Abstract
In this paper, a study of simultaneous adsorption of mixture dyes of Basic Blue 41 and Basic Yellow 28 in binary system was done using two types of activated carbon and compared with a single dye system in a batch mode. A competitive adsorption between the two cationic dyes was observed and it was noticed that Basic Blue 41 was favored. Kinetics of each dye in single and binary systems was found to follow pseudo-second-order rate kinetic model, with a good correlation (higher than 0.99). The single component equilibrium data were analyzed using the Langmuir and Freundlich isotherms. Overall, the Langmuir isotherm showed a better fitting for all adsorptions under investigation in terms of correlation coefficient. As the binary adsorption is competitive, extended Langmuir models could not predict the binary component isotherm well. The essential parameters which affect the removal efficiency of binary mixture solution such as pH, temperature and adsorbent type were optimized using full factorial design methodology. Effect of parameters and interaction were analyzed using Pareto chart, main effect and interaction effect. In various industrial effluents like textile industries and plant-produced water, dyes are existent in mixture form. So, this work might be of great benefit in knowing to remove the used dyes.
1 Introduction
When analyzing the wastewater which is produced from Industrial textile, it was observed that many dyes coexist simultaneously. The removal of these dyes from water can be achieved via several techniques such as bio-treatment (CitationEl-Sheekh et al., 2009; CitationKhataee et al., 2012a–Citationc, Citation2011a,Citationb), adsorption (Eren et al., Citation2010; Dogan et al., Citation2007; Karaca et al., Citation2013), flocculation-coagulation (CitationCanizares et al., 2006), photo catalytic degradation (CitationRezaee et al., 2012; CitationKhataee et al., 2012a–Citationc, Citation2011a,Citationb), chemical oxidation (Khataee et al., Citation2011a,Citationb; Sun et al., Citation2012) etc.
The adsorption technique has proven to be an effective and attractive process for the treatment of these dye-bearing wastewaters (CitationCrini et al., 2006). Earlier, the production of activated carbon using various agricultural residues like, coconut shell, groundnut shell, cassava peel, corn cob, olive stones and walnut shells, palm kernel shell, coir pith, pecan shell, fruit stones and nutshells, rubber seed coats has been attempted (Singh and Arora, Citation2011; Ioannidou and Zabaniotou, Citation2007; Amuda et al., Citation2007; Malik et al., Citation2007; Sudaryanto et al., Citation2006; Cao et al., Citation2006; Martinez et al., Citation2006; Jumasiah et al., Citation2005; Bansode et al., Citation2004) because of their high surface area, microporous character and the chemical nature of their surface.
Recently, for ongoing program research we have used some materials of animal origin to remove dyes from aqueous media (El Haddad et al., Citation2014; Slimani et al., Citation2014, Citation2011).
Much of the work on the adsorption of dyes by various kinds of adsorbents has been focused on the uptake of single dyes. Since industrial effluents may contain several dyes, it is necessary to study the simultaneous sorption of two or more dyes and to quantify the interference of one dye with the sorption of the other. Thus, the studies on the equilibrium and kinetics of dyes adsorption from multi-component systems are very important. In this fact, factorial design is employed to reduce the total number of experiments in order to achieve the best overall optimization of the system.
Factorial design experiments allow the simultaneous study of the effects that several factors may have on the optimization of a particular process. On the other hand, factorial design allows measuring the interaction between different group of factors. The high and low levels defined for the 23 factorial designs are listed in .
Table 1 Factors and levels used in factorial design study.
The aim of the present paper is to achieve the followings studies such, the feasibility of using Persea Americana Nuts Carbon (C-PAN) and Ziziphus Mauritiana Nuts Carbon (C-ZMN) as adsorbents for the individual and simultaneous removal of BB41 and BY28 dyes from aqueous solutions, kinetics for the two dyes in single and binary solutions, Equilibrium isotherms for the single adsorption of dyes were analyzed using the Langmuir and Freundlich models, in binary mixture solution the data were analyzed using the extended Langmuir model and finally to examine in binary solution, the effects and the interactions by optimization of various parameters such as pH, temperature and type of adsorbent.
2 Materials and methods
2.1 Materials
The Persea americana and Ziziphus mauritiana nuts were used to prepare the activated carbon of C-PAN and C-ZMN successively. The mentioned Nuts were washed with distilled water and dried in a drier at ambient temperature for several days. These later were dubbed the names PAN and ZMN. The carbonization of PAN and ZMN was carried out using an appropriate weight and 25 mL concentrated phosphoric acid with a mass ratio (1:4) The activation was completed by heating at temperature 500 °C for 1 h producing a black carbonaceous residue. The activated carbon was repeatedly washed with hot distilled water until the pH of the washing solution reached 6–6.5. The product was dried at 105 °C for 2 h and kept in tightly closed plastic container.
Basic Blue 41 and Basic Yellow 28 were used as received without any purification; their colors are stable within the pH range of the study. The chemical structures of BB41 and BY28 are given in . A stock solution of 100 mg/L was prepared by dissolving the required amount of each dye in distilled water. The characterization of adsorbents achieved by FT-IR spectroscopy and Scanning Electronic Microscopy (SEM) images were obtained with HITACHI-S4100 equipment operated at 20 kV. Adsorption–desorption isotherms of nitrogen at −196 °C were measure with an automatic adsorption instrument (NOVA-1000 Gas Sorption analyzer) in order to determine surface areas and total pore volumes.
2.2 Adsorption experiments
Dye removal experiments were performed by mixing known weights of each adsorbent in 50 mL of single dye solution in flasks at 25 °C. The effects of initial dye concentrations (25–100 mg/L) on adsorption capacity were investigated. The required combinations of Basic Yellow 28 and Basic Blue 41 were obtained for binary pollutants mixture by diluting 1000 mg/L of the stock solutions and mixing them.
The percentage of the removal efficiency of each dye in binary system was compared with that of their pure state. In this investigation of mixture dyes removal using C-PAN and C-ZMN, the percentage removal may depend on pH, temperature and adsorbent type. The other variables such as the initial concentration of each dye (25 mg/L), time of agitation which is suitable for equilibrium at 30 min and adsorbent dosage (0.2 g/L) were kept constant. At any time, the residual dye concentration in the reaction mixture was analyzed by centrifuging the reaction mixture and then measuring the absorbance by UV–Visible spectroscopy of the supernatant at the wavelength that is corresponding to the maximum absorbance of 438 nm for BY28 and 606 nm for BB41 respectively. The removal dye percentage (%) can be calculated as follows:
The amount of equilibrium adsorption qe (mg/g) was calculated using the formula:(1) where Ce (mg/L) is the liquid concentration of dye at equilibrium, C0 (mg/L) is the initial concentration of the dye in solution. V is the volume of the solution (L) and W is the mass of dye biosorbent (g)
(2) where C0 is the initial dye concentration and Ce (mg/L) is the concentrations of dye at equilibrium. The results of the experimental design were analyzed using Minitab 15 statistical software to evaluate the effects as well as the statistical parameters.
3 Results and discussion
3.1 Characterization of adsorbents
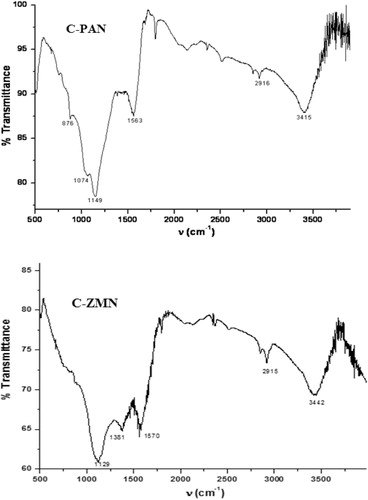
FTIR spectra of the adsorbents with its most abundant functional groups responsible for adsorption is given in , it can be observed that surface of two adsorbents are practically similar hydroxyl group at 3415 and 3442 cm−1 (CitationLiang et al., 2011), unsymmetrical aliphatic C–H stretching at 2016 and 2015 cm−1 and C=C stretching of aromatic rings at 1563 and 1570 cm−1. Band at 1125 in C-ZMN surface is ascribed to C–O stretching in alcohol or ether (He et al., Citation2002; Pavia et al., Citation1987) and the band in 876 cm−1 C-PAN surface is attributed to symmetrical vibration in a chain of P-O-P and to P-C phosphorus-containing compound (Liu et al., Citation2012; Wang et al., Citation2011).
The surface morphology of the C-PAN and C-ZMN adsorbents is shown in . The SEM of C-PAN and C-ZMN indicates that the surfaces are relatively smooth and contain many pores, which can be taken as a sign for effective adsorption of dye molecules.
The BET surface area and the total pore volumes of the obtained Persea Americana activated carbon(C-PAN) and Ziziphus Mauritiana nuts carbon (C-ZMN) were found to be (1593 m2/g, 1.053 cm3/g) and (480 m2/g, 0.73 cm3/g) respectively.
3.2 Competitive adsorption and kinetics
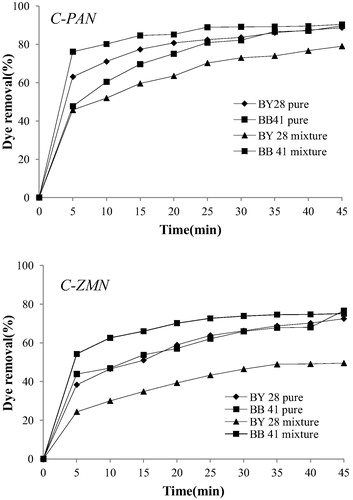
Whenever there is an adsorption from a mixture of substances, a competition occurs between the various adsorptive for the interface. The presence of other solutes in the mixture adversely affects the adsorption of the first, leading to a much more rapid breakthrough of this material. BB41 and BY28 adsorption in single and binary adsorption systems onto C-PAN and C-ZMN were studied and are illustrated in . Equilibrium is reached at about 30 min of adsorption. In binary system, BB41 was adsorbed well in surface of adsorbent in comparison with BY28. Adsorption efficiency of BB41 increases when going from pure BB41 solution to a mixture solution and vice versa when it comes to BY28 as it is seen that BY28 removal percentage decreases in binary system in comparison with single system. This result can be explained by the fact that BB41 attraction to the interfaces is stronger than that of BY28 and that the presence of both dyes in solution enhance a competitive adsorption in the system. Also, it was found that C-PAN adsorbs dyes better than C-ZMN; this is due to the larger surface area of the first.
Figure 4 Kinetic study of dyes in single and binary system by C-PAN and C-ZMN. (V = 50 mL, m = 15 mg, C = 25 mg/L and T = 298 K).
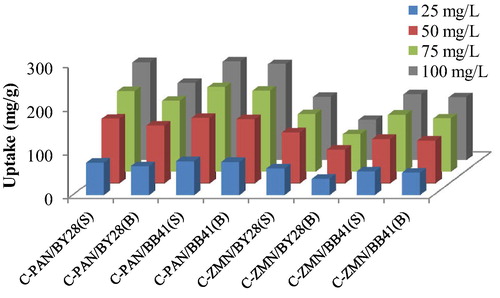
Values of experimental sorption capacity (mg/g) of C-PAN and C-ZMN for used dyes at different concentrations, in single and binary systems are shown in . Results revealed that the sorption capacity of C-PAN and C-ZMN for both dyes increases with an increase in initial concentration and the simultaneous Presence of the two dyes caused more significant change of uptake in binary BB41-BY28 system indicating a competitive adsorption onto adsorbent sites.
Figure 5 Adsorption capacity of used adsorbent for BY28 BB41 in single and binary system (t = 45 min, V = 50 mL, m = 15 mg and at ambient temperature).
The pseudo-first-order model was proposed by CitationLagergren (1898) and used to fit the experimental results obtained herein. The integrated form of the model is:(3) where, qe is the amount of dye biosorbed at equilibrium (mg/g), qt is the amount of dye biosorbed at time t (mg/g), k1 is the first-order rate constant (min−1) and t is time (min). Hence, a linear trace is expected between the two parameters, log (qe – qt) and t, , provides the adsorption following first kinetics. The values of k1 and qe can be determined from the slope and intercept.
Figure 6 Pseudo-first-order plots for different dye solutions using C-PAN and C-ZMN. (m = 15 mg; V = 50 mL, C = 25 mg/L and T = 298 K).
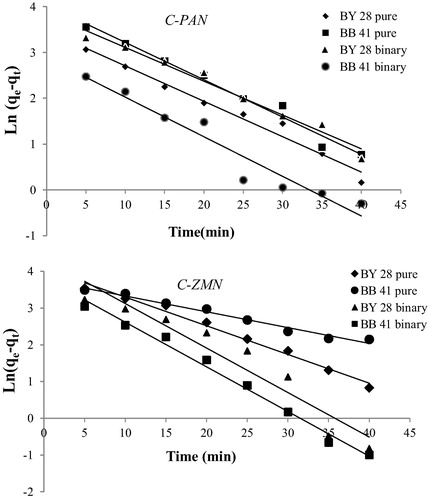
The adsorption may also be described by pseudo-second-order kinetic model (CitationHo and McKay, 1999) is the adsorption does not follows first order kinetics. The linearized form of the pseudo-second-order model is:(4) where, k2 is the pseudo-second-order rate constant (g/mg.min), shown in . The results of kinetics are given in . Result shows that second order model is suitable to describe the adsorption efficiency. For BB41 removal, the pseudo second order rate constant increases from pure to binary system which can be taken as prove of the increase of BB41 removal efficiency, but for BY28 removal percentage decreases from pure to binary solution. It can be explained by the decrease of the pseudo second order rate constant.
Figure 7 Pseudo-second-order plots for different dye solutions removal using C-PAN and C-ZMN adsorbents. (m = 15 mg, C = 25 mg/L, V = 50 mL and T = 298 K).
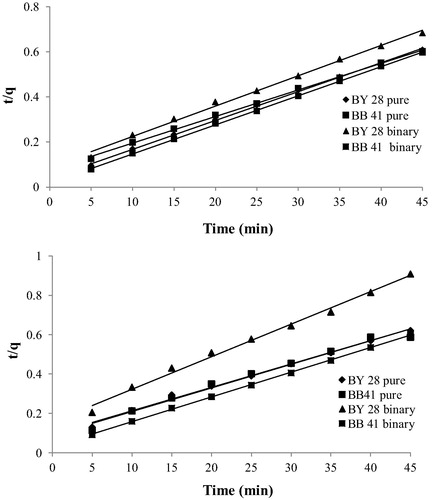
Table 2 The pseudo-first-order and pseudo-second-order kinetic parameters for BB41 pure, BY28 pure and mixture of BB41 and BY28 using C-PAN and C-ZMN.
3.3 Modeling of adsorption isotherms
Two known isotherm models (Langmuir and Freundlich) have been applied to describe the experimental data in a single dye system. The classical non-competitive Langmuir isotherm model for adsorption of one component is expressed as (CitationSafa and Bhatti 2011):(5) where Ce (mg/L) is the equilibrium concentration of dye, qe (mg/g) is the amount of dye adsorbed par unit mass of adsorbent. KL (L/mg) and qm (mg/g) are Langmuir constants related to rate of adsorption and adsorption capacity, respectively.
The Freundlich isotherm is expressed as (CitationDeniz and Karaman 2011):(6) where Kf and n are Freundlich isotherm constants. The non-linear correlation obtained () shows that, the experimental data also obeyed Langmuir suggesting monolayer coverage.
Table 3 Langmuir and Freundlich isotherm parameters for single component adsorption of BY28 and BB41 onto C-PAN and C-ZMN.
A model for competitive sorption based on the Langmuir equation was first developed by Butler and Ockrent to describe the sorption equilibrium in multi-component systems. This isotherm (extended Langmuir) is applicable when each component obeys Langmuir behavior in a single-solute system. It is widely used to calculate the Langmuir constant (qm), the amount of solute adsorbed per unit weight of adsorbent, in the multi-component systems. This model assumed a homogeneous surface with respect to the energy of adsorption, no interaction between adsorbed species and that all adsorption sites are equally available to all adsorbed species. This model can be written as follows (Yang et al., Citation2011; Oladipo and Gazi, Citation2015):(7) where i is the number of components, Ce,i is the equilibrium concentration of the component i in the multi-component solution (mg/L), qe,i is the equilibrium uptake of the component i (mg/g). The parameters for the simultaneous adsorption of BB41 and BY28 on C-PAN and C-ZMN are shown in . Significant differences between calculated and experimental data were observed, which indicate that extended –Langmuir equation failed to explain the adsorption of the binary mixture of BB41and BY28 on the two adsorbents. The failure of the model suggested that the binary adsorption might be competitive.
Table 4 Extended Langmuir parameters for simultaneous sorption of BY28 BB41 by C-PAN C-ZMN.
3.4 Statistical analysis of factorial design experiments
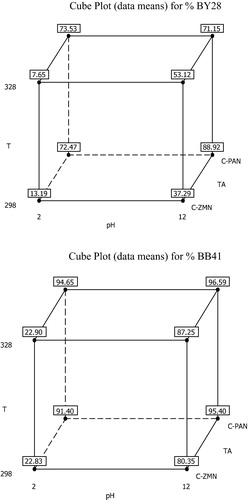
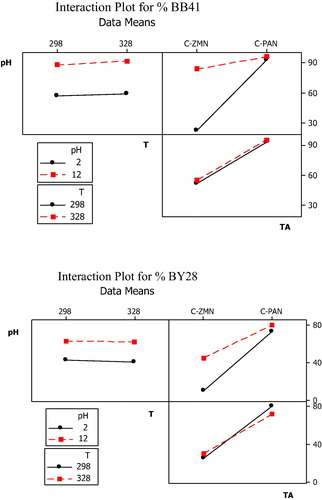
The design matrix of coded values for factors and adsorption efficiency of BB41 and BY28 in each factorial experiment in binary systems are shown in . The mean of the experimental results for the respective high and low levels is shown in . Factors that influence the adsorbed percentage of these dyes onto the two types of activated carbon were evaluated by using factorial plots: main effect, interaction effect, Pareto and normal probability plots.
Table 5 Design matrix and the results of the 23 full factorial design.
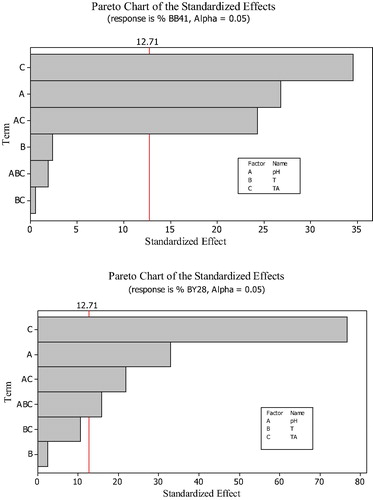
Main and interaction effect, coefficients of the model, standard deviation of each coefficient, and probability for the full 23 factorial designs are presented in . Interaction pH.T has the smallest effect for both dyes. Thus, it can be discarded. Whereas All BB41 effects were significant with an exception of T and T.TA, the only effect that was insignificant was T in BY28. In addition, the model presented an adjusted square correlation coefficient R2 (adj) of 99.72% for BB41 and 99.91% for BY28.
Table 6 Estimated effects and coefficients for adsorption efficiency.
The dyes removal efficiency could be expressed, after discarding pH.T using the following equations:(8)
(9)
This function describes how the experimental variables and their interactions influence the dyes adsorption. The coefficients above are given in .
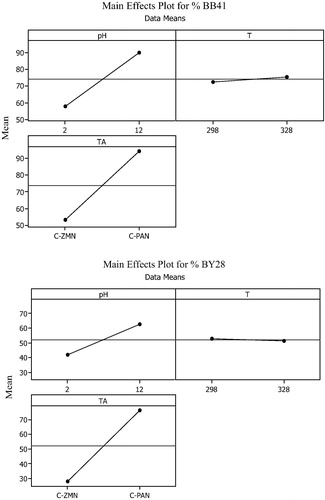
The main effects which are helpful in visualizing which factors most affect the response of each parameter represent deviations of the average between high and low levels of each one of them as shown in . Each level of the factors affects the response differently. If the slope is close to zero, then the magnitude of the main effect will be small. As the results show, for both dyes, TA appears to have a greater effect on the response, as indicated by a steeply slope due to the great surface area of C-PAN in comparison with C-ZMN, followed by the solution pH, an increase in the pH from 2 to 10 resulted in a 34% increase in adsorption efficiency due that at higher pH value, the surface is negatively charged which increases the electrostatic attraction of the positively charged cationic dye. The results also indicate that temperature has almost no effect on adsorption efficiency.
The interaction effects of each parameter on the dyes adsorption are shown in . If the interactions between the lines are not parallel, the interaction among control factors is strong and vice versa. This figure shows a lot of interactions: pH.T, pH.TA and T.TA.
The important interaction is between pH and type of activated carbon (pH.TA) and less important is T.TA while pH.T paralleled lines display the unimportance of the interaction. These results can also be explained by the values found in .
The relative importance of the main effects and their interactions was also observed on the Pareto chart as shown in . The values that exceed the reference line are considered significant values and those which do not are considered insignificant (CitationMathialagan and Viraraghavan, 2005). According to this figure, for BB41 only TA, pH and their interaction are significant and as for BY28, the significant factors are TA, pH, TA.pH and pH.T.TA.
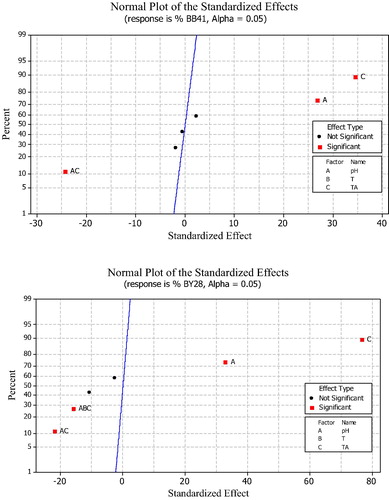
A normal probability plot is used. One point on the plot is assigned to each effect. According to the normal probability plots, the points which are close to a line fitted to the middle group of points represent those estimated factors that do not demonstrate any significant affect on the response variables. Points far away from the line likely represent the authentic factor effects (CitationPalanikumar and Dawim, 2009). shows the normal probability plot of the standardized effects. for BB41, the main factors (pH and TA) and their interactions (pH*TA) are very far away from the straight line and are therefore considered to be significant, TA.pH exists on the left of the line which proves that it has a negative effect whereas TA and pH exist on the right of the line which means they have a positive effect. In the same way, for BY28, TA and pH have positive effects and the interactions TA.pH and pH.T.TA have negative effects. These results confirm the previous Pareto chart analysis and the values of .
4 Conclusion
Adsorption was an effective process for decolorization of textile basic dyes and C-PAN gives strong adsorption efficiency of dyes in comparison with C-ZMN. In binary dyes mixture, the removal percentage of BB41 increases in comparison to its pure state, the removal percentage of BY28 decreases in comparison to its pure state. BB41 has more affinity to the pore of adsorbent and that is due to, perhaps, the interaction force between cationic dyes BB41 and surface of adsorbent. Adsorption of both dyes follows the pseudo second order in single and binary systems. Langmuir gives the best fit of the experimental data in single component system and extended Langmuir did not accurately predict the adsorption equilibrium of dyes in a binary mixture. For both dyes, the most significant parameters were found to be TA, pH and TA.pH.
Notes
Peer review under responsibility of University of Bahrain.
References
- O.S.AmudaA.A.GiwaI.A.BelloRemoval of heavy metal from industrial wastewater using modified activated coconut shell carbonBiochem. Eng. J.362007174181
- R.R.BansodeJ.N.LossoW.E.MarshallR.M.RaoR.J.PortierPecan shell-based granular activated carbon for treatment of chemical oxygen demand (COD) in municipal wastewaterBioresour. Technol.942004129135
- P.CanizaresF.MartınezC.JimenezJ.LobatoM.A.RodrigoCoagulation and electrocoagulation of wastes polluted with dyesEnviron. Sci. Technol.40200664186424
- Q.CaoK.C.XieY.K.LvW.R.BaoProcess effects on activated carbon with large specific surface area from corn cobBioresour. Technol.972006110115
- G.CriniNon-conventional low-cost adsorbents for dye removal: a reviewBioresour. Technol.97200610611085
- F.DenizS.KaramanRemoval of Basic Red 46 dye from aqueous solution by pine tree leavesChem. Eng. J.17020116774
- M.DoganR.Y.OzdemiM.AlkanAdsorption kinetics and mechanism of cationic methyl violet and methylene blue dyes onto sepioliteDyes Pigments752007701713
- M.El HaddadA.RegtiR.LaamariR.SlimaniR.MamouniS.El AntriS.LazarCalcined mussel shells as a new and eco-friendly biosorbent to remove textile dyes from aqueous solutionsJ. Taiwan Inst. Chem. Eng.452014533540
- M.M.El-SheekhM.M.GhariebG.W.Abou-El-SouodBiodegradation of dyes by some green algae and cyanobacteriaInt. Biodeterior. Biodegrad.632009699704
- E.ErenO.CubukH.CiftciB.ErenB.CaglarAdsorption of basic dye from aqueous solutions by modified sepiolite: equilibrium, kinetics and thermodynamics studyDesalination25220108896
- J.HeW.MaJ.HeJ.ZhaoJ.C.YuPhotooxidation of azo dye in aqueous dispersions of H2O2/FeOOHAppl. Catal. B Environ.392002211220
- Y.S.HoG.McKayPseudo-second-order model for sorption processesProcess Biochem.341999451465
- O.IoannidouA.ZabaniotouAgricultural residues as precursors for activated carbon production-a reviewRenewable Sustainable Energy Rev.11200719662005
- A.JumasiahT.G.ChuahJ.GimbonT.S.Y.ChoongI.AzniAdsorption of basic dye onto palm kernel shell activated carbon: sorption equilibrium and kinetics studiesDesalination18620055764
- S.KaracaA.GursesA.HassaniM.KıransK.YıkılmazModeling of adsorption isotherms and kinetics of Remazol Red RB adsorption from aqueous solution by modified clayDesalin. Water Treat.51201327262739
- A.R.KhataeeG.DehghanM.ZareiE.EbadiM.PourhassanNeural network modeling of biotreatment of triphenylmethane dye solution by a green macroalgaeChem. Eng. Res. Des.892011172178
- A.R.KhataeeM.FathiniaS.AberKinetic study of photocatalytic decolorization of C.I. Basic Blue 3 solution on immobilized titanium dioxide nanoparticlesChem. Eng. Res. Des.89201121102116
- A.R.KhataeeA.MovafeghiS.TorbatiS.Y.SalehiLisarM.ZareiPhytoremediation potential of duckweed (Lemna minor L.) in degradation of C.I. Acid Blue 92: artificial neural network modelingEcotoxicol. Environ. Saf.802012291298
- A.R.KhataeeM.SafarpourM.ZareiS.AberCombined heterogeneous and homogeneous photodegradation of a dye using immobilized TiO2 nanophotocatalyst and modified graphite electrode with carbon nanotubesJ. Mol. Catal. A Chem.36336420125868
- A.R.KhataeeM.SafarpourA.NaseriM.ZareiPhotoelectro Fenton/nanophotocatalysis decolorization of three textile dyes mixture: response surface modeling and multivariate calibration procedure for simultaneous determinationJ. Electroanal. Chem.67220125362
- S.LagergrenZur theorie der sogenannten adsorption gelöster stoffeKungliga Svenska, Vetenskapsakademiens Handlingar241898139
- S.LiangX.GuoQ.TianAdsorption of Pb2+ and Zn2+ from aqueous solutions by sulfured orange peelDesalination2752011212216
- H.LiuJ.ZhangN.BaoC.ChengL.RenC.ZhangTextural properties and surface chemistry of lotus stalk-derived activated carbons prepared using different phosphorus oxyacids: adsorption of trimethoprimJ. Hazard. Mater.235–2362012367375
- R.MalikD.S.RamtekeS.R.WateAdsorption of malachite green on groundnut shell waste based powdered activated carbonWaste Manage.27200711291138
- M.L.MartinezM.M.TorresC.A.GuzmanD.M.MaestriPreparation and characteristics of activated carbon from olive stones and walnut shellsInd. Crops Prod.2320062328
- T.MathialaganT.ViraraghavanBiosorption of pentachlorophenol by fungal biomass from aqueous solutions: a factorial design analysisEnviron. Technol.52005571580
- A.A.OladipoM.GaziMicrowaves initiated synthesis of activated carbon-based composite hydrogel for simultaneous removal of copper (II) ions and direct red 80 dye: a multi-component adsorption systemJ. Taiwan Inst. Chem. Eng.472015125136
- J.PalanikumarP.DawimAssessment of some factors influencing tool wear on the machining of glass fibre-reinforced plastics by coated cemented carbide toolsJ. Mater. Process Technol.2092009511519
- D.L.PaviaG.M.LampmanG.S.KaizIntroduction to Spectroscopy: A Guide for Students of Organic Chemistry1987W.B. Saunders Company
- A.RezaeeH.MasoumbeigiR.D.C.SoltaniA.R.KhataeeS.HashemiyanPhotocatalytic decolorization of methylene blue using immobilized ZnO nanoparticles prepared by solution combustion methodDesalin. Water Treat.442012174179
- Y.SafaH.N.BhattiKinetic and thermodynamic modeling for the removal of Direct Red 31 and Direct Red 26 dyes from aquoeus solutions by rice huskDesalination2732011313322
- K.SinghS.AroraRemoval of synthetic textile dyes from wastewaters: a critical review on present treatment technologiesCrit. Rev. Environ. Sci. Technol.412011807878
- R.SlimaniA.AnouzlaY.AbroukiS.El AntriR.MamouniS.LazarM.El HaddadRemoval of a cationic dye -Methylene Blue- from aqueous media by the use of animal bone meal as a new low cost adsorbentJ. Mater. Environ. Sci.220117787
- R.SlimaniI.El OuahabiM.El HaddadA.RegtiR.LaamariS.El AntriS.LazarCalcined eggshells as a new biosorbent to remove basic dye from aqueous solutions: thermodynamics, kinetics, isotherms and error analysisJ. Taiwan Inst. Chem. Eng.45201415781587
- Y.SudaryantoS.B.HartonoW.IrawatyH.HindarsoS.IsmadjiHigh surface area activated carbon prepared from cassava peel by chemical activationBioresour. Technol.972006734739
- J.H.SunS.P.SunG.L.WangL.P.QiaoDegradation of azo dye Amido black 10B in aqueous solution by Fenton oxidation processDyes Pigm.742007647652
- Z.WangE.NieJ.LiY.ZhaoX.LuoZ.ZhengCarbons prepared from Spartina alterniflora and its anaerobically digested residue by H3PO4 activation: Characterization and adsorption of cadmium from aqueous solutionsJ. Hazard. Mater.18820112936
- Y.YangD.JinG.WangS.WangX.JiaY.ZhaoCompetitive biosorption of acid blue 25 and acid red 337 onto unmodified and CDAB-modified biomass of Aspergillus oryzaeBioresour. Technol.1022011742974736