?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A simple and rapid stability-indicating LC-analytical method was developed for the simultaneous determination of flucloxacillin (fluc) and amoxicillin (amox) in bulk and pharmaceutical dosage form. A chromatographic separation of the two drugs was achieved with a Thermosil C18 (4.6 mm × 250 mm, 5 μm) analytical column using potassium dihydrogen phosphate buffer (adjusted to pH 3 by ortho phosphoric acid):methanol (70:30%, v/v) in isocratic mode at a flow rate of 1 mL/min and column at ambient temperature. The detection was monitored at 225 nm using a PDA detector. The stressed samples were analyzed for the degradation study in acid, base, peroxide, thermal, photolytic and validated as per ICH guideline. This proposed method was found to be specific and stability-indicating as no interfering peaks of degradation compounds and excipients were noticed. The described method shows excellent linearity over a range of 20–100 μg/mL for both drugs. The correlation coefficient for fluc and amox was 0.9992 and 0.9993, respectively. The mean recovery value for fluc and amox was 99.9% and 99.7%, respectively. The limit of detection for fluc and amox was 0.018 and 0.009 μg/mL and the limit of quantification was 0.06 and 0.03 μg/mL, respectively. The retention time was observed at 2.582 and 3.407 min for amox and fluc, respectively. The robustness study and percentage of assay of the formulation were found within limit as per ICH guidelines. The proposed method was suitable for quantitative determination and it can be applied in quality control department in industries, approved testing laboratories, bio-pharmaceutics and bio-equivalence and clinical pharmacokinetic studies.
1 Introduction
Flucloxacillin sodium [Citation1] chemically is (2S, 5R, 6R)-6-({[3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-yl]carbonyl}amino)-3, 3-dimethyl-7-oxo-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylicacid and amoxicillin trihydrate [Citation2] (2S, 5R, 6R)-6-[[(2R)-2-amino-2-(4-hydroxy phenyl)acetyl]amino]-3, 3-dimethyl-7-oxo-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylicacid tri hydrate. Flumox is a combination of two bactericidal penicillins: amoxycillin (broad-spectrum penicillin) and flucloxacillin (penicillin-resistant penicillin), to produce a wider spectrum of activity. Flucloxacillin exerts a bactericidal action on penicillinase-producing microorganisms including most staphylococci. This combination exhibits bactericidal activity against a wide range of gram-positive and gram-negative microorganisms including penicillinase and non-penicillinase-producing Staphylococci, Streptococcus pyogenes, pneumoniae, and faecalis, Corynebacterium diphtheriae, Clostridia spp., Bacillus anthracis; Haemophilus influenzae, Moraxella (Branhamella) catarrhalis, Neisseria gonorrheae and meningitidis, Escherichia coli, Proteus mirabilis, Salmonella, Bordetella pertussis and Bacteroides melaninogenicus. Literature review reveals that few spectrophotometric and potentiometric methods have been reported for analysis of flucloxacillin alone [Citation3–Citation5] or method development in combination with ampicillin in LC–MS [Citation6] and in LC [Citation7] or with amoxicillin by liquid chromatography without forced degradation study [Citation8,Citation9] for pharmaceutical injection [Citation10] and ion-pairs along with gradient elution HPLC method [Citation11]. Although two LC simultaneous methods for pharmaceutical dosage and one for injections were reported in the literature review, existing methods were not subjected to forced degradation study. Moreover reported methods were not much cost-effective in terms of solvent consumption and total run time of the analysis, so we decided to perform rapid, selective and precise stability-indicating isocratic RP-HPLC assay method for simultaneous determination of flucloxacillin and amoxicillin in solid dosage form which was not developed so far.
2 Experimental
2.1 Chemicals and reagents
Flucloxacillin standards were obtained from Surya Pharmaceutical Limited and amoxicillin trihydrate from ABL-Life Care Limited. Methanol (HPLC-grade) was obtained from Merck Fine Chemicals (Mumbai, India). Sodium hydroxide (NaOH), hydrochloric acid (HCl) and hydrogen peroxide (H2O2) were from SD Fine Chemicals, Finar Chemicals and Alpha Pharma Limited, respectively. The 0.45-μm pump nylon filter was obtained from advanced micro devices (Ambala Cantt, India). The flucamox (Sedico Pharmaceuticals) and flumox (Eipico) capsules of the combination of flucloxacillin and amoxicillin were purchased commercially. Double-distilled water was used throughout the experiment. Other chemicals used were of analytical or HPLC-grade.
2.2 HPLC instrumental condition
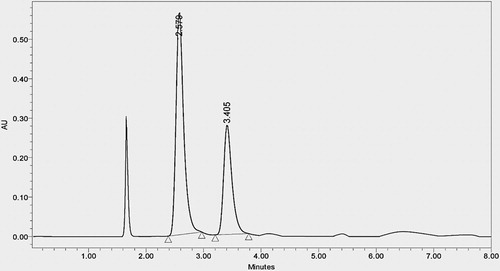
Instrument used in the study was Shimadzu-LC equipped with Auto Sampler, DAD or UV detector and Empower 2 software. A chromatographic separation of the two drugs was achieved with a Thermosil C18 (4.6 mm × 250 mm, 5 m) analytical column using potassium dihydrogen phosphate buffer (adjusted to pH 3 by ortho phosphoric acid):methanol (70:30%, v/v) in isocratic mode at a flow rate of 1 mL/min and column at ambient temperature. All the solvents were filtered through 0.45-μm nylon filter and degassed in ultra-sonic bath prior to use. Measurements were made with injection volume 20 μL and detection at 225 nm. For analysis of forced degradation samples, the PDA detector was used in scan mode with a scan range of 200–400 nm. The peak homogeneity was expressed in terms of peak purity and was obtained spectral analysis report using previously mentioned software. Final optimization was performed with pure analytical standard which was obtained from above mentioned pharmaceutical industry ().
Table 1 Optimized chromatographic parameters.
2.3 Standard solution preparation
Accurately weigh and transfer 10 mg of amox and 10 mg of fluc working standard into a 10 mL clean dry volumetric flask. Then, add about 7 mL of diluents and sonicate to dissolve it completely and make volume up to the mark with the same solvent (stock solution). Further pipette 0.6 mL of amox and fluc in the above stock solution into a 10 mL volumetric flask and dilute up to the mark with mobile phase.
2.4 Analysis of formulations
20 capsules of two different brands were taken and their average weight was calculated. Dose equivalent to 10 mg of amox and fluc sample was transferred into a 100 mL clean dry volumetric flask, add about 70 mL of diluent and sonicate to dissolve it completely and make volume up to the mark with the same solvent (stock solution). Further pipette 6 mL of amox and fluc from the above stock solution into a 10 mL volumetric flask and dilute up to the mark with diluents ( and ).
Table 2 Assay of formulation.
2.4.1 Calculations
where
AS = average peak area counts of standard preparation;
AT = average peak area counts of sample preparation;
WS = weight of working standard taken in mg;
WT = weight of working test taken in mg;
P = percentage purity of working standard;
DT = dilution of test solution;
DS = dilution of standard solution;
LC = label claim of sample; avg. wt = average weight of capsule in mg.
2.4.2 Sample details
The flucamox (Sedico Pharmaceuticals) and flumox (Eipico) (each capsule 500 mg contains amox 250 and fluc 250 mg).
2.5 Preparation of calibration curve
Calibration curve was established for fluc and amox concentration ranging from 20 to 100 μg/mL, respectively.
2.6 Forced degradation studies
The International Conference on Harmonization (ICH) guideline entitled stability testing of new drug substances and products requires that stress testing is carried out to elucidate the inherent stability characteristics of the active substance [Citation12]. The aim of this work was to perform the stress degradation studies on the amoxicillin and flucloxacillin using the proposed method.
2.7 Standard solution preparation
Standard solution of 60 μg/mL of fluc and amox was prepared for forced degradation study and it was injected into the HPLC system.
2.8 Hydrolytic degradation under acidic condition
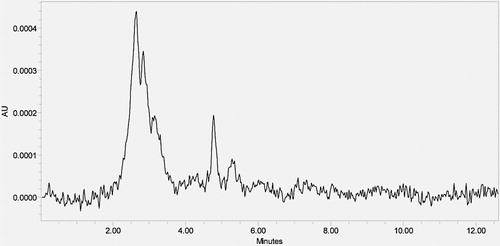
From 100 μg/mL stock solution, 6 mL of stock solution and 1 mL of 0.1 N HCl were added in 10 mL volumetric flask. Then, the volumetric flask was kept at 60–70 °C reflux condition for 90 min and neutralized with 0.1 N NaOH and 10 mL with diluent was made to get concentration of 60 μg/mL of fluc and amox. Cool the solution to room temperature. Filter the solution with 0.45 μm syringe filters and place in vials of the HPLC system. No degradant peaks were reported in the retention time of fluc and amox ().
2.9 Hydrolytic degradation under alkaline condition
From 100 μg/mL stock solution, 6 mL of stock solution and 1 mL of 0.1 N NaOH were added in 10 mL volumetric flask. Then, the volumetric flask was kept at 60–70 °C reflux condition for 90 min and neutralized with 0.1 N HCl and 10 mL with diluent was made to get concentration of 60 μg/mL of fluc and amox. Cool the solution to room temperature. Filter the solution with 0.45 μm syringe filters and place in vials of the HPLC system. No degradant peaks were reported in the retention time of fluc and amox ().
2.10 Oxidative degradation
From 100 μg/mL stock solution, 6 mL of stock solution and 1 mL of 3% (w/v) of hydrogen peroxide were added in 10 mL volumetric flask. Volumetric flask was then kept at 60–70 °C reflux condition 90 min and volume was made up to the mark with diluents to get concentration of 60 μg/mL of fluc and amox. Cool the solution to room temperature. Filter the solution with 0.45 μm syringe filters and place in vials of the HPLC system. No degradant peaks were reported in the retention time of fluc and amox ().
2.11 Thermal induced degradation
From 100 μg/mL stock solution, 6 mL of stock solution was added in 10 mL volumetric flask. Then, volumetric flask was kept at 60–70 °C in hot air oven for 90 min and the volume was made up to the mark with diluents to get concentration of 60 μg/mL of fluc and amox. Cool the solution to room temperature. Filter the solution with 0.45 μm syringe filters and place in vials of the HPLC system. No degradant peaks were reported in the retention time of fluc and amox ().
2.12 Photo degradation
From 100 μg/mL stock solution, 6 mL of stock solution was added in 10 mL volumetric flask. Above samples were transferred into a petri dish and kept in photostability chamber 200 W h/m2 in UV light and 1.2 million l × h in visible light for 5 h. Then, the volumetric flask was made up to mark with diluents to get concentration of 60 μg/mL of fluc and amox. Cool the solution to room temperature. Filter the solution with 0.45 μm syringe. Filter and place in vials of the HPLC system. No degradant peaks were reported in the retention time of fluc and amox ().
3 Validation of chromatographic method
3.1 Accuracy or recovery
Accuracy is represented and determined by recovery experiments. In this method, recovery studies was performed at three different percentage levels namely 80, 100 and 120 and finally analyzing amount recovered of both drugs during studies.
3.2 Specificity
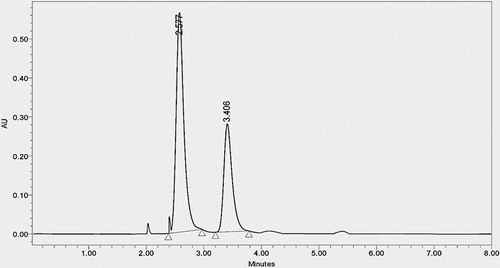
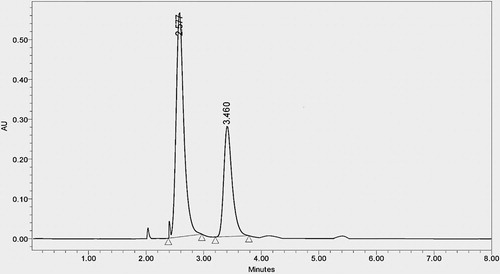
To assess the method's specificity, working blank solution (placebo) without amox, fluc, standard solution and sample solution were injected into the system. It was found that chromatogram of working blank (placebo) solution did not show any interference at the retention time of both amox and fluc. Specificity of the method was also confirmed by forced degradation study. In peak purity analysis with photo diode detector, purity angle was less than purity threshold for both the analytes ( and ).
3.3 Precision
Precision and intermediate precision/ruggedness of the analytical method were established for both systems and method/procedure at 60 μg/mL for both drugs. System precision was determined by six replicate injections of standard solution injected into the HPLC system. Method precision was determined by the six individual sample preparations injected to the HPLC system. The relative standard deviation was found to be less than 2 ().
Table 3 Precision study.
3.4 Linearity
Linearity was demonstrated by preparing and analyzing the standard preparation at five different concentrations for fluc and amox 20–100 μg/mL. From this experiment coefficient of correlation, intercept and slope were calculated.
3.5 Limit of detection (LOD) and limit of quantification (LOQ)
The detection limit and quantitation limit were determined as signal to noise. The limit of detection for fluc and amox was 0.018 and 0.009 μg/mL and the limit of quantification was 0.06 and 0.03 μg/mL, respectively.
3.6 System suitability study
System suitability tests were carried out on freshly prepared standard solution of flucloxacillin and amoxicillin to check the various parameters such as plate count, resolution and tailing factor ().
Table 4 Summary of validation parameters.
3.7 Robustness study
The robustness of the method was studied by small changes in the method like altering the mobile phase pH, flow rate, column temperature variation and changes in mobile phase composition. It was observed that there were not much changes in the chromatograms ().
Table 5 Robustness study.
4 Results and discussion
4.1 Optimization of chromatographic conditions
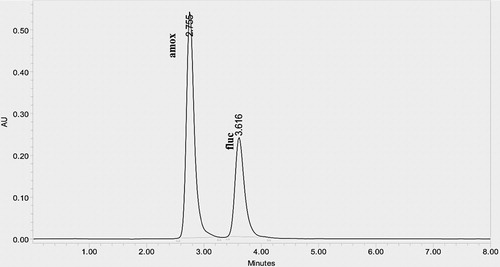
As there is a growing demand of flucloxacillin and amoxicillin in pharmaceutical market, it is required to develop fast, cost-effective, stable, precise and sensitive analytical method LC for the simultaneous estimation of both drugs. The primary target in developing this stability-indicating RP-HPLC method is to achieve the optimum resolution between the fluc and amox and its degradation products. To develop a stability-indicating method different makes of C18, C8 column (Xterra, Hypersil and Thermosil) and different mobile phases containing buffers (pH 3–5):methanol in different ratios were tried but failed to get optimum resolution between drugs. This challenge was met by using dihydrogen phosphate buffer (adjusted to pH 3 by ortho phosphoric acid):methanol (70:30, %v/v) where optimum resolution and good symmetric peaks were observed by using Thermosil C18 (4.6 mm × 250 mm, 5 μm) analytical column in isocratic mode at a flow rate of 1 mL/min and column at ambient temperature. Under the above optimized conditions, the retention time reported for formulation was 2.582 min for amox and 3.407 min for fluc (). The linearity of an analytical procedure was demonstrated by preparing and analyzing the standard preparation at five different concentrations of amox and fluc (20–100 μg/mL). The calibration curve constructed for amoxicillin by plotting the peak area versus concentration yielded coefficient of regression R2 = 0.9993. The mean recovery value for fluc and amox was 99.9 and 99.7, respectively. The limit of detection for fluc and amox was 0.018 and 0.009 μg/mL and the limit of quantification was 0.06 and 0.03 μg/mL, respectively. The robustness study and percentage of assay of the formulation were found within limit as per ICH Guidelines [Citation13]. The resolution was more than 3, theoretical plates were more than 2500 and tailing factor was less than 1.5 for the flucloxacillin and amoxicillin peak. Specificity of the method was proved as the chromatogram of working blank (placebo) solution does not show any interference at the retention time of amox and fluc. Therefore, the developed method was free from the inference of diluents and excipients used in the formulation.
4.2 Forced degradation study
Degradation studies indicate the specificity of developed method in the presence of degradation products. Degradation was carried out in combination of two drugs and purity of drug peaks was confirmed by purity angles. Formulation drug products were exposed to thermal, hydrolytic under acid and base, oxidative, photolytic stress conditions. During degradation study, fluc has been degraded slightly higher in acidic condition when compared to alkaline condition and was found to be more stable in thermal and photolytic conditions but amox degraded more in thermal and photolytic conditions. Although unknown degradant peaks were observed in acid, base, peroxide, photolytic and thermal study, no degradant peaks were reported in the retention time of amox and fluc. Hence amox and fluc stable up to specified period of time (90 min) in the proposed method or they are susceptible towards acid, alkali, heat, hydrogen peroxide and photolytic degradation ().
Table 6 Forced degradation study.
4.3 Significance of the developed method
Developed isocratic stability-indicating method has many advantages over reported methods: (a) the method was simple because mobile phase used was cheap and easily available; (b) total run of chromatogram was 8 min and both drugs were eluted within 3.5 min indicating that very less amount of mobile phase was consumed; so the method was cost-effective whereas in reported methods it was more than 4.5 min; (c) the limit of detection for flucloxacillin and amoxicillin was 0.018 and 0.009 μg/mL and the limit of quantification was 0.06 and 0.03 μg/mL, respectively, indicating that the method was sensitive and rapid; (d) specificity study ( and ) and forced degradation study () indicate that the method was very specific, stable in the proposed method. Moreover, forced degradation study was neither performed or nor reported elsewhere so it was the first stability-indicating LC method for amox and fluc; (e) mode of separation is isocratic which means it is easy to operate throughout the process without any complications whereas one of the reported method was gradient elution techniques without stability study; (f) simultaneous estimation of different brands gives precise results indicating that developed method is compatible to estimate in different pharmaceutical formulations; (g) validation of the developed method as per ICH guideline indicates that the method was highly precise, rapid, simple, economical, sensitive accurate, robust and specific for simultaneous estimation of fluc and amox in bulk and pharmaceutical dosage form.
5 Conclusion
This method was proved to be simple, rapid, precise, selective and economical which can applied in different commercially available popular combinations in pharmaceutical market used as antibiotic namely in flummox, flucamox, suprapen without the interferences of degradants product or excipients in bulk and pharmaceutical dosage form. The developed method was validated as per ICH Guidelines and stability study in different stress condition shows that the developed method was highly specific and robust so that it can be effectively applied for routine analysis in research institutions, in bio-analytical lab, in therapeutic drug monitoring for clinical trials, in quality control department of pharmaceutical industries, in approved testing laboratories, in bio-equivalence studies and in clinical pharmacokinetic studies.
Notes
Peer review under responsibility of Taibah University
References
- European Pharmacopoeia, Strasbourg Supplement5th ed.2005Council of Europe pp. 2925, 3440
- British Pharmacopoeia2007British Pharmacopoeia Commission, TSOLondon pp. 140, 876
- P.VipinK.Sarif NiroushS.GeradDevelopment and validation of spectrophotometric method for the estimation of flucloxacillinIJPSR320121806
- L.Amr SaberA.MohmedA.ElmosallamyM.Hamada KillaM.Mohmed GhoneimSelective potentiometric method for determination of flucloxacillin antibioticJ. Taibah Univ. Sci.72013195
- D.SuddhasattyaVaithiyanathanB.HimansuVikram reddyBala krishnaY.Anil reddyG.Navin kumarSpectrophotometric, method developed for the estimation of flucloxacillin in bulk and dosage form using UV–vis spectrophotometric methodIJPBS1201035
- C.HuangJ.GaoL.MiaoSimultaneous determination of flucloxacillin and ampicillin in human plasma by ultra performance liquid chromatography–tandem mass spectrometry and subsequent application to a clinical study in healthy Chinese volunteersJ. Pharm. Biomed. Anal.592012157
- K.Sarif niroushT.Jane JacobStability indicating isocratic RP-HPLC DAD method for the simultaneous estimation of flucloxacillin and ampicillin in pharmaceutical dosage form: application for routine drug analysis in quality control laboratories and clinical pharmacokinetic studiesInventi Impact: Pharm Anal. Qual. Assur.32014158
- P.ShanmugasundaramR.Kamal RajJ.Mohan ranganG.DevdassM.ArunadeviR.MaheswariM.Vijey anandhiSimultaneous estimation of amoxicillin and flucloxacillin in its combined capsule dosage form by HPLCRasayan J. Chem.2200957
- S.Dhiraj NikamG.Chandrakant BondeS.J.SuranaG.VenkateshwarluP.G.DekateDevelopment and validation of RP-HPLC method for simultaneous estimation of amoxicillin trihydrate and flucloxacillin sodium in capsule dosage formInt. J. PharmTech Res.12009935
- H.L.Hongwu WangV.Bruce SunderlandAn isocratic ion exchange HPLC method for the simultaneous determination of flucloxacillin and amoxicillin in a pharmaceutical formulation for injectionJ. Pharm. Biomed. Anal.372005395
- Y.ChenT.HuaAssay of amoxicillin sodium and flucloxacillin sodium for injection and their related substances by ion-pairs along with gradient elution HPLC methodChin. J. Antibiot.2920041518
- ICH Harmonized Tripartite Guideline, Stability Testing of New Drug Substances and Products: Q1A (R2)2003
- ICH Harmonized Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology: Q2 (R1)2005