?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The photo-stabilization of polymers derived from poly(vinyl chloride) (PVC) films was investigated. The PVC and modified PVC films were produced by the casting method from tetrahydrofuran (THF) solvent. The photostabilization activities of these compounds were determined by monitoring the carbonyl, polyene and hydroxyl indices with irradiation time. The changes in viscosity average molecular weight of PVC with irradiation time were also tracked (using THF as a solvent). The quantum yield of the chain scission (Φcs) of these complexes in PVC films was evaluated and found to range between 4.79 × 10−8 and 7.50 × 10−8. Results obtained showed that the rate of photostabilization of PVC in the presence of the additive followed the trend:
1 Introduction
Amines are organic compound and functional group that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group [Citation1]. Important amines include amino acids, biogenic amines, aniline and trimethylamine. Poly(vinyl chloride), otherwise known as PVC is a thermoplastic and the third largest production polymer in the world [Citation2,Citation3].
A wide variety of synthetic and naturally occurring high polymers absorb solar ultraviolet radiation and undergo photolytic, photo-oxidative, and thermo-oxidative reactions that result in the degradation of the material [Citation4–Citation7]. The degradation suffered by these materials can range from mere surface discoloration affecting the esthetic appeal of a product to extensive loss of mechanical properties, which severely limits their performance.
The low cost and excellent performance of poly(vinyl chloride) (PVC) make it a very attractive and suitable plastic for a wide variety of applications. With respect to the production and consumption of synthetic materials, it stands third in the world after polyethylene and polypropylene. However, PVC suffers from poor thermal and light stability. It undergoes rapid autocatalytic dehydrochlorination upon exposure to heat and light [Citation8–Citation10] during its molding and use, respectively. As a result, conjugated polyene sequences are formed from the beginning of the reaction, and they give rise to discoloration of the polymer and seriously change its physical properties [Citation11,Citation12]. Degradation also causes a drastic change in the mechanical properties of the polymer, which is accompanied by a decrease or increase in its average molecular weight as a result of either chain scission or cross-linking of the polymer molecules, respectively [Citation13,Citation14]. Various defect sites in the polymer chain are thought to be responsible for this instability. Possible defect structures in PVC chains are allylic chlorine, tertiary hydrogen and chlorine atoms, end groups such as double bonds, oxygen-containing groups, peroxide residues, and head-to-head structures [Citation15,Citation16]. In addition to these abnormalities, the steric order of the monomer units (tacticity) may have some influence on the degradation. The dehydrochlorination most likely proceeds by a chain mechanism involving radical intermediates. However, ultimate user acceptance of the PVC products for outdoor building applications will depend on their ability to resist photodegradation over long periods of sunlight exposure. To ensure weatherability, the PVC resin needs to be compounded and processed properly using suitable additives, leading to a complex material whose behavior and properties are quite different from the PVC resin by itself [Citation17–Citation20]. Recently, scientists have used substituted benzothiazole and benzimidozole ring [Citation21] as photostabilizers for rigid PVC. They have also used 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives as novel photostabilizers for rigid PVC [Citation22]. In this article we report the designing of some PVC – Amiens modified polymers and studied their use as photostabilizing reagent.
2 Experimental
2.1 Materials
The following polymers derived from poly(vinyl chloride) were all prepared by the method previously described by Al-Daeif et al., 2012 [Citation23]. Synthesis of PVC-Ligand compounds (0.1 g) of PVC was dissolved in dry THF (20 ml), then add (0.05 mole) from appropriate amine. The reaction mixture was heated under reflux for 6 h. The hot mixture was transferred to pit-reddish, the precipitate modified polymer separated as film by evaporated the solvent.
P1 > P2 > P3 > P4 > P5
Table
2.2 Experimental techniques
2.2.1 Films preparation
A solution of poly(vinyl chloride) solution or modified poly(vinyl chloride) (5 g/100 ml) in tetrahydrofuran was used to prepare (30 μm) thickness of polymer films, (measured by a micrometer type 2610 A, Germany). The films were prepared by evaporation technique at room temperature for 24 h. To remove the possible residual tetrahydrofuran solvent, film samples were further dried at room temperature for 3 h under reduced pressure.
2.2.2 Irradiation experiments
2.2.2.1 Accelerated testing technique
Accelerated weatherometer Q.U.V. tester (Q. panel, company, USA), was used for irradiation of polymers films. The accelerated weathering tester contains stainless steel plate, which has two holes in the front side and a third one behind. Each side contains a lamp (type Fluorescent Ultraviolet Lights) 40 Watt each. These lamps are of the type UV-B 313 giving spectrum range between 290 and 360 nm with a maximum wavelength at 313 nm. The polymer film samples were vertically fixed parallel to the lamps to make sure that the UV incident radiation is perpendicular to the samples. The irradiated samples are rotated from time to time to ensure that the intensity of light incident on all samples is the same.
2.2.3 Photodegradation measuring methods
2.2.3.1 Measuring the photodegradation rate of polymer films using infrared spectrophotometery
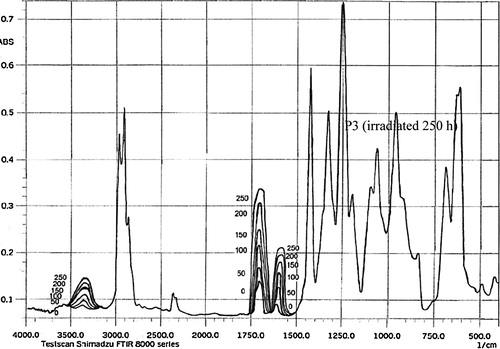
The degree of photodegradation of polymer film samples was followed by monitoring FTIR spectra in the range 4000–400 cm−1 using FTIR 8300 Shimadzu spectrophotometer. The position of carbonyl absorption is specified at 1722 cm−1, polyene group at 1602 cm−1 and the hydroxyl group at 3500 cm−1 [Citation10,Citation24]. The progress of photodegradation during different irradiation time was followed by observing the changes in carbonyl and polyene peaks. Then carbonyl (Ico), polyene (Ipo) and hydroxyl (IOH) indices were calculated by comparison of the FTIR absorption peak at 1722, 1602 and 3500 cm−1 with reference peak at 1328 cm−1, respectively. This method is called band index method which includes.(1)
(1) where As = absorbance of peak under study, Ar = absorbance of reference peak, and Is = index of group under study.
Actual absorbance, the difference between the absorbance of top peak and base line (A top peak – A base line) is calculated using the base line method.
2.2.3.2 Determination of average molecular weight 
 using viscometry method
using viscometry method
The viscosity property was used to determine the average molecular weight of polymer, using the Mark Houwink relation [Citation25].(2)
(2) [η] = the intrinsic viscosity.
K and α are constants depend upon the polymer–solvent system at a particular temperature.
The intrinsic viscosity of a polymer solution was measured with an Ostwald U-tube viscometer. Solutions were made by dissolving the polymer in a solvent (g/100 ml) and the flow times of polymer solution and pure solvent are t and t0 respectively. Specific viscosity (ηsp) was calculated as follows:(3)
(3) ηre = relative viscosity.
(4)
(4)
The single-point measurements were converted to intrinsic viscosities by the relation Equation(2)(2)
(2) .
(5)
(5) C = concentration of polymer solution (g/100 ml).
By applying Eq. Equation(5)(5)
(5) , the molecular weight of degraded and virgin polymer can be calculated. Molecular weights of PVC with and without additives were calculated from intrinsic viscosities measured in THF solution using the following equation:
(6)
(6)
The quantum yield of main chain scission (Φcs) [Citation26] was calculated from viscosity measurement using the following relation Equation(7)(7)
(7) .
(7)
(7) where C = concentration; A = Avogadro's number;
the initial viscosity − average molecular weight; [ηo] = Intrinsic viscosity of PVC before irradiation; Io = incident intensity and t = irradiation time in second.
3 Results and discussion
The polymers derived from PVC were used as additives for the photostabilization of PVC films. In order to study the photochemical activity of these additives for the photostabilization of PVC films, the carbonyl and polyene indices were monitored with irradiation time using IR spectrophotometry. The irradiation of PVC films with UV light of wavelength, λ = 313 nm led to a clear change in the FTIR spectrum, as shown in . Appearance of bands at 1772 cm−1 and 1724 cm−1, were attributed to the formation of carbonyl groups related to chloroketone and to aliphatic ketone, respectively. A third band was observed at 1604 cm−1, related to polyene group. The hydroxyl band appeared at 3500 cm−1 was annotated to the OH– of the hydroperoxides group as shown in .
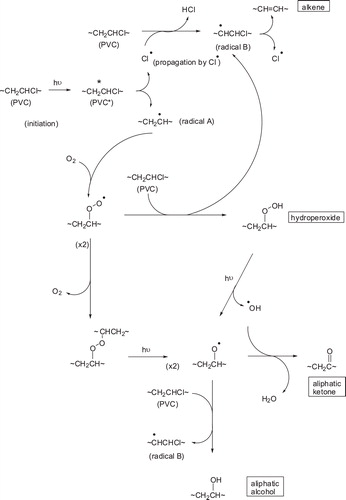
Scheme 1 Some of the photooxidation processes of PVC; reactions of radical B with molecular oxygen, akin to those of radical A, would give rise to chloroketone, chloroalcohol and chlorohydroperoxide moieties; further reactions of such reactive functionalities may lead to chain cleavage and reduction in molecular weight.
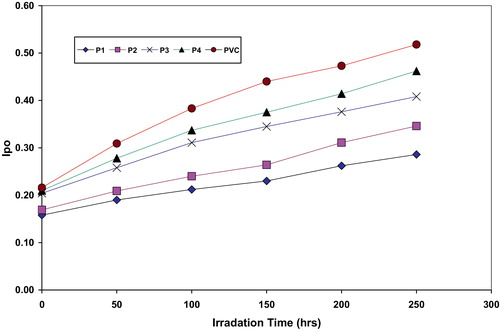
The absorption of the carbonyl, polyene and hydroxyl groups was used to follow the extent of polymer degradation during irradiation. This absorption was calculated as carbonyl index (Ico), polyene index (IPO) and hydroxyl index (IOH). It is reasonable to assume that the growth of carbonyl index is a measure to the extent of degradation. However, in , the Ico of P4, P3, P2 and P1 showed lower growth rate with irradiation time with respect to the PVC control film without additives. Since the growth of carbonyl index with irradiation time is lower than PVC control, as seen in , it is suitable to conclude that these additives might be considered as photostabilizers of PVC polymer. Since efficient photostabilizer shows a longer induction period, therefore, the P1 is considered as the most active photostabilizer, followed by P2, P3 and P4 which is the least active. Just like carbonyl, polyene compounds are also produced during photodegradation of PVC. Therefore, polyene index (IPO) could also be monitored with irradiation time in the presence and absence of these additives. Results are shown in .
Fig. 2 The relationship between the carbonyl index and irradiation time for PVC films and modified PVC films (30 μm thickness).
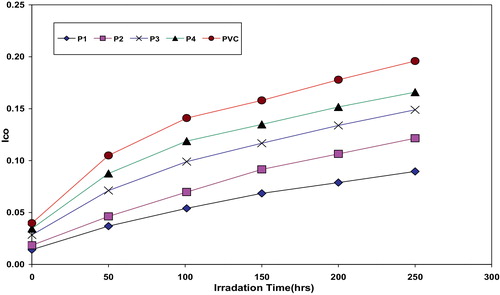
Fig. 3 The relationship between the polyene index and irradiation time for PVC films and modified PVC films (30 μm thickness).
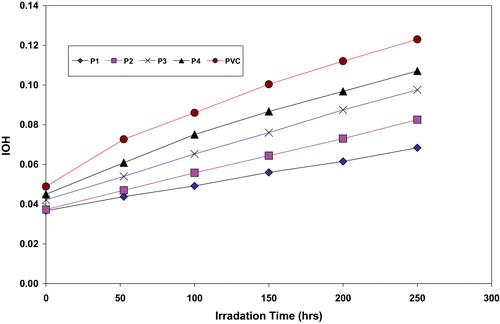
Hydroxyl species were produced during photodegradation of PVC. Therefore, hydroxyl index (IOH) was monitored with irradiation time for PVC and with additives. From the P4, P3, P2 and P1 showed lower growth rate of hydroxyl index with irradiation time compared to PVC blank.
Fig. 4 The relationship between the hydroxyl index and irradiation time for PVC films and modified PVC films (30 μm thickness).
3.1 Variation of PVC molecular weight during photolysis of modified polymers
Analysis of the relative changes in viscosity average molecular weight , has been shown to provide a versatile test for random chain scission. shows the plot of
versus irradiation time for PVC film modified polymers, with absorbed light intensity of 1.065 × 10−8 ein. dm−3 s−1.
is measured using Eq. Equation(4)
(4)
(4) with THF as a solvent at 25 °C.
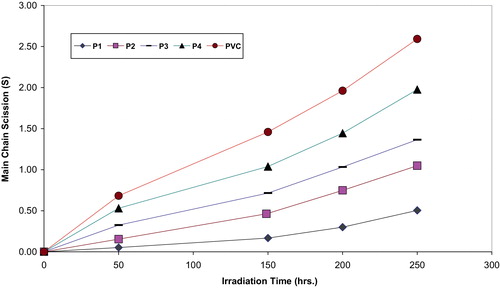
It is worth mentioning that traces of the films with additives are not soluble in THF indicating that cross-linking or branching in the PVC chain does occur during the course of photolysis. For better support of this view, the number of average chain scission (average number cut per single chain) (S) [Citation27] was calculated using the relation Equation(8)(8)
(8) :
(8)
(8) where
and
are viscosity average molecular weight at initial (0) and t irradiation time respectively. The plot of S versus time is shown in . The curve indicates an increase in the degree of branching such as that might arise from cross-linking occurrence. It is observed that insoluble material was formed during irradiation which provided additional evidence to the idea that cross-linking does occur.
Fig. 5 Changes in the viscosity-average molecular weight during irradiation of PVC films (30 μm) (control) and modified PVC films (30 μm thickness).
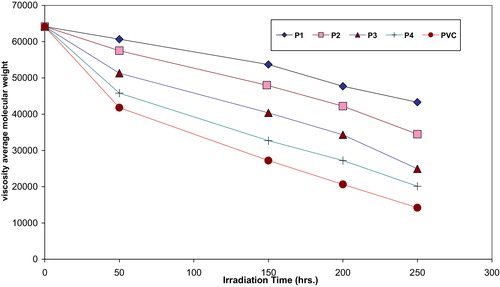
Fig. 6 Changes in the main chain scission (S) during irradiation of PVC films (30 μm) (control) and with modified PVC films (30 μm thickness).
For randomly distributed weak bond links, which break rapidly in the initial stages of photodegradation, the degree of deterioration α [Citation28] is given as:(9)
(9) where m is the initial molecular weight.
The plot of α as a function of irradiation time is shown in .
Fig. 7 Changes in the degree of deterioration (α) during irradiation of PVC films (30 μm) (control) and modified PVC films (30 μm thickness).
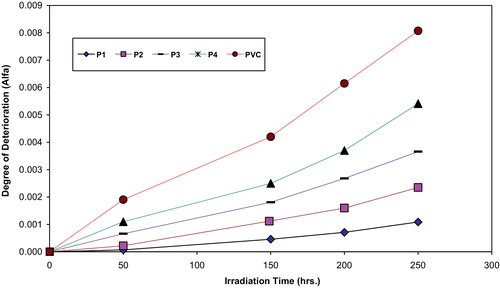
The values of α of the irradiated samples are higher when additives are absent and lower in the presence of additives compared to the corresponding values of the additive free PVC. In the initial stages of photodegradation of PVC, the value of α increases rapidly with time, this indicates a random breaking of bonds in the polymer chain.
3.2 Surface morphology as a criterion of the photostabilizing compounds
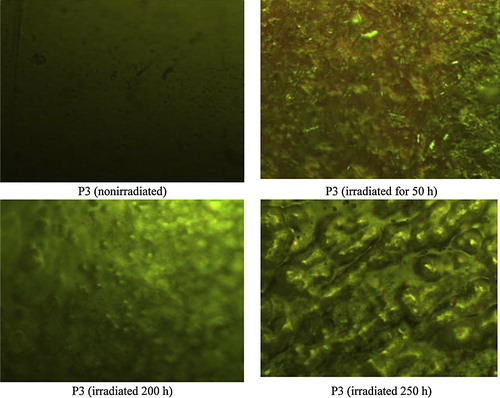
The morphological study for the surface of polymers gives a clear Photo about some of the physical properties of the polymer For example, the crystalline case, irregular surface molecules, smoothing the surface and how to build it. And also shows the nature of the surface defects resulting from Photons of light interaction with the polymer molecule [Citation29,Citation30]. The morphological study also can follow photodecomposition or stabilization of polymers that exposed to the UV light through that appear on the surface of polymers, and see whether that the decomposition process can occur on chain scission or Decomposition for substitutes groups. The morphologic studies also gives a clear indication for the polymers resistance to weathering and how to produce tough polymers as well as to know deformation that occur when polymer exposed to weathering conditions.
The surface morphology of films of the nonirradiated PVC blank, PVC and modified PVC in the presence of P1, P2, P3 and P4 (irradiated for 0 h, 50 h. 100 h, 150 h, 200 and 250 h) was studied with Biolab microscope. The PVC (blank) film surface was smooth and empty of any white spots indicating degradation, whereas the PVC film surface irradiated for 250 h was full of white spots indicating the holes or grooves in which HCl evolved. In the case of modified polymers the surface was almost smooth, and fewer white spots appeared; this indicated the great stabilizing efficiency of the investigated the stabilizer and how much it protected the polymer surface from deterioration via dehydrochlorination ().
A method of degradation reaction characterization is the measurement of the quantum yield of the chain scission (Φcs) [Citation31]. The quantum yield for chain scission was calculated for PVC and modified polymers mentioned above using Eq. Equation(5)(5)
(5) . The Φcs values for complexes are tabulated in .
Table 1 Quantum yield (Φcs) for the chain scission for PVC films (30 μm) thickness with and modified polymers after 250 h irradiation time.
The Φcs values for modified polymers less than that of free PVC (blank), which increase in the order:
The explanation for low values of Φcs is that in macromolecule of PVC, the energy is absorbed at one site, and then the electronic excitation is distributed over many bonds so that the probability of a single bond breaking is small, or the absorbed energy is dissipated by non-reactive processes.
It is well established that the quantum yield (Φcs) increases with increasing temperature around the glass transition temperature, (Tg) of the amorphous polymer, and around the melting temperature of crystalline polymers. In the study presented in this work, the photolysis of PVC film is carried out at a temperature 35–45 °C well below the glass transition temperature (Tg of PVC = 80 °C). Therefore, the Φcs dependency on temperature is not expected to be observed.
4 Conclusions
In the work described in this paper, the photostabilization of modified polymers of poly(vinyl chloride) films were studied. These modified polymers behave successfully as photostabilizer for PVC films. The additives take the following order in photostabilization activity according to their decrease in carbonyl, polyene and hydroxyl indices for PVC films.
P1 > P2 > P3 > P4
These additives stabilize the PVC films through UV absorption or screening, peroxide decomposer and radical scavenger mechanisms. The P1 was found to be the most efficient in photostabilization process according to the photostability and mechanisms mentioned above. The results obtained support the idea of using modified PVC as commercial stabilizer.
Acknowledgment
We are grateful for the financial support provided by Al-Nahrain University and Universiti Kebangsaan Malaysia for funding (codes UKM-GUP-NBT-08-27-113, UKM-GGPM-NBT-164-2010 and UKM-OUP-2012-139) Malaysia.
Notes
Peer review under responsibility of Taibah University.
References
- Mc.MurryE.JohnOrganic Chemistry3rd ed.1992WadsworthBelmont2012 ISBN 0-534-16218-5
- M.AllsoppG.VianelloPoly (Vinyl Chloride) in Ullmann's Encyclopedia of Industrial Chemistry2012Wiley-VCHWeinheim
- M.SabaaR.MohamedE.OrabyVanillin–Schiff's bases as organic thermal stabilizers and co-stabilizers for rigid poly(vinyl chloride)Eur. Polym. J.45200930723080
- A.AndradyS.HamidX.HuA.TorikaiEffects of increased solar ultraviolet radiation on materialsJ. Photochem. Photobiol. B: Biol.46199896103
- E.YousifJ.SalimonN.SalihMechanism of photostabilization of poly(methy methacrylate) films by 2-thioacetic acid benzothiazol complexesArab. J. Chem.72014206311
- S.RabieM.Abd-El-GhaffarA.AhmedA.E.M.SabaaPolyester condensation adducts as novel photostabilizers for polystyreneJ. Vinyl Addit. Technol.192013293301
- S.RabieA.AhmedM.SabaaM.Abd El-GhaffarMaleic diamides as photostabilizers for polystyreneJ. Ind. Eng. Chem.19201318691878
- E.YousifS.AliwiA.AmeerJ.UkalImproved photostability of PVC films in the presence of 2-thioacetic acid-5-phenyl-1, 3, 4-oxadiazole complexesTurk. J. Chem.332009339410
- M.SabaaE.OrabyA.Abdel NabyR.MohamedN-phenyl-3-substituted 5-pyrazolone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photodegradationJ. Appl. Polym. Sci.101200615431555
- E.YousifA.HasanUltra-violet spectra studies of photostabilization rate in PVC films by using some transition metal complexesArab. J. Phys. Chem.220142338
- I.Pimentel RealA.FerrariaA.Botelho do RegoComparison of different photo-oxidation conditions of poly(vinyl chloride) for outdoor applicationsPolym. Test.272008743751
- S.RabieA.KhalilAntimicrobial agents as photostabilizers for rigid poly (vinyl chloride)Polym. Adv. Technol.32201213941402
- E.YousifN.SalihJ.SalimonImprovement of the photostabilization of PVC films in the presence of 2N-salicylidene-5-(substituted)-1,3,4-thiadiazoleJ. Appl. Polym. Sci.120201122072214
- D.BraunS.RabieN.KhaireldinM.Abd El-GhaffarPreparation and evaluation of some benzophenone terpolymers as photostabilizers for rigid PVCJ. Vinyl Addit. Technol.17201147155
- S.CrawleyI.McNeillPreparation and degradation of head-to-head PVCJ. Polym. Sci. Polym. Chem. Ed.10197825932606
- M.SabaaS.RabieR.MohamedNovel antimicrobial and antitumor organic thermal stabilizers for rigid poly(vinyl chloride)J. Therm. Anal. Calorim.109201215031513
- R.RasheedH.MansoorE.YousifA.HameedY.FarinaA.GraisaPhotostabilizing of PVC films by 2-(aryl)-5-[4-(aryloxy)-phenyl]-1,3,4-oxadiazole compoundsEur. J. Sci. Res.302009464477
- S.RabieA.KhalilA.NadaDiamide derivatives as photostabilizers for plasticized poly(vinyl chloride)J. Vinyl Addit. Technol.142008191196
- D.BraunE.RichterS.RabieA.NadaM.Abd-El-GhaffarA.YassinGlucoside derivatives as novel photostabilizers for rigid PVCDie Angewandte Makromolekulare Chemie271199993100
- M.SabaaS.RabieR.MohamedNovel antimicrobial and antitumor organic thermal stabilizers for rigid poly (vinyl chloride)J. Therm. Anal. Calorim.109201215031513
- E.YousifTriorganotin(IV) complexes photo-stabilizers for rigid PVC againstJ. Taibah Univ. Sci.720137987
- E.YousifA.HameedE.BakerSynthesis and photochemical study of poly(vinyl chloride)-1,3,4-oxadiazole and 1,3,4-thiadiazoleJ. Al-Nahrain Univ. Sci.12007711
- Y.Al-DaeifE.YousifM.Abdul-NabiSynthesis of new polymers derived from poly (vinyl chloride) and study their optical propertiesJ. Al-Nahrain Univ. Sci.1520127983
- E.YousifN.SalihJ.SalimonImprovement of the photostabilization of PVC films in the presence of thioacetic acid benzothiazole complexesMalays. J. Anal. Sci.1520118192
- J.MarkPhysical Properties of Polymers Handbook2007SpringerNew York
- J.RabekB.RanbyPhotodegradation, Photooxidation and Photostabilization of Polymers1975WileyNew York
- E.YousifJ.SalimonN.SalihNew stabilizers for polystyrene based on 2-thioacetic acid benzothiazol complexesJ. Appl. Polym. Sci.125201219221927
- H.AdilE.YousifJ.alimonNew Stabilizers for PVC Based on Benzothiazole Complexes2011LAMBERT Academic PublishingGermany
- J.SchultzDeformation Morphologies and Toughening of Polymer System1998349368 (Chapter 8)
- G.ScottJ.E.GuilletPolymers and Ecological Problemsvol. 31973Plenum pressNew York2735
- E.YousifJ.SalimonN.SalihA.AhmedImprovement of the photostabilization of PMMA films in the presence 2N-salicylidene-5-(substituted)-1,3,4-thiadiazoleJ. King Saud Univ. (Sci.)2420121117