehyde and formic acid (CitationOstlie et al., 2003).
4 Conclusion
The addition of tea extracts in yogurt had no significant effect on the post-acidification during 21 days of refrigerated storage. All three types of tea yogurt maintained a high level of S. thermophilus (108-–109 CFU/ml), and Lactobacilllus spp. (106–107 CFU/ml) counts throughout the refrigerated storage period, and these values could be a desirable characteristic of the product to confer health benefits to consumers upon regular consumption. Further studies are needed to investigate the viability of these bacteria in yogurt after simulated gastrointestinal digestion (SGD).
Conflicts of interest
The author declares no conflicts of interest.
Notes
Peer review under responsibility of University of Bahrain.
References
- L.H.AnalieC.V.BennieYogurt as probiotic carrier foodInt. Dairy J.112001117
- A.S.BabaA.NajarianA.B.ShoriK.W.LitG.A.KengIn vitro inhibition of key enzymes related to diabetes and hypertension in Lycium barbarum-yogurtArabian J. Sci. Eng.39201453555362
- C.BealJ.SkokanovaE.LatrilleN.MartinG.CorrieuCombined effects of culture condition and storage time on acidification and viscosity of stirred yogurtJ. Dairy Sci.821999673681
- S.M.El-DiebF.H.R.Abd RaboS.M.BadranA.M.Abd El-FattahF.M.F.ElshaghabeeThe growth behavior and enhancement of probiotic viability in bioyoghurtInt. Dairy J.2220124447
- H.GaudreauC.P.ChampagneG.E.RemondettoL.BazinetM.SubiradeEffect of catechins on the growth of oxygen-sensitive probiotic bacteriaFood Res. Int.532013751757
- A.GondoinD.GrussuD.StewartG.J.McDougallWhite and green tea polyphenols inhibit pancreatic lipase in vitroFood Res. Int.43201015371544
- F.GuarnerG.PerdigonG.CorthierS.SalminenB.KoletzkoL.MorelliShould yogurt cultures be considered probiotic?Br. J. Nutr.932005783786
- Y.HilalU.EngelhardtCharacterisation of white tea – comparison to green and black teaJ. Verbr. Lebensm.22007414421
- D.HorzicD.KomesA.BelščakK.K.GanićD.IvekovićD.KarlovićThe composition of polyphenols and methylxanthines in teas and herbal infusionsFood Chem.1152009441448
- Y.H.HuiR.C.ChandanS.ClarkN.A.CrossJ.C.DobbsW.J.Hurstet alHandbook of Food Products Manufacturing2007Wiley-InterscienceHoboken, N.J
- I.JaziriM.B.SlamaH.MhadhbiM.C.UrdaciM.HamdiEffect of green and black teas (Camellia sinensis L.) on the characteristic microflora of yogurt during fermentation and refrigerated storageFood Chem.1122009614620
- K.KailasapathyI.HarmstorfM.PhillipsSurvival of Lactobacillus acidophilus and Bifidobacterium animalis ssp. lactis in stirred fruit yogurtsLWT Food Sci. Technol.41200813171322
- A.L.KellyF.O’FlahertyP.F.FoxIndigenous proteolytic enzymes in milk: a brief overview of the present state of knowledgeInt. Dairy J.162006563572
- Y.KimK.L.GoodnerJ.ParkJ.ChoiS.T.TalcottChanges in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentationFood Chem.129201113311342
- W.KneifelD.JarosF.ErhardMicroflora and acidification properties of yogurt and yogurt-related products fermented with commercially available starter culturesInt. J. Food Microbiol.181993179189
- K.LeeJ.LeeY.-H.KimS.-H.MoonY.-H.ParkUnique properties of four lactobacilli in amino acid production and symbiotic mixed culture for lactic acid biosynthesisCurr. Microbiol.432001383390
- H.C.LeeA.M.JennerC.S.LowY.K.LeeEffect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiotaRes. Microbiol.1572006876884
- López de Lacey, A.M., 2012. Diseño, desarrollo y aplicación de envases comestibles potencialmente bioactivos (Design, Development and Applying of Potentially Bioactive Food Packaging) (doctoral thesis).
- A.M.López de LaceyE.Pérez-SantìnM.E.López-CaballeroP.MonteroSurvival and metabolic activity of probiotic bacteria in green teaLWT Food Sci. Technol.552014314322
- A.Lourens-HattinghB.C.ViljoenYogurt as probiotic carrier foodInt. Dairy J.112001117
- A.R.MadureiraM.AmorimA.M.GomesM.E.PintadoF.X.MalcataProtective effect of whey cheese matrix on probiotic strains exposed to simulated gastrointestinal conditionsFood Res. Int.442011465470
- P.MarteauM.MinekusR.HavenaarJ.H.J.Huis In’t VeldSurvival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bileJ. Dairy Sci.80199710311037
- M.MichaelR.K.PhebusK.A.SchmidtImpact of a plant extract on the viability of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus in a non-fat yogurtInt. Dairy J.202010665672
- V.MishraD.N.PrasadApplication of in vitro methods for selection of Lactobacillus casei strains as potential probioticsInt. J. Food Microbiol.1032005109115
- D.Najgebauer-LejkoEffect of green tea supplementation on the microbiological, antioxidant, and sensory properties of probiotic milksDairy Sci. Technol.942014327339
- D.Najgebauer-LejkoM.SadyT.GregaM.WalczyckaThe impact of tea supplementation on microflora, pH and antioxidant capacity of yoghurtInt. Dairy J.212011568574
- R.P.S.OliveiraP.PeregoM.N.OliveiraA.ConvertiEffect of inulin as a prebiotic to improve growth and counts of a probiotic cocktail in fermented skim milkLWT Food Sci. Technol.442011520523
- M.OstlieM.H.HellandJ.A.NarvhusGrowth and metabolism of selected strains of probiotic bacteria in milkInt. J. Food Microbiol.8720031727
- C.S.RanadheeraC.A.EvansM.C.AdamsS.K.BainesIn vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat’s milk ice cream and yogurtFood Res. Int.492012619625
- G.RusakD.KomesS.LikicD.HorzicM.KovacPhenolic content and antioxidative capacity of green and white tea extracts depending on extractionFood Chem.1102008852858
- M.SaxelinProbiotic formulations and applications, the current probiotics market, and changes in the marketplace: a European perspectiveClin. Infect. Dis.462008S76S79
- M.SaxelinR.KorpelaA.Mayra-MakinenIntroduction: classifying functional dairy productsT.Mattila-SandholmM.SaarelaFunctional Dairy Products2003Woodhead Publishing LimitedCambridge, England116
- H.SchneiderA.SchwiertzM.D.CollinsM.BlautAnaerobic transformation of quercetin-3-glucoside by bacteria from the human intestinal tractArch. Microbiol.17119998191
- N.P.ShahProbiotic bacteria: selective enumeration and survival in dairy productsJ. Dairy Sci.832000894907
- N.P.ShahW.K.DingM.J.FallourdG.LeyerImproving the stability of probiotic bacteria in model fruit juices using vitamins and antioxidantsJ. Food Sci.752010M278M282
- A.B.ShoriAntioxidant activity and viability of lactic acid bacteria in soybean-yogurt made from cow and camel milkJ. Taibah Univ. Sci.72013202208
- A.B.ShoriNutritional and therapeutical values of chickpea water extract enriched yogurt made from cow and camel milkAm. J. Drug Discovery Dev.320134759
- A.B.ShoriA.S.BabaViability of lactic acid bacteria and sensory evaluation in Cinnamomum verum and Allium sativum-bio-yogurts made from camel and cow milkJ. Assoc. Arab Univ. Basic Appl. Sci.1120125055
- A.B.ShoriA.S.BabaEffects of inclusion of Allium Sativum and Cinnamomum Verum in milk on the growth and activity of lactic acid bacteria during yogurt fermentationAmerican–Eurasian J. Agric. Environ. Sci.1311201314481457
- C.StephaineC.NichaelB.FloydA.MaryThe Sensory Evaluation of Dairy Products2009Springer Science and Business Media, LLC233 Spring Street, New York, NY 10013, USA
- R.TabascoF.Sánchez-PatánM.MonagasB.BartoloméM.V.Moreno-ArribasC.PeláezT.RequenaEffect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: resistance and metabolismFood Microbiol.28201113451352
- C.G.VinderolaN.BailoJ.A.ReinheimerSurvival of probiotic microflora in Argentinian yogurts during refrigerated storageFood Res. Int.33200097102
- G.VinderolaM.CespedesD.MateolliP.CardenasM.LescanoN.AimarettiJ.einheimerChanges in gastric resistance of Lactobacillus casei in flavoured commercial fermented milks during refrigerated storageInt. J. Dairy Technol.642011269275
- T.S.YamS.ShahJ.M.T.Hamilton-MillerMicrobiological activity of whole and fractionated crude extracts of tea (Camellia sinensis), and of tea componentsFEMS Microbiol. Lett.1521997169174
