Abstract
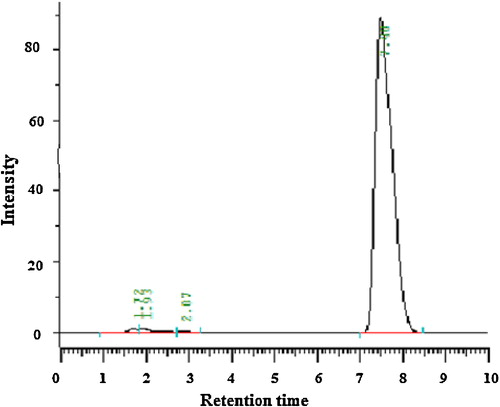
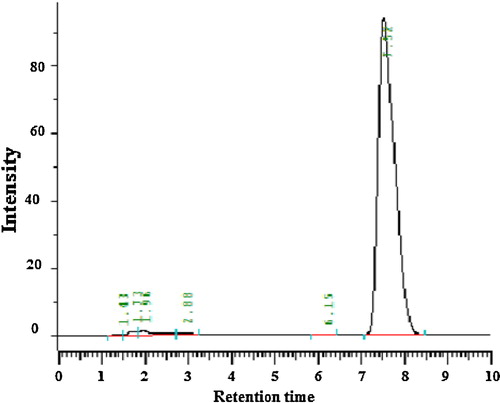
A novel, simple, economical reverse-phase high-performance liquid chromatography (RP-HPLC) method was developed for quantifying clopidogrel in bulk and tablet form with greater precision and accuracy. Separation was achieved on a Develosil ODS HG-5 RP C18 (15 cm × 4.6 mm, i.d. 5 μm) column in isocratic mode with a mobile phase consisting of acetonitrile:phosphate (65:35) buffer (pH 2.85) with a flow rate of 1 mL/min. Detection was carried out at 225 nm. The retention time of clopidogrel was 7.48 min. The method was validated as per the guidelines of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Linearity was established for clopidogrel in the range 10–60 μg/mL, with an r2 value of 0.999. The recovery of clopidogrel was 99.71–100.03%. The high recovery and low relative standard deviation confirm the suitability of the proposed method for estimating the concentrations of the drug in bulk and tablet dosage forms. Validation studies demonstrated that the proposed RP-HPLC method is simple, specific, rapid, reliable and reproducible for the determination of clopidogrel for quality control.
Keywords:
1 Introduction
Clopidogrel bisulfate was officially registered in the United States Pharmacopeia in 2007. Its chemical name is (+)-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetic acid methyl ester sulfate. It inhibits adenosine diphosphate (ADP)-induced platelet aggregation () and acts by direct inhibition of ADP binding to its receptor and of subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex. It is used effectively to reduce the incidence of ischaemic strokes, heart attacks and claudication due to vascular diseases such as atherosclerosis [Citation1]. A literature survey revealed various assays for determining clopidogrel in pharmaceutical dosage forms, including chemometry [Citation2], spectrophotometry [Citation3], thin-layer chromatography [Citation4], high-performance thin-layer chromatography [Citation5,Citation6], high-performance liquid chromatography (HPLC) [Citation6–Citation15], liquid chromatography–mass spectrometry [Citation16] and voltammetry [Citation17].
Analysis of the carboxylic acid metabolite of clopidogrel in plasma and serum has been reported with HPLC [Citation17,Citation18], liquid chromatography–tandem mass spectrometry [Citation19] and gas chromatography–mass spectrometry [Citation20]. The aim of the present work was to develop and validate a sensitive reverse-phase (RP)-HPLC method for estimating the concentration of clopidogrel in bulk and in tablet dosage forms.
2 Experimental
2.1 Materials
Pure clopidogrel, used as a working standard, was obtained from Aurobindo Pharma, Hyderabad, India. Tablets containing clopidogrel (Clopilet® and Plavix®) were obtained from Apollo Pharmaceuticals Pvt. Ltd., Visakhapatnam, India, and used within their shelf life. Acetonitrile and water (HPLC grade) were purchased from Merck, India. All other chemicals and reagents employed were of analytical grade and purchased from Desai Chemicals, Visakhapatnam, India.
2.2 Instrumentation
The chromatographic system used was an Analytical Technologies Ltd UV 2230 UV-Vis detector. Data were integrated with A-4000 version software. Samples were injected into a Develosil ODS HG-5 RP C18 (15 cm × 4.6 mm, i.d. 5 μm) column. An Analytical Technologies Ltd. sonicator was used to enhance dissolution of the compounds. A Wenster digital pH meter was used to adjust the pH.
2.3 Chromatographic conditions
The HPLC system used was operated isocratically, with the column temperature maintained at 30 °C and a mobile phase composed of acetonitrile:phosphate (65:35, v/v) buffer (pH adjusted to 2.85 with o-phosphoric acid) at a flow rate of 1.0 mL/min and a run time of 10 min. Before use, the mobile phase was degassed in an ultrasonic bath and filtered through a Millipore vacuum filter system equipped with a 0.45-μm vacuum filter. The drug was detected and quantified at 225 nm.
2.4 Preparation of standard solutions
The stock solution was prepared by transferring 100 mg of clopidogrel into a 100-mL volumetric flask, to which a small amount of diluent was added; the mixture was sonicated to dissolution and made up to volume with the mobile phase. Final concentrations of 10–60 μg/mL were prepared from the stock solution for calibration of the standard curve.
2.5 Assay of clopidogrel in marketed tablets
Twenty tablets of each marketed formulation were accurately weighed and crushed to a fine powder in a mortar. Then, 100 mg of the powder were transferred into a 100-mL volumetric flask, to which 25 mL of diluent were added, followed by 10 mL of o-phosphoric acid. The mixture was sonicated to dissolve the excipients and then made up to volume with mobile phase. After 15 min of mechanical shaking, the solution was maintained in an ultrasonic bath for 15 min and then filtered through 0.45-μm filter paper. Suitable aliquots of the filtered solution were transferred to a volumetric flask and made up to volume with mobile phase (40 μg/mL) to yield six concentrations of clopidogrel. A 20-μL volume of the sample solution was injected into the chromatographic system six times under optimized chromatographic conditions. The peak areas were measured at 225 nm, and the concentrations in the samples were determined by interpolation from previously obtained calibration plots for each drug.
3 Method validation
The method was validated in accordance with the guidelines of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) [Citation14]. The parameters assessed were linearity, accuracy, limit of detection (LOD), limit of quantification (LOQ), precision, reproducibility, robustness and system suitability.
3.1 Accuracy
Accuracy was best determined by the standard addition method. Samples of clopidogrel previously analyzed for active pharmaceutical ingredient (API) were added with standard drug solutions and analyzed by the proposed method. Recovery (%), relative standard deviation (RSD) (%) and bias (%) were calculated for each concentration [Citation21].
3.2 Precision
Precision was determined as both repeatability and intermediate precision, in accordance with ICH guidelines. The repeatability of sample injection was determined as intra-day and intermediate variation. For these determinations, a single concentration (40 μg/mL) of clopidogrel API was tested at different intervals and on different days.
3.3 Robustness
The concept of the robustness of an analytical procedure has been defined by the ICH as “a measure of its capacity to remain unaffected by small but deliberate variations in method parameters”. To determine the robustness of the method, experimental conditions are purposely altered, and chromatographic characteristics are evaluated. The influence of small changes in chromatographic conditions, such as in flow rate (±0.1 mL/min), the wavelength for detection (±2 nm) and acetonitrile content in the mobile phase (±2%), were studied to determine the robustness of the method.
3.4 Limit of detection and limit of quantification
The LOD of an analytical method may be defined as the concentration that gives rise to an instrument signal that is significantly different from the blank. The LOQ is the concentration that can be quantified reliably with a specified level of accuracy and precision and represents the concentration of analyte that would yield a signal:noise ratio of 10 [Citation21].
4 Results and discussion
4.1 Optimization of chromatographic conditions
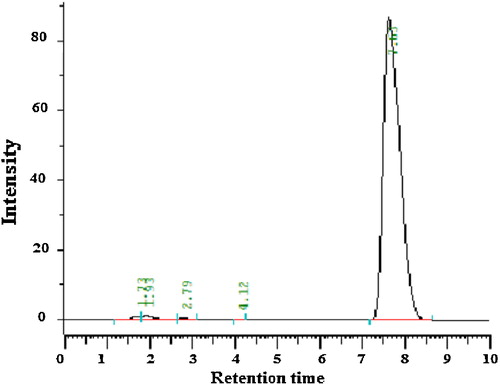
The chromatographic conditions were optimized by using different columns, different mobile phases, different flow rates, different detection wavelengths and different diluents for standard drug and marketed tablets, as summarized in . The chromatograms obtained are shown in –.
Table 1 Results of optimization of the method.
4.2 Linearity and range
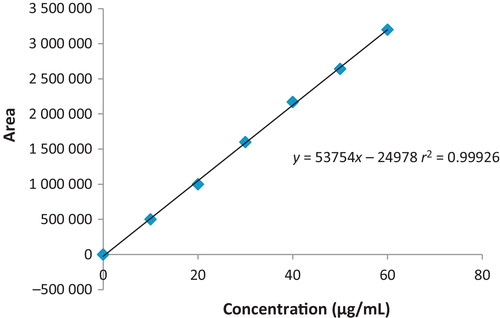
The calibration curve () showed good linearity in the range 10–60 μg/mL for clopidogrel API, with a correlation coefficient (r2) of 0.999. A typical calibration curve for clopidogrel fitted the regression equation y = 53754x − 24978.
4.3 Accuracy: recovery study
The recovery of the method, determined by adding a previously analyzed test solution with additional drug standard solution at three concentrations, was 99.71–100.03%. The values for recovery, RSD and bias listed in indicate that the method is accurate and better than all previous methods reported [Citation6–Citation15].
Table 2 Recovery of the method.
4.4 Precision: intra-assay and inter-assay
The intra- and inter-day variation of the method was measured. The high mean values and the low standard deviation and RSD (<2%) show that the proposed method is precise ().
Table 3 Intra- and inter-assay variation.
4.5 Robustness
The influence of small changes in chromatographic conditions, such as flow rate (±0.1 mL/min), wavelength of detection (±2 nm) and acetonitrile content in the mobile phase (±2%) (; RSD < 2%), show the robustness of the method for the analysis of Clopidogrel API.
Table 4 Robustness of proposed assay.
4.6 LOD and LOQ
The minimum concentrations at which the analyte can be reliable detected (LOD) and quantified (LOQ) were 0.0003 and 0.001 μg/mL, respectively, which are lower than those found with previously reported methods [Citation6–Citation15], indicating an advantage of our method.
4.7 Assays of clopidogrel in tablet form
The results of assays performed according to the regression equation (y = 53754x − 24978, r2 = 0.999) obtained from the standard curve of clopidogrel API are shown in . Assay of Clopilet® and Plavix® tablets showed a clopidogrel content of 100.00% and 99.96%, respectively ( and ).
Table 5 Assay of clopidogrel tablets.
5 Conclusion
A new RP-HPLC method for the assay of clopidogrel in bulk and in pharmaceutical dosage forms is reported. The method is simple, reliable, linear, accurate, sensitive and reproducible as well as cost-effective for quantitative analysis of clopidogrel in bulk and in tablet formulations. The method was completely validated and showed satisfactory results for all the method validation parameters tested; the method is free from interference from the other active ingredients and additives used in the formulations. The method showed linearity in the range 10–60 μg/mL with a regression coefficient of 0.999. The recovery is nearly 100%. The RSD was < 0.1%, which is an added advantage over previous methods. In previous studies, only single marketed formulations were assayed, whereas we tested two different formulations. The method is suitable for use in routine quality control of clopidogrel in API or pharmaceutical dosage forms.
Acknowledgement
The authors acknowledge Yalamarty Pharmacy College, Tarluwada, Anandapuram, Visakhapatnam, Andhra Pradesh, India, for providing the necessary facilities to carry out the research.
Notes
Peer review under responsibility of Taibah University
References
- K.JoseP.JayaseharStability indicating HPLC determination of clopidogrel bisulfate in pharmaceutical dosage forms pharmacyInt. J. Compr. Pharm.1201315
- S.J.RajputR.K.GeorgeB.D.RuikarChemometric simultaneous estimation of clopidogrel bisulfate and aspirin from combined dosage formIndian J. Pharm. Sci.702008450454
- P.B.ChaudhariP.D.PawarK.P.NarkhedeStability indicating spectrophotometric method for determination and validation of clopidogrel bisulfate in tablet dosage formInt. J. Res. Ayurveda Pharm.12010418423
- D.AnticS.FilipicD.AgbabaA simple and sensitive TLC method for determination of clopidogrel and its impurity SR 26334 in pharmaceutical productActa Chromatogr.182007199206
- A.HimaniK.NeerajA.R.ParadkarK.R.MahadikStability indicating HPTLC determination of method for clopidogrel bisulfate as bulk drug and in pharmaceutical dosage formJ. Pharm. Biomed. Anal.612003581589
- R.B.PatelM.B.ShankarM.R.PatelK.P.BhatSimultaneous estimation of acetylsalicylic acid and clopidogrel bisulfate in pure powder and tablet formulations by HPLC and HPTLCJ. AOAC Int.912008750755
- Y.GomezE.AdamsJ.HoogmartensAnalysis of purity in 19 drug product tablets containing clopidogrel: 18 copies versus the original brandJ. Pharm. Biomed. Anal.342004341348
- N.A.AlarfajStability indicating liquid chromatography for determination of clopidogrel bisulfate in tablets: application to content uniformity testingJ. Saudi Chem. Soc.1620122330
- N.P.GosaviM.U.BhajaneV.V.PatilV.R.PatilDevelopment and validation of analytical and method for the simultaneous estimation of clopidogrel bisulphate and atorvastatin calcium in bulk and in tabletRes. J. Pharm. Biol. Chem. Sci.3201210651071
- M.A.Al-KhayatS.HaidarH.MandoDevelopment and validation of RP-HPLC method for determination of clopidogrel in tabletsInt. J. Pharm. Sci. Rev. Res.14201215
- G.R.K.ReddyV.S.N.RaoJ.N.SateeshDevelopment and validation of stability indicating related substances method for clopidogrel sulphate by normal phase HPLCJ. Global Trends Pharm. Sci.22011367379
- S.KulsumS.RamyaT.SnehalathaM.KanakadurgaD.ThimmareddyDevelopment and validation of RP-HPLC method for the simultaneous estimation of amlodipine, besylate and clopidogrel in bulk and tablet dosage formsInt. J. PharmTech4201243374349
- A.SrinivasB.JayapaulA.MadhukarR.V.KumarSimple and sensitive analytical method development and validation of clopidogrel by RP-HPLCPharm. Sci.1201257
- A.MounikaN.SriramMethod development and validation of clopidogrel bisulphate by reverse phase-HPLC in bulk and pharmaceutical dosage formsInt. J. Pharm. Anal. Res.1201217
- S.S.PandaIon-pairing RP-HPLC method for simultaneous determination of aspirin and clopidogrel bisulphate in tablet and capsule dosage formInt. J. PharmTech Res.22010269273
- A.MitakosI.PanderiA validated LC method for the determination of clopidogrel in pharmaceutical preparationsJ. Pharm. Biomed. Anal.282002431438
- S.DermisE.AydoganElectrochemical study of clopidogrel and its determination using differential pulse voltammetry in bulk drug and pharmaceutical preparationsPharmazie652010175181
- E.SouriH.JalalizadehA.KebriacezadehM.ShekarchiA.DalvandiValidated HPLC method for determination of carboxylic acid metabolite of clopidogrel in human plasma and its application to a pharmacokinetic studyBiomed. Chromatogr.20200613091314
- S.S.SinghK.SharmaD.BarotP.R.MohanV.B.LoharyEstimation of carboxylic acid metabolite of clopidogrel in Wistar rat plasma by HPLC and its application to a pharmacokinetic studyJ. Chromatogr. B8212005173180
- N.K.PatelG.SubhaiahH.ShahM.MohanS.P.ShrivastavRapid LC–ESI-MS–MS method for the simultaneous determination of clopidogrel and its carboxylic acid metabolite in human plasmaJ. Chromatogr. Sci.462008867875
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), Harmonized Triplicate Guideline on Validation of Analytical Procedures: Methodology Recommended for Adoption at Step 4 of the ICH Process on November 1996 by the ICH Steering Committee, International Federation of Pharmaceutical Manufacturers and Associations, Geneva.