?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Ampicillin [(2S,6R)-6-(2-(aminomethyl)benzamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0) heptanes-2-carboxylic acid], an antibiotic drug was investigated as corrosion inhibitor for mild steel in HCl using gravimetric method. The results obtained showed that various concentrations of ampicillin studied inhibited the corrosion of mild steel in solutions of HCl. The inhibition efficiency increased with increase in the concentrations of ampicillin and decreased with increase in temperature of which the inhibitor of concentration 5 × 10−3 M at 30 °C had the highest inhibition efficiency of 75.85%. Its adsorption was found to be physical, exothermic and spontaneous as confirmed by values of activation energy and free energy of adsorption (around −20 kJ mol−1 for adsorption and below 80 kJ mol−1 for activation energy) and also fitted the Langmuir adsorption model. Quantum chemical calculations results show that ampicillin possesses a number of active centers concentrated mainly on the nitrogen atoms and the neighboring C atoms. The HOMO and LUMO plots of ampicillin further present ampicillin as an effective corrosion inhibitor.
1 Introduction
Corrosion is the deterioration of metal by chemical attack or reaction with its environment. It is a constant and continuous problem, often difficult to eliminate completely. Prevention would be more practical and achievable than complete elimination. Corrosion processes develop fast after disruption of the protective barrier and are accompanied by a number of reactions that change the composition and properties of both the metal surface and the local environment, for example, formation of oxides, diffusion of metal cations into the coating matrix, local pH changes, and electrochemical potential. The study of corrosion of mild steel and iron is a matter of tremendous theoretical and practical concern and as such has received a considerable amount of interest. Acid solutions, widely used in industrial acid cleaning, acid descaling, acid pickling, and oil well acidizing, require the use of corrosion inhibitors in order to restrain their corrosion attack on metallic materials [Citation1].
The use of inhibitors is one of the best options of protecting metals against corrosion. Several inhibitors in use are either synthesized from cheap raw material or chosen from compounds having heteroatoms in their aromatic or long-chain carbon system [Citation2]. However, most of these inhibitors are toxic to the environment. This has prompted the search for green corrosion inhibitors. Green corrosion inhibitors are biodegradable and do not contain heavy metals or other toxic compounds. Green inhibitors include plant extracts and drugs [Citation3–Citation11].
Recently, quantitative structure activity relationship (QSAR) has been a subject of intense interest in many disciplines of chemistry. The development of semi-empirical quantum chemical calculations emphasizes the scientific approaches involved in the selection of inhibitors by correlating the experimental data with quantum-chemical properties. Energy of the highest occupied molecular orbital (EHOMO), energy of the lowest unoccupied molecular orbital (ELUMO), separation energy (ELUMO − EHOMO), charges on reactive center, substituent constant (Log P), polarizability, dipole moment (μ) and conformations of molecules have been used to achieve the appropriate correlations [Citation2].
The use of ampicillin as corrosion inhibitor as been sparely reported, thus a deeper investigation into its use as corrosion inhibitors.
Therefore the objective of this study is to present an experimental and theoretical approach on the adsorption, electronic and molecular structure of ampicillin as corrosion inhibitor.
2 Experimental techniques
2.1 Materials
Materials used for the study were mild steel sheets of composition (wt.%); Mn (0.6), P (0.36), C (0.15), Si (0.03) and Fe (98.86). Each sheet was mechanically pressed cut to form different coupons, each of dimension 2 cm × 2 cm × 0.036 cm. Each coupon was degreased by washing with ethanol, dipped in acetone and allowed to air dry before they were preserved in a desiccator. All reagents used for the study were Analar grade and distilled water was used for their preparation. An aqueous solution of 1 M HCl was used as a blank solution. Ampicillin was added to the acid in concentrations ranging from 1 × 10−3 to 5 × 10−3 M.
2.2 Gravimetric (weight loss) method
In the gravimetric experiment, a previously weighed mild steel coupon was completely immersed in 50 mL of the test solution in an open beaker. The beaker was inserted into a water bath maintained at 303 K (30 °C). The coupons were then retrieved after 8 h of immersion. Each coupon, at retrieval was washed in a solution containing 50% NaOH and 100 g/L of zinc dust. The washed coupons were dipped in acetone and allowed to air dry before re-weighing. The difference in weight was taken as total weight loss. The experiments were repeated at 333 K (60 °C). From the weight loss results, the inhibition efficiency (%IE) of the inhibitor, degree of surface coverage (θ) and corrosion rates (CR) were calculated using Eqs. (1)–(3), respectively [Citation12,Citation13] and displayed in :(1)
(1)
(2)
(2)
(3)
(3) where W1 and W2 are the weight losses (g) for mild steel in the presence and absence of the inhibitor in HCl solution, θ is the degree of surface coverage of the inhibitor, A is the area of the mild steel coupon (in cm2), t is the period of immersion (in hours) and W is the weight loss of mild steel after time, t. All the measurements were performed in triplicate and the mean value recorded.
Table 1 Corrosion parameters for mild steel in 1 M HCl in the presence and absence of different concentrations of ampicillin at different temperatures.
2.3 Theoretical approach
All the quantum chemical calculations were performed with SPARTAN’10 V112 semi empirical program using AM1 method. The following quantum chemical indices were considered: Energy of the highest occupied molecular orbital (EHOMO), Energy of the lowest unoccupied molecular orbital (ELUMO), separation energy (ELUMO − EHOMO), dipole moment (μ), and atomic charges of ampicillin.
3 Results and discussion
3.1 Effect of concentration and temperature
The weight loss method of monitoring corrosion rate is useful because of its simple application and reliability [Citation14]. The corrosion rate of mild steel in the absence and presence of ampicillin at 30 °C and 60 °C was studied using the weight loss method. shows the calculated values of corrosion rates (mg cm−2 h−1), inhibition efficiency (IE%) and the degree of surface coverage for dissolution of mild steel in 1 M HCL in the presence and absence of ampicillin. From the values obtained, it is obvious that there is a decrease in the corrosion rate of mild steel in the presence of ampicillin when compared to the blank. The corrosion rate also decreases with an increase in the concentration of ampicillin. This indicates that the ampicillin in the solution inhibits the corrosion of mild steel in HCL and that the extent of corrosion inhibition depends on the amount of ampicillin present. Furthermore, an increase in temperature leads to an increase in corrosion rate regardless of the concentration of inhibitor (ampicillin) present. This can be attributed to the fact that the rate of chemical reaction increases with increase in temperature.
From , it is observed that the IE (%) for ampicillin reaches a maximum value of 75.85% inhibition with the highest concentration at 5 × 10−3 M at 30 °C. Also, it is observed that there is an increase in inhibition efficiency with increase in the concentration of ampicillin but a decrease with increase in temperature. The decrease in inhibition efficiency with increase in temperature is suggestive of physical adsorption mechanism (physiosorption) and may be attributed to increase in the solubility of the protective film of any of the reaction products precipitated on the surface of the mild steel that may otherwise inhibit the corrosion process. A possible shift in the adsorption–desorption equilibrium toward desorption of adsorbed inhibitor due to an increase in the kinetic energy of the molecules of the solution, may be another possible cause of the above observed decrease in the inhibition efficiency of ampicillin with an increase in temperature. Thus, as the temperature increases, the number of adsorbed molecules decreases, leading to a decrease in the inhibition efficiency.
Propositions have been made to show that inhibitors slow corrosion processes by:
| (1) | Increasing the anodic or cathodic polarization behavior (Tafel Slopes), | ||||
| (2) | Reducing the movement or diffusion of ions to the metallic surface, and | ||||
| (3) | Increasing the electrical resistance of the metallic surface [Citation14]. | ||||
By convention the entire surface of the corroding metal is affected by organic inhibitors when present in appreciable amount.
3.2 Adsorption isotherm
Adsorption isotherm study describes the adsorptive behavior of organic inhibitors to know the adsorption mechanism [Citation15]. It depends mainly on the nature and charge of the metal surface, adsorption of solvent and other ionic species, electronic characteristics of the metal surface, temperature of the corrosion reaction and the electrochemical potential at solution interface.
The description of adsorption processes can be achieved via the adoption of adsorption isotherms. The most frequently used isotherms include: Langmuir, Frumkin, Hill de Boer, Flory-Huggins, Parsons–Temkin, Dhar–Flory-Huggins, and Bockris–Swinkels. The establishment of adsorption isotherms that describe the adsorption of a corrosion inhibitor can provide important clues to the nature of the metal–inhibitor interaction. Adsorption of the organic molecules occurs as the interaction energy between molecule and metal surface is higher than that between the water molecule and the metal surface ().
In order to obtain the adsorption isotherm, the degree of surface coverage (θ) for various concentrations of the inhibitor has been calculated using Eq. Equation(4)(4)
(4) . Langmuir isotherm was tested for its fit to the experimental data. Langmuir isotherm is given by
(4)
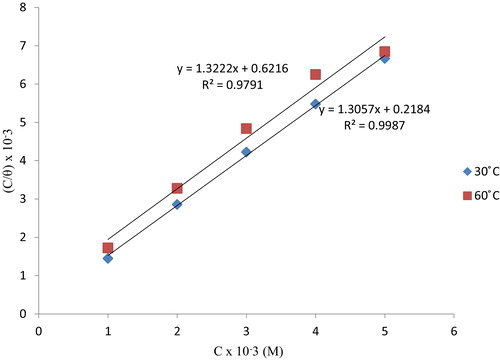
(4) where θ is the degree of surface coverage, C the molar inhibitor concentration in the bulk solution and Kads is the equilibrium constant of the process of adsorption. The plot of C/θ versus C was linear according to with a correlation of 0.998 at 30 °C and 0.979 at 60 °C, with a negligible deviation of the slopes from unity (). This seems to suggest that the Langmuir adsorption isotherm model provides a good description of the adsorption behavior. Langmuir isotherm assumes that:
| (1) | The metal surface contains a fixed number of adsorption sites and each site holds one adsorbate. | ||||
| (2) | ΔGads is the same for all sites and it is independent of θ. | ||||
| (3) | The adsorbates do not interact with one another, i.e. there is no effect of lateral interaction of the adsorbates [Citation13]. | ||||
Table 2 Calculated parameters from Langmuir isotherm.
3.3 Kinetic and thermodynamic considerations
The relationship between the percentage inhibition efficiency (IE%) of an inhibitor, temperature and the activation energy of in the presence of an inhibitor was given as follows [Citation16]:
| (1) | Inhibitor whose IE (%) decreases with increase in temperature. The value of activation energy (Ea) found is greater than that in the uninhibited solution. | ||||
| (2) | Inhibitors with IE (%) that does not change with variation in temperature (i.e. either an increase or decrease). The activation energy (Ea) does not change in the presence or absence of inhibitors. | ||||
| (3) | Inhibitors whose IE (%) tend to increase with an increase in temperature. The value of activation energy (Ea) found is less than that of the uninhibited solution. | ||||
A higher value of the activation energy (Ea) of the process in an inhibitor's presence when compared to that in its absence can be attributed to physiosorption.
To further show ampicillin adsorbed to mild steel via physiosorption, the values of activation energy (Ea) were calculated with the help of Arrhenius equation(5)
(5) where CR1 and CR2 represent the corrosion rates at temperature T1 and T2, respectively. The values are as shown in . An increase in the value of Ea in the presence of ampicillin in comparison to that in its absence, and the decrease of its IE% with temperature increase can be interpreted as an indication of physical adsorption. Also, according to Ameh [Citation17], it is expected that for chemical adsorption, the values of activation energy should be 80 kJ mol−1 greater. With the range of Ea observed in (38.00–41.16 kJ mol−1) it further confirms physical adsorption.
Table 3 Calculated values of activation energy (Ea) and heat of adsorption (Qads) for mild steel dissolution in 1 M HCl in the absence and presence of ampicillin at 30 and 60 °C.
An estimate of heat of adsorption was also obtained for the trend of surface coverage with temperature as follows [Citation18]:(6)
(6) where θ1 and θ2 are the degrees of surface coverage and the temperatures T1 and T2.
The calculated values for Qads are presented in . Values of Qads calculated through Eq. Equation(6)(6)
(6) ranged from −2.91 to −13.35 kJ mol−1. These values are negative indicating that the adsorption of ampicillin on mild steel is exothermic [Citation17].
The values of the adsorption equilibrium constant obtained from the intercept of the Langmuir adsorption isotherms are related to the free energy of adsorption according to Eq. Equation(7)(7)
(7) [Citation2]:
(7)
(7) where
is the free energy of the adsorption, R is the gas constant and T is the temperature of the system. Calculated values of the free energies are also presented in . The free transfer of charge from the inhibitor to the metal surface. Therefore, the adsorption of ampicillin was spontaneous.
Generally, values of up to −20 kJ mol−1 signify physiosorption, the inhibition acts due to electrostatic interaction between the charge molecules and the charged metal, while values around −40 kJ mol−1 or more are associated with chemisorption as a result of sharing or transfer of electrons from the organic molecules to the metal surface to form a coordinate type of bond (chemisorption) [Citation2]. The values obtained from this research ranged from −12.44 kJ mol−1 to −14.00 kJ mol−1, which supports the mechanism of physical adsorption.
3.4 Quantum chemical studies
In general, it is unusual to include quantum chemical calculations in corrosion investigation. However, quantum chemical calculations have obvious advantages compared to existing methods of synthesis and selection of corrosion inhibitors. This approach is not restricted closely to the related compound, as is often the case with group theoretical, topological and others, and it makes interpretation of quantitative structure–activity relationships more straightforward. In addition, the results of quantum chemical calculations could be obtained without laboratory measurements, thus saving time and equipment, alleviating safety and disposal concerns. Besides, the correlations between inhibition efficiency and molecular parameters can be used for pre-selection of new inhibitors, which are, at the moment, taken essentially from empirical knowledge. These facts have made quantum chemical calculation to be very powerful tool for studying corrosion inhibition mechanism [Citation14].
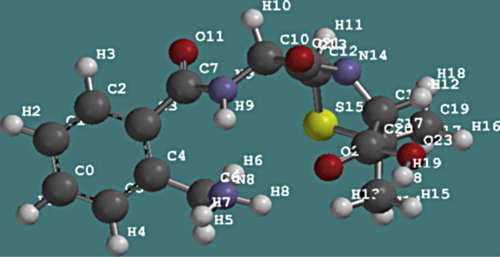
The quantum chemical parameters of ampicillin were calculated and listed in . The AM1 optimized geometry of ampicillin is shown in . Information about the adsorption centers showing the calculated total partial atomic charges (TPAC) in ampicillin molecule is presented in .
Table 4 Quantum parameters of ampicillin obtained by SPARTAN’10.
Table 5 The charge densities of the ampicillin molecule.
Frontier orbital theory was useful in predicting the adsorption centers of the inhibitor molecule responsible for the interaction with surface atoms [Citation14]. The energy of the highest occupied molecular orbital (EHOMO) measures the tendency toward the donation of electron by a molecule. Therefore, higher values of EHOMO indicate better tendency toward the donation of electron, enhancing the adsorption of the inhibitor on mild steel and therefore better inhibition efficiency. On the other hand, the energy of the lowest unoccupied molecular orbital (ELUMO) indicates the tendency toward the acceptance of electron. Therefore, the lower the value of ELUMO, the better the expected inhibition efficiency. The difference between the ELUMO and EHOMO is the energy gap of the molecule (i.e. EL–H = ELUMO − EHOMO). This separation energy is also responsible for the softness or hardness of the molecule. Soft molecules are more reactive than hard molecules [Citation12]. Thus the HOMO and LUMO properties of ampicillin were plotted as shown in and . It is evident from that ampicillin showed a high value of HOMO energy and a low value of LUMO energy, which was in favor of bonding with metal surface [Citation19,Citation20].
Fig. 4 HOMO energy density of ampicillin molecule showing electron rich centers on the ring containing the sulphur atom.
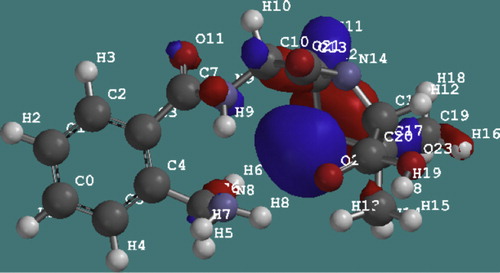
Fig. 5 LUMO energy of the ampicillin molecule showing electron deficient molecules at the benzene ring.
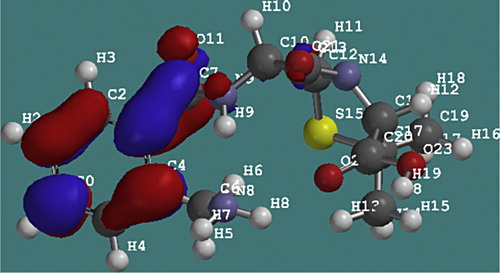
As EHOMO is often associated with the electron donating activity of the molecule, high values of EHOMO are likely to indicate a tendency of the molecule to donate to appropriate acceptor molecule with low energy and empty molecular orbital. The electronic configuration of Iron is 1s22s22p63s23p63d64s2. The incompletely filled 3d orbital of the Iron could bond with the HOMO of ampicillin while its filled 4s orbital could interact with the LUMO of ampicillin.
From the values of the atomic charges (), it can be concluded that the negative charge is concentrated on the N atoms and also around the C atoms both on and surrounding the five membered ring containing the sulphur atom. Accordingly, the ampicillin molecule can be adsorbed on the surface of the mild steel leading to corrosion inhibition action.
The obtained results from this quantum study of the ampicillin molecule show that more than one adsorption site could be obtained from one molecule of ampicillin. This result is in good agreement with the Langmuir plots obtained from the experimental observations of ampicillin.
4 Conclusions
The following conclusions were drawn for this study:
| (1) | Ampicillin was found to be a good inhibitor of mild steel corrosion in acid medium. | ||||
| (2) | Inhibition efficiency increased with an increase in ampicillin concentration but decreased with increase in temperature. | ||||
| (3) | The inhibition efficiency increased with increase in the concentrations of ampicillin and decreased with increase in temperature. In this study the inhibitor of concentration 5 × 10−3 M at 30 °C had the highest inhibition efficiency of 75.85%. | ||||
| (4) | The free energy of adsorption indicates that the process was spontaneous. | ||||
| (5) | Quantum chemical calculation showed that ampicillin had multiple sites of adsorption. | ||||
Notes
Peer review under responsibility of Taibah University.
References
- A.OstovariS.M.HoseiniehM.PeikariS.R.ShadizadehS.J.HashemiCorrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (lawsone, gallic acid, a-d-glucose and tannic acid)Corros. Sci.51200919351949
- E.E.EbensoD.A.IsabiryeN.O.EddyAdsorption and quantum chemical studies on the inhibition potentials of some thiosemicarbazides for the corrosion of mild steel in acidic mediumInt. J. Mol. Sci.11201024732498
- B.E.Amitha RaniB.B.BharathiGreen inhibitors for corrosion protection of metals and alloys: an overviewInt. J. Corros.20122011115
- N.O.EddyE.E.EbensoQuantum chemical studies on the inhibition potentials of some penicillin compounds for the corrosion of mild steel in 0.1 M HClInt. J. Mol. Sci.1162011 b24732498
- A.Y.El-EtreInhibition of aluminum corrosion using Opuntia extractCorros. Sci.4511200324852495
- U.J.EkpeE.E.EbensoU.J.IbokInhibitory action of Azadirachta indica leaves extract on the corrosion of mild steel in H2SO4West Afr. J. Biol. Appl. Chem.3719941330
- O.K.AbiolaN.C.OforkaE.E.EbensoN.M.NwinukaEco-friendly corrosion inhibitors: the inhibitive action of Delonix regia extract for the corrosion of aluminium in acidic mediaAnti-Corros. Methods Mater.5442007219224
- M.KliskicJ.RadoservicS.GudicV.KatalinicAqueous extract of Rosmarinus officinalis L. as inhibitor of Al–Mg alloy corrosion in chloride solutionJ. Appl. Electrochem.3072000823830
- N.O.EddyE.E.EbensoAdsorption and quantum chemical studies on cloxacillin and halides for the corrosion of mild steel in acidic mediumInt. J. Electrochem. Sci.562010731750
- S.K.ShuklaA.K.SinghI.AhamadM.A.QuraishiStreptomycin A commercially available drug as corrosion inhibitor for mild steel in hydrochloric acid solutionMater. Lett.632009819822
- P.C.OkaforE.E.EbensoInhibitive action of Carica papaya extracts on the corrosion of mild steel in acidic media and their adsorption characteristicsPigment Resin Technol.3632007134140
- O.N.EddyB.I.ItaN.E.IbisiE.E.EbensoExperimental and quantum chemical studies on the corrosion inhibition potentials of 2-(2-oxoindoline-3-ylideneamino) acetic acid and indoline-2,3-dioneInt. J. Electrochem. Sci.6201110271044
- A.A.El MaghrabyT.Y.SororQuaternary ammonium salt as effective corrosion inhibitor for carbon steel dissolution in sulphuric acid mediaAdv. Appl. Sci. Res.122003143155
- I.B.ObotN.O.Obi-EgbediS.A.UmorenAdsorption characteristics and corrosion inhibitive properties of clotrimazole for aluminum corrosion in hydrochloric acidInt. J. Electrochem. Sci.42009863877
- D.B.HmamouR.SalghiA.ZarroukM.MessaliH.ZarrokM.ErramiB.HammoutiL.BazziA.ChakirInhibition of steel corrosion in hydrochloric acid solution by chamomile extractDer Pharma Chem.4201214961505
- P.M.NiameinA.A.KoffiA.TrokoureyAdsorption and inhibitive effects of 2-thiobenzylbenzimidazole (TBBI) at aluminum alloy AA3003/1 M hydrochloric acid interfaceInt. Res. J. Pure Appl. Chem.42012268304
- P.O.AmehL.MagajiT.SalihuCorrosion inhibition and adsorption behavior for mild steel by Ficus glumosa gum in H2SO4 solutionJ. Pure Appl. Chem.62012100106
- A.A.SiakaN.O.EddyS.O.IdrisA.MohammedC.M.ElingeF.A.AtikuFTIR spectroscopic information on the corrosion inhibition potentials of ampicillin in HCL solutionInnov. Sci. Eng.220124148
- P.UdhayakalaT.V.RajendiranS.GunasekaranTheoretical assessment of inhibitive behaviour of some benzohydrazide derivatives on mild steelJ. Adv. Sci. Res.320128288
- H.El SayedA.El NemrA.SamyS.RagabCorrosion inhibitors part 31: quantum chemical studies on the efficiencies of some aromatic hydrazides and Schiff bases as corrosion inhibitors of steel in acidic mediumArkivoc62006205220