Abstract
In the present research study, 3-heteroarylazo 4-hydroxy coumarin derivatives were synthesized and evaluated in vitro for their preliminary antibacterial activities against four different pathogenic bacterial strains such as Staphylococcus aureus, Escherichia coli, Bacillus subtilis and Pseudomonas aeruginosa. Antibacterial activity of each compound was compared with standard drug, Ampicillin. The compounds were interpreted by UV, IR, 1H NMR, mass spectroscopy and X-ray diffraction studies. Solvatochromic behaviour of these compounds was also investigated by UV–vis spectra. Zone of inhibition and minimum inhibitory concentration revealed that all the products exhibited greater antibacterial potential against all bacterial strains except 4g.3-Thiazolylazo and 3-(4-phenyl thiazolylazo) of 4-hydroxy coumarin, which have been exhibiting good zone of inhibition against both gram +ve strains and gram −ve strains, where as the compound pyrazolone azo analogue 4e has tremendous antibacterial activity. Finally we concluded that the compounds having thiazole, pyrazole and triazole nucleus in individual molecular structure clubbed with potent antibacterial pharmacophore of 4-hydroxy coumain showed antibacterial activities.
1 Introduction
Nowadays, the use of nitrogen bearing heterocyclic intermediates in the synthesis of azo disperse dye is well established and the resultant dyes give higher tinctorial strength and are brighter than those aromatic primary amine based diazo components [Citation1]. Dyes synthesized from heterocyclic amines produce pronounced bathochromic effect, when compared to the corresponding aniline compounds [Citation2]. Azo dyes based on heterocyclic amines have been studied widely due to their excellent thermal [Citation3], optical [Citation4] and medicinal properties, such as antibacterial [Citation5], antiviral [Citation6], anti-fungal [Citation7] and antioxidant activities [Citation8]. Incorporation of hydrazino and azo group has been reported to enhance the pharmacological activity of heterocyclic compounds [Citation9]. Azo dyes are the most important group of synthetic colourants. Furthermore, thiazoles are important compounds that have many derivatives with wide range of pharmacological properties. 2-Amino thiazole derivatives can be obtained by the reaction of acetophenone with thiourea in the presence of iodine [Citation10]. 4-Hydroxycoumarin is a structurally a Benz[α]pyrone derivative and was an intermediate synthetic precursor in the preparation of potent anticoagulant drug such as warfarin. The aryl substitution at C-3 of 4-hydroxy coumarin is very essential for exhibiting broad biological actions such as anti-viral [Citation11], anti-bacterial [Citation12], anticancer [Citation13], anticoagulant [Citation14] and antioxidant activities [Citation15,Citation16]. Insertion of aryl/hetero aryl azo in C-3 of coumarin has been reported to have a good antimicrobial activity [Citation17,Citation18]. The 4-hydroxy 3-hetroaryl coumarin moiety is found in many natural and synthetic products and also could be useful in significant biological actions [Citation19]. A part of our investigation has focused on the synthetic and medicinal utilities of newly prepared heterocyclic azo compounds. According to these medicinal values in the present study, a series of linear azodyes were synthesized by coupling of 4-hydroxy coumarin with diazonium salts of potent antimicrobial pharmacophores of heterocyclic amine moieties, such as derivatives of 2-amino thiazole, 2-amino pyridine, 3-amino triazole and 4-amino pyrazolone.
2 Experimental
2.1 Instruments and methods
The chemicals used in the present studies were of synthetic grade, and some were sourced from Merck Company Ltd. The products were characterised by IR (JASCO FT/IR 4100 Spectrophotometer using K Br disc), 1H NMR (Bruker 1H NMR 400MHZ) using TMS as an internal standard, UV (JASCO V-630 Spectrophotometer), LC–MS (Shimadzu-Mass spectrophotometer) and elemental analysis was carried out by using Perkins Elmer-2400C H N S Analyser system. An X-ray diffraction (XRD) pattern of silica was obtained with Cu Ka X-ray source including and a step of 0.02(2θ) and run 2θ = 6–80° at room temperature. The melting points were determined by open capillary method and remained uncorrected. The purity of prepared compounds was checked by TLC using silica gel with appropriate solvents ethyl acetate and cyclohexane in 1:1. Elemental (C, H, N, S) analysis indicated that the calculated and found values were within the acceptable limits (±0.4%).
2.2 General method of synthesis of 4-hydroxy-3-(heteroaryl-2-yldiazenyl)-2H-chromen-2-one (4a, 4b, 4c, 4d, 4e, 4g) [Citation20]
A cold solution of sodium nitrite (0.207 g, 3 mmol) was added dropwise into the solution of six different individual substituted hetero aromatic amines (3 mmol) with conc. Sulphuric acid (8–9 mmol) and water (5 ml) were kept on an ice bath. The temperature of the reaction was maintained up to 5 °C. When addition was completed, the solution was kept for 15 min with occasional stirring to complete the diazotization. Then it was poured into an ice-cold solution of 4-hydroxy coumarin (0.543 g, 3 mmol) in 10 ml of acetate buffer solution (pH = 5) in ethanol. Then resultant mixtures were stirred at 0–5 °C and allowed to stand in an ice bath for 1 h. The colour products obtained were filtered and washed with water. Finally the obtained products were dried and recrystallised by ethanol.
2.3 Synthesis of 4-[(4-hydroxy-2-oxo-2H-chromen-3-yl) diazenyl]-N-(5-methylisoxazol-3-yl) benzenesulfonamide (4f)
A cold solution of sodium nitrite (0.207 g, 3 mmol) was added dropwise into the solution of sulfamethoxazole (3 mmol) with conc. Hydrochloric acid (8–9 mmol) and water (5 ml)were kept on an ice bath. The temperature of the reaction was maintained up to 5 °C. When addition was completed, the solution was kept for 15 min with occasional stirring to complete the diazotization. Then it was poured into an ice-cold solution of 4-hydroxy coumarin (0.543 g, 3 mmol) in 20 ml of 10% sodium hydroxide solution. Then resultant mixture was stirred and kept on an ice bath for 30 min at a temperature of 5 °C. The pH was maintained at about 5–6. The obtained products were filtered and washed with cold distilled water. Finally the obtained product was dried and recrystallised by ethanol.
2.3.1 4-Hydroxy-2H-chromen-2-one
White solid, m.p. (°C): 207–210 (lit. 211–213 °C) [Citation21]; UV–vis (λmax, ethanol): 294 nm; IR (K Br) cm−1: 3417 (O–H str), 2993 (Ar–H), 1698 (C=O str. lactone carbonyl), 1610 (C=C str), 1198 (=C–O–H bending), 1102 (C–O str); 1H NMR (DMSO-d6) δ: 15.56 (s, 1H, 4-OH) 7.58 (d, 1H, J = 7.2 Hz), 7.42–7.58 (m, 1H), 7.15–7.23 (m, 2H), 5.45 (s, coumarin H-3) [Citation22].
2.3.2 4-Hydroxy-3-(thiazol-2-yldiazenyl)-2H-chromen-2-one (4a)
IR (K Br) cm−1: 3418 (O–H str), 2928 (Ar–H), 1696 (C=O str. lactone carbonyl), 1609 (C=C str), 1554 (–N=N–), 1197 (C–O str), 1198 (=C–O–H bending), 947 (C–S str); 1H NMR (DMSO-d6) δ: 7.92 (d, coumarin H-5, J = 8.1 Hz), 7.59 (m, coumarin H-6), 7.63 (m, coumarin H-7), 7.36 (d, coumarin H-8, J = 8.1 Hz), 7.22–7.34 (d, 2H, thiazole-H), 13.56 (s, 1H, 4-OH); analysis calcd% for C12H7N3O3S: C, 52.74; H, 2.58; N, 15.38, S, 11.70; Found%: C, 52.68; H, 2.46; N, 15.45; S, 11.65; m/z; 273.27 (100%), 274.02 (14.9%).
2.3.3 4-Hydroxy-3-(pyridin-2-yldiazenyl)-2H-chromen-2-one (4b)
IR (K Br) cm−1: 3419 (O–H str), 3028 (Ar–H), 1710 (C=O str. lactone carbonyl), 1608 (C=C str. coumarin), 1560 (–N=N–), 1286 (C–O str), 1188 (=C–O–H bending), 1019 (C–N str), 846,780 (Hetero aryl –CH bending); 1H NMR (CDCl3) δ: 7.89 (d, coumarin H-5, J = 8.1 Hz), 7.45 (m, coumarin H-6), 7.65 (m, coumarin H-7), 7.53 (d, coumarin H-8, J = 7.8 Hz), 7.15–8.52 (m, 5H, Pyridine-H), 13.65 (s, 1H, 4-OH); Analysis calcd % for C14H9N3O3: C, 62.92; H, 3.39, N, 15.72, Found %: C, 62.78; H, 3.43, N, 15.91; m/z; 267.05 (100%), 268.07 (19.97%).
2.3.4 3-((4H-1, 2, 4-triazol-3-yl) diazenyl)-4-hydroxy-2H-chromen-2-one (4c)
IR (K Br) cm−1: 3419 (O–H str), 3028 (Ar–H), 1710 (C=O str. lactone carbonyl), 1608 (C=C str. coumarin), 1560 (–N=N–), 1286 (C–O str), 1188 (O–H bending); 1H NMR (CDCl3) δ: 7.42–7.96 (m, 4H, coumarin-H), 7.8 (s, 1H, 1,2,4-Triazole-NH); Analysis calcd% for C11H7N5O3: C, 51.37; H, 2.74, N, 27.23, Found %: C, 51.27; H, 2.31, N, 27.41; m/z; 257.05 (97.13%), 258.07 (19.97%).
2.3.5 4-Hydroxy-3-[(4-phenylthiazol-2-yl) diazenyl]-2H-chromen-2-one (4d)
IR (K Br) cm−1: 3418 (O–H str), 3050, 2998 (Ar–H), 1700 (C=O str. lactone carbonyl), 1610 (C=C str. coumarin), 1544 (–N=N–), 1220 (C–O str), 1100 (O–H bending), 998 (C–S str); 1H NMR(DMSO-d6) δ: 7.92 (d, coumarin H-5, J = 8.1 Hz), 7.59 (m, coumarin H-6), 7.63 (m, coumarin H-7), 7.36 (d, coumarin H-8, J = 8.1 Hz), 7.42–7.78 (m, 5H, ArH), 8.15 (s, 1H, thiazole-H), 13.56 (s, 1H, 4-OH); Analysis calcd% for C18H11N3O3S: C, 61.88; H, 3.17, N, 12.03, S, 9.18; Found %: C, 61.78; H, 2.97, N, 12.33, S, 9.15; m/z; 349.05 (100%), 350.06 (19.97%).
2.3.6 4-[(4-Hydroxy-2-oxo-2H-chromen-3-yl) diazenyl]-1, 5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one (4e)
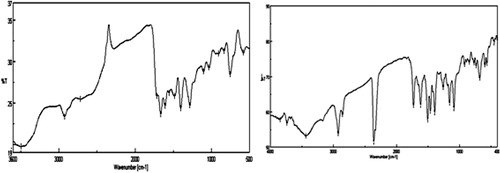
IR (KBr) cm−1:3495 (O–H str), 2925 (Ar–CH3), 1716 (C=O str. lactone carbonyl), 1664 (C=O str Pyrazolone), 1607 (C=C str), 1557 (–N=N–), 1483 (–C=N str), 1282 (C–O str), 1132 (O–H bending); 1H NMR (CDCl3) δ: 8.08 (d, coumarin H-5, J = 8.1 Hz), 7.41 (m, coumarin H-6), 7.64 (m, coumarin H-7), 7.43 (d, coumarin H-8, J = 8.1 Hz), 7.64–7.99 (m, 5H, ArH), 3.19 (s, 3H, N-CH3), 2.65 (s, 3H, C-CH3), 13.56 (s, 1H, 4-OH); Analysis calcd% for C20H16N4O4: C, 63.82; H, 4.28; N, 14.89, Found %: C 63.75; H 4.25; N 14.95, m/z; 377.18 (90.15%), 274.02 (14.9%).
2.3.7 4-[(4-Hydroxy-2-oxo-2H-chromen-3-yl)diazenyl]-N-(5-methylisoxazol-3-yl) benzenesulfonamide (4f)
IR (K Br) cm−1: 3438 (N–H str), 3179 (O–H str), 2925 (Ar–CH3), 1728 (C=O str. lactone carbonyl), 1617 (C=C str), 1506 (–N=N–), 1390, 1157 (SO2 str), 1260 (C–O str), 920 (S–N str), 1H NMR (CDCl3) δ: 7.93 (d, coumarin H-5, J = 8.1 Hz), 7.54 (m, coumarin H-6), 7.65 (m, coumarin H-7), 7.42 (d, coumarin H-8, J = 8.1 Hz), 2.43 (s, 3H, C-CH3), 5.62 (s, 1H, isoxazolyl-H-4), 13.56 (s, 1H, 4-OH), 10.55 (s, 1H, N-H); Analysis calcd % for C19H14N4O6S: C, 53.52; H, 3.31; N, 13.14; S, 7.52; Found %: C 53.47; H 3.36; N 13.21; S,7.49; m/z; 427.18 (100%).
2.3.8 4-Hydroxy-3-(benzo[d]thiazol-2-yldiazenyl)-2H-chromen-2-one (4g)
IR (K Br) cm−1: 3370, 3193 (O–H str), 2884 (Heteroaryl-H), 1704 (C=O str. lactone carbonyl), 1648 (C=N str.), 1616 (C=C str. coumarin), 1551 (–N=N–), 1187 (C–O str), 1245 (=C–O–H bending), 939 (C–S str. benzthiazole); 1H NMR (DMSO-d6) δ: 7.92 (d, coumarin H-5, J = 8.1 Hz), 7.59 (m, coumarin H-6), 7.63 (m, coumarin H-7), 7.36 (d, coumarin H-8, J = 8.1 Hz), 7.46–8.10 (m, 4H, benzthiazole-H); Analysis calcd% for C16H9N3O3S: C, 59.44; H, 2.81; N, 13.00,S, 9.92; Found %: C, 59.41; H 2.79; N 12.97; S, 9.89; m/z; 323.27 (100%), 324.02 (18.9%).
3 Antimicrobial activity
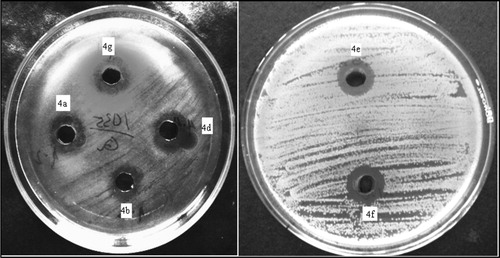
The seven (4a–g) newly synthesized compounds were screened for their antibacterial activity against Escherichia coli (MTCC 614), Staphylococcus aureus (subcultured), Pseudomonas aeruginosa (MTCC 1035) and Bacillus subtilis (subcultured) by cup and plate method using Himedia Nutrient Agar as medium [Citation23]. Antibacterial activities were evaluated by measuring the diameter of zones of inhibition. Ampicillin was used as reference standard for all the compounds. Compounds having the zone of inhibition of 20 mm or more were considered as highly active and that having a zone of inhibition less than 20 mm was considered moderately active.
3.1 Determinations of minimum inhibitory concentration
1 mg/1 ml stock solution of synthesized compounds as well as reference compound was prepared using 10% DMF solution (distilled water). Further four different concentrations of (250 μg/ml, 125 μg/ml, and 62.5 μg/ml 31.25 μg/ml) were prepared by serial dilution method. All the different concentrations for respective compounds were poured into the cups and the minimum concentration at which the zone of inhibition was observed, and indicated as the MIC.
4 Results and discussion
4.1 Chemistry
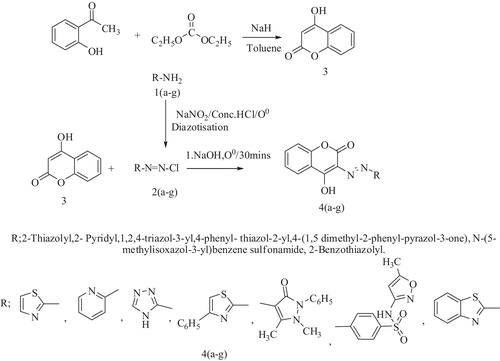
A series of 4-hydroxy-3-(hetero aryl Azo) coumarin compounds were synthesized by coupling of diazonium salt of seven different hetero aryl amine derivatives with 4-hydroxy coumarin in the presence of acetate buffer (). Diazotisation was carried out in the presence of nitrosyl sulphuric acid and excess nitrous acid is destroyed by the addition of urea. The compound 4f was synthesized by coupling of diazotised sulfamethoxazole with 4-hydroxy coumarin in presence of 10% sodium hydroxide. The crude products were recrystallized from 50% ethanol. The coupling starting material 4-hydroxy coumarin (3) was prepared by the claisen condensation of 2-hydroxy acetophenone with diethyl carbonate in sodium hydride as previously reported [Citation21]. The infrared spectra of the prepared starting material (3) showed strong absorption band at 3417 cm−1 corresponding to –OH group and band at 1698 cm−1 with respect to lactone carbonyl of 4-hydroxy coumarin. 2-Amino thiazole and its derivatives were synthesized by Hanztsch reactions, in which the reaction proceeds between α-halo carbonyl compounds and thiourea or thioamides. The coupling reactions initially generate strong N2+ electrophiles from a different heteroarylamine, then finally react and couple at C-3 position of 4-hydroxy coumarin to produce 5-heteroaryl azohydroxy coumarin. The physical data of all prepared compounds are given in . The structures of prepared compounds have been confirmed by FTIR, 1H NMR, UV, LCMS and elemental analysis. The purity of compounds was checked by TLC using silica gel with suitable solvents such as ethyl acetate: cyclohexane (40:60). XRD analysis of synthetic compounds was to characterize the shape, size and internal stress of crystalline regions, average spacing between crystal atoms, orientation of a crystal and also to determine the crystal structure. The predicted molecular weight of synthesized compounds was confirmed by LCMS. The compound (4f) at retention time 2.210 together with a and having molecular ion peak 427.0 strongly reveals the predicted molecular formula ().
Table 1 Physical characteristics of newly synthesized azo-coumarin analogues.
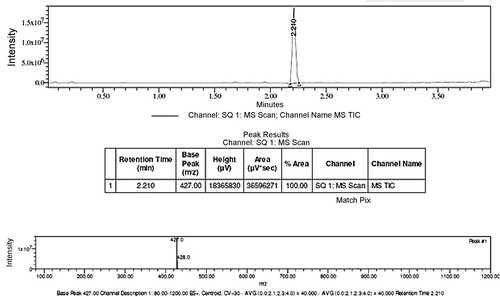
The 1H NMR spectra of aromatic protons of compounds in the 4-hydroxy coumarin proton ring, appeared at δ 7.92–7.84 doublet, δ 7.42–7.36 doublet, δ 7.65–7.55 multiplet and 7.55–7.42 multiplet. The coumarin H-6 and H-8 protons were more shielded and appeared multiplet at δ 7.42–7.59 ppm [Citation24]. The infrared spectra of the prepared compounds 4a–4g showed a strong and broad band within the range 3495–3418 cm−1 corresponding to 4-Hydroxy coumarin. The FTIR spectra of synthesized compounds 4a–4g were shown within the range 1720–1698 cm−1 due to lactone carbonyl stretching that predominantly signifies the presence of a coumarin moiety. This broad value reveals that hydroxyl was involved due to both intermolecular and intramolecular H bondings. In case of compound 4f, the NH proton of the SO2NH– group was observed in 1H NMR at δ 10.33 ppm as singlet and the two sharp strong vibrational SO2 strs assigned, and at 1386, 1157 cm−1 in FTIR spectra (). The signal of amine group was not observed in the 1H NMR spectral data and also not shown the absorption band of amine that was in FTIR spectral data in all the synthetic dyes 4a–4g. The heteroarylazo coumarin compounds 4e showed characteristic peaks δ 3.197 singlet and 2.65 singlet, which can attribute to the presences of proton N–CH3 and =C–CH3 respectively (). 1H NMR spectra showed board singlet at δ 13.45–13.65, which suggests the presence of enolic OH in all the prepared compounds except 4d, 4g.
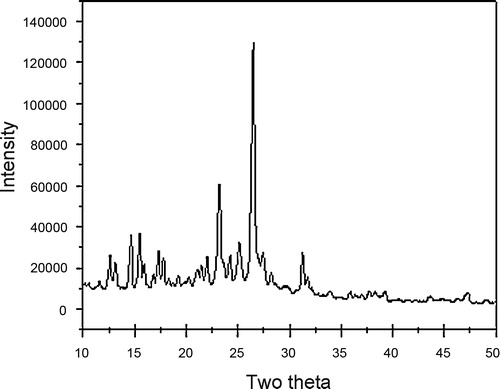
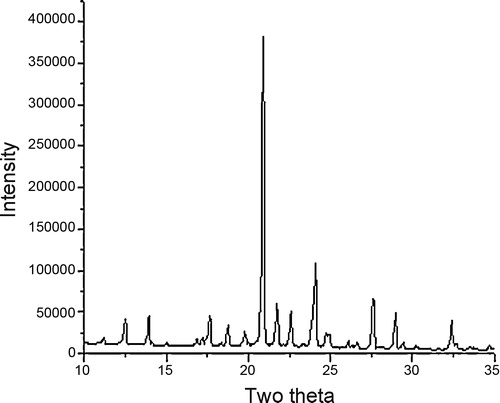
X-ray diffraction pattern of compound 4f shows twelve reflections, between 2θ ranges from 10 to 35° with maximum at 20.884° corresponding to inter-planar distance d = 4.25 Å and compound 4e shows ten reflections, between 2θ ranges from 10 to 50° with maximum at 26.623° (d = 3.56 Å) ( and ). Main peaks have been indexed by trial and error method [Citation25].
4.2 Solvatochromic study of newly synthesized compounds
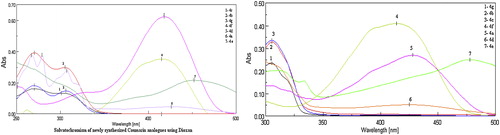
The solvent effects of the products were studied on UV–vis spectrophometer. The absorption spectra of these dyes 4a–4g were recorded in different solvents at a concentration of 10−5–10−6 M, and results are summarized in . According to illustrated data in , the synthesized dye 4e shows bathochromic shift in 1, 4-dioxane and THF with respect to the λmax as compared to the other polar solvents. Synthetic dyes 4a–4g were shown Solvatochromic effect for different hetero aryl substitutions in 4-hydroxy coumarin using 1, 4-dioxane and THF (). These bathochromic shifts can be attributed to the interaction H-atom of amino proton of dyes with polar aprotic solvent such as THF because of increase in polarity of the dyes system, generally in the excited state. Introduction of 4-(-2-phenyl, 1, 5 dimethyl 3-oxo) pyrazolyl substituent into the coumarin at the C-3 position gives largest bathochromic shift compared to other heteroaryl azo dyes in all the solvents used. On the other hand, the presence of 4-phenyl thiazolyl 4d at the C-3 position gives rise to bathochromic shift, when compared to thiazolyl 4a in using aprotic solvent such as THF.
Table 2 UV–vis spectral data (λmax, nm) of newly synthesized Coumarin analogues (4a–4g) using different solvents.
4.3 Antibacterial activity
The antibacterial activity potentials were qualitatively assessed by the presence or absence of inhibition zones, zone diameters and MIC values. The results of antibacterial activity of the tested compounds 4a–4g are summarized in . All the synthesized compounds except 4c were tremendously inhibited against S. aureus (). The compound 4e showed greater antibacterial potential against all bacterial strains. The maximum inhibition zones (mm) produced by 4e against E. coli (MTCC 614), S. aureus (sub cultured), P. aeruginosa (MTCC 1035) and B. subtilis (sub cultured) were 18, 25, 18 and 23 respectively. How ever, all the synthesized coumarin analogues except 4g showed good antibacterial activity against P. aeruginosa. The values of the MIC against pathogenic micro-organisms are depicted in . The results showed significant inhibitory effects, with the majority of the compounds with their MIC values 31.25–62.5 μg/ml. Compound 4 g has no response against all the four organisms at a concentration level less than 1gm/ml. Compound 4e has excellent antimicrobial response against the above four organisms, even at a concentration level 31.25 μg/ml and may be comparable to the standard drug Ampicillin. However, the compound 4a and 4b also exhibited remarkable antimicrobial activity against the same strains.
Table 3 Antibacterial activity of synthesized compounds (4a–g) zone of inhibition (mm), MIC (μg/ml).
Investigation of the chemical structure of the targeted molecules suggested that the targeted compounds could be divided into two moieties that is, 4-hydroxy coumarin and substituted hetero aryl azo functional group. The two chemical portions have been reported to have significant broad-spectrum antibacterial activities which might contribute to good results which obtained by testing them as antibacterial agents. Moreover, the substitution of heteroarylamine from 4-hydroxy coumarin moiety with azo at C-3 position might also helped in obtaining good antibacterial results and also indicated that the thiazolyl, substituted thiazolyl, and pyrazolyl in coumarin ring systems are capable of inducing some synergistic antibacterial actions which are found to be present in same structural components of the synthesized compounds.
5 Conclusion
Present research work involves synthesis of 4-hydroxy coumarin bearing hetero aryl diazo derivatives to explore their antimicrobial activities. All the newly synthesized compounds are characterized by different modern analytical techniques and purity is checked by TLC. These compounds can be used as ligands for new azo metal complex dyes. Most of the synthesized compounds possess excellent antimicrobial activities against gram +ve and gram −ve strains. Hence it may be suggested for topical antimicrobial evaluation of the synthesized compounds because the prepared compounds are tremendously active against P. aeruginosa. All the compounds were showing good Solvatochromic effect studied by using UV–vis spectrophotometer.
Conflict of interest
All authors have none to declare.
Support information
Download MS Word (265.7 KB)Acknowledgements
Authors are grateful to Siksha ‘O’ Anusandhan University for financial and technical support. The authors are also thankful to the Director, NISER and IMMT, Bhubaneswar for providing 1H NMR and XRD data of the synthesized compounds reported herein. They are again thankful to the Director of Piramal Healthcare, Ahmadabad for providing LC–MS data.
Notes
Peer review under responsibility of Taibah University
References
- T.Liang FuI.Jing WangSynthesis and substituent effects of some novel dyes derived from indeno [2,1-b] thiophene compoundsDyes Pigments762008158164
- J.GriffithsRecent developments in the colour and constitution of organic dyesRev. Prog. Color.1119813757
- M.S.RefatM.Y.EI-SayedA.M.A.AdamCu (II), Co (II), and Ni (II) complexes of new Schiff base ligand: synthesis, thermal and spectroscopic characterizationsJ. Mol. Struct.103820136272
- S.WangS.ShenH.XuSynthesis, spectroscopic and thermal properties of a series of azo metal chelate dyesDyes Pigments4432000195198
- I.M.AwadA.A.AlyA.M.Abdel AlimR.A.AbdeS.H.AhmedSynthesis of some 5-azo(4′-substituted benzene-sulphomoyl)-8-hydroxyquinolines with antidotal and antibacterial activitiesJ. Inorg. Biochem.33219887789
- M.TonelliI.VazzanaB.JassoV.BoidaF.Sparatoreet alAntiviral and cytotoxic activities of aminoarylazo compounds and aryltriazene derivativesBiorg. Med. Chem.17200944254440
- K.R.RaghavendraK.Ajay KumarSynthesis of some novel Azodyes and their dyeing. Redox and antifungal propertiesInt. J. ChemTech Res.52201317561760
- A.A.H.KadhamA.A.Al-AmieryA.Y.MusaA.B.MohamadThe antioxidant activity of new coumarin derivativesInt. J. Mol. Sci.12201157475761
- T.A.FarghalyZ.A.AbdullaSynthesis azo-hydrazone tautomerism and antitumor screening of N-(3-ethoxy carbonyl-4,5, 6, 7-tetrahydro-benz[b] thien-2-yl)-2-arylhydrazono-3-oxobutanamide derivativesARKIVOCxvii2008295305
- L.Hui-LingL.ZongchengA.ThorleifSynthesis and fungicidal activity of 2-Imino-3-(4 arylthiazol-2-yl)-thiazolidine-4-ones and their 5-arylidene derivativesMolecules5200010551061
- R.GargS.P.GuptaH.GaoM.S.BabuA.K.DebanathC.HanschComparative quantitative structure minus sign activity relationship studies on anti-HIV drugsChem. Rev.99199935253602
- P.LaurinD.FerrondM.KlichC.D.HamelinP.MavaisP.LassaigneA.BonnefoyB.MusicksSynthesis and in vitro evaluation of novel highly potent coumarin inhibitors of gyrase BBiorg. Med. Chem. Lett.9199920792084
- N.TamerB.SamirY.MahmoudAnticancer activity of new coumarin substituted hydrazide–hydrazone derivativesEur. J. Med. Chem.7692014539548
- J.Jae-ChulP.Oee-SookSynthetic approaches and biological activities of 4′-hydroxycoumarin derivativesMolecules14200947904803
- A.S.Al-AyedN.HamdiA new and efficient method for the synthesis of novel 3-acetyl coumarins oxadiazoles derivatives with expected biological activityMolecules1912014911924
- N.HamdiF.BouabdallahA.RomerosaA novel class of potential antibacterial and antioxidant agentsC. R. Chim.13201012611268
- P.SharmaS.PritimaniSynthesis characterisation and antimicrobial studies of some Novel 3-Arylazo-7-hydroxy-4-methylcoumarinsInd. J. Chem.38B199911391142
- P.ManojkumarT.K.RaviS.GopalkrishnanAntioxidant and antibacterial activities of arylazopyrazoles and arylhydrazopyrazolones containing Coumarin moietyEur. J. Med. Chem.4411200946904694
- S.ValentinaM.DimitrisM.GeorgiaA.Antreaset alFunctionalized 4-hydroxy coumarins; novel synthesis, crystal structure and DFT calculationsMolecules162011384402
- M.R.YazdanbakhshA.GhanadzadehE.MoradiSynthesis of some new azo dyes derived from 4-hydroxy coumarin and spectrometric determination of their acidic dissociation constantsJ. Mol. Liq.1362007165168
- J.C.JungY.J.JungO.S.ParkA convenient one-pot synthesis of 4-hydroxy coumarin, 4-hydroxy thiocoumarin and 4-hydroxy quinolin-2(1H)-oneSynth. Commun.31200111951200
- S.J.ParkJ.C.LeeK.L.LeeA facile synthesis of 4-hydroxycoumarin and 4-hydroxy-2-quinolone derivativesBull. Korean Chem. Soc.287200712031205
- H.W.SeeleyP.J.Van DenmarkA Laboratory Manual of Microbiology2nd ed.1975D. B. Taraporewala Sons and Co.Bombay5580
- B.R.DekicN.S.RadulovicV.S.DekicR.D.VukicevicR.M.PalicSynthesis and antimicrobial activity of new 4-hetero arylamino heteroatomsMolecules15201022462256
- B.D.CullityElements of X-ray Diffractions1959Wesley publishing Co.England8588
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jtusci.2014.08.001.