Abstract
Soil enzyme activities provide a unique biochemical means for assessing soil function as an indicator of soil fertility, which can be altered by a profusion of fluoride in the soil and seasonal changes. Seven sites were chosen in the fluoride-affected area of Nasipur, Birbhum District, West Bengal, India, to compare seasonal changes in enzymes (urease, amylase, cellulase and invertase), fluoride content, physicochemical characteristics and the availability of microbes in the soil with a control. The activity of all the enzymes varied with season. Urease had greater activity in the summer, followed by winter; it showed marginal differences from the control area during the winter (p < 0.002) and summer (p < 0.110) but a significant (p < 0.000) difference during the rainy season. Soil pH had a negative impact on urease activity during both winter and summer. Cellulase activity was accelerated by the organic matter and organic carbon content of the soil. Fluoride therefore had the greatest activity against urease activity during the rainy, summer and winter seasons. The microbial population of the soil also showed a negative impact of fluoride, which may in turn affect the soil enzymes and characteristics.
1 Introduction
Fluoride is a common geo-genic contaminant of drinking-water, and its effects on humans are well known [Citation1]. An elevated level of fluoride results mainly from weathering of rocks and leaching of fluoride-bearing minerals [Citation2,Citation3]. Fluorosis due to the high fluoride content of water and soil in the villages of Birbhum District in West Bengal, India, has been reported [Citation4,Citation5]. Fluoride can have a negative impact on soil by disrupting soil structure, alkalization, changing the soil adsorption complex and increasing the mobility of humic substances, which promote carbon and nitrogen mineralization [Citation6].
Measurement of the activity of several enzymes has been used to establish indices of soil fertility [Citation7–Citation9] and the bio-geochemical cycling of carbon, nitrogen, phosphorus, sulfur and other nutrients [Citation10]. Soil enzymes originate mainly from plant residues [Citation11], both as intra- and extracellular enzymes [Citation12,Citation13]. Enzymes play the main biochemical role in decomposition of organic matter in soil systems [Citation14,Citation15], and soil microbes reflect soil quality [Citation16]. Some soil enzymes are involved in transformation of carbon (e.g. invertase), nitrogen (e.g. urease) and phosphorus (e.g. acid phosphatase) [Citation17]. Soil enzyme activities therefore provide a unique integrative biochemical assessment of soil function and condition and are useful indicators of soil functional diversity [Citation18]. Extracellular soil enzymes responsible for the initial processing of detrital carbon and organic-bound nutrients [Citation19] should indicate the initial functional response of the microbial community to crop harvesting or soil disturbance. Several studies have suggested organic matter, microbial biomass and activity parameters as indicators of soil quality [Citation20–Citation22]. Three common enzymes in soil are invertase, amylase and cellulase, their substrates being the naturally occurring carbohydrates sucrose, starch and cellulose, respectively. These enzymes, with urease, are important for understanding the carbon load and fertility of field soil. The microclimate, soil chemical factors and substrate availability are the main factors that control enzyme activity [Citation23] and may be the cause of seasonality.
Fluoride can change metabolism by binding directly to some enzymes, e.g. those containing haem groups, or other metal enzymes. The activity of phosphatase enzyme can be enhanced or depressed by the complex-forming ability of fluoride with aluminium or beryllium, which affects the phosphate content of soil [Citation24]. Fluoride also inhibits urease activity, which increases in an acid environment [Citation25]. Elevated levels of fluoride are found in soils in which large amounts of phosphorus fertilizers are used, and these soils also contain less microbial biomass and dehydrogenase activity [Citation26]. Several physicochemical parameters of soil and water have a substantial effect on the fluoride concentration [Citation4,Citation5], which in turn affects the soil enzymes.
Studies have been reported on the response of soil microbes and enzyme activities to fluoride pollution [Citation27] and on inhibition of soil enzymes activity by fluoride [Citation24]; however, few studies have been published on seasonal variations in enzyme activity and microbial populations under fluoride stress. As soil enzymes reflect the fertility of soil, we chose the agriculture-based area of Nasipur, a known fluoride-endemic area, in Birbhum District. The aim of the study was to assess the stress effect of fluoride on soil microbial properties and soil enzyme activity, to determine the influence of fluoride on soil properties and to quantify the influence of season on enzyme activities.
2 Materials and methods
2.1 Study site
The study was based in two areas, a fluoride-prevalent region and a control region in which fluoride pollution has not been reported (). The polluted region, Nasipur (24°17′33.7″ N and 87°45′13.6″ E) is located in Nalhati I block of Birbhum District West Bengal, India, where fluoride contamination was first detected in 1997. The geophysical cause of fluoride pollution in this region has been reviewed [Citation28,Citation29]. Geographically, the district lies at the north-eastern end of Chhotanagpur plateau and slopes down to merge with the alluvial plains of the Ganges. The climate is dry and extreme in the western part of the district but is relatively milder on the eastern side. The temperature rises well above 40 °C in summer and it drops to around 10 °C in winter. The average annual rainfall in this district is 1405 mm. Sandy, hard red soil of the alfisoil type and latterite soil are the most interesting aspects of the geology. Bore wells and open wells are the main sources of water for domestic and agricultural purposes in this arid region.
The Burdwan University seed multiplication farm and a nearby locality (23°15′12″ N and 77°50′51″ E) of Burdwan District were chosen as control areas for the study. The average temperature in this area ranges from 10 to 38 °C, and the average annual rainfall is 1320 mm. Burdwan District has various soil types in different topographical, biological, hydrological and geological conditions. In the study area, alluvial soil attains extreme thickness due to alluvium brought down by the Damodar and numerous other rivers. These soils are sandy, well drained and slightly acidic.
2.2 Soil sampling
Seventy soil samples were collected from the top layer (10 cm depth) in seven study sites in Nasipur during three distinct seasons: rainy, winter and summer. Samples were collected in the same manner from Burdwan University agricultural farm during the same three seasons. The soil samples were collected in sterilized polythene zipper packets and were immediately transported in thermo boxes to the laboratory and stored at 4 °C for subsequent enzyme analysis. A portion of each sample was air-dried, sieved through a <2-mm sieve, homogenized and stored at room temperature for determination of physical and chemical properties.
2.3 Physicochemical analysis
Soil pH was determined in a 1:2.5 soil:water suspension by dipping a digital pH meter (Systronics-335, Systronic Pvt. Ltd). The same suspension was used to measure electrical conductivity in a digital conductivity meter (Model 304-Systronics Pvt. Ltd). Soil organic carbon content was estimated by Walkley–Black's [Citation30] rapid titration method, available nitrogen according to Subbiah and Asija [Citation31] and available phosphorus by Olsen's method [Citation32]. Available soil potassium was estimated [Citation33] with neutral normal NH4OAc, standard stock potassium solutions (1000 mg/L).
2.4 Soil enzyme assay
The carbohydrate reducing enzymes amylase (EC 3.2.1.1), cellulase (EC 3.2.1.4) and invertase (EC 3.2.1.26) were estimated by the 3,5-dinitrosalicylic acid method [Citation34] with starch, carboxymethyl cellulose and sucrose. After incubation of 3 g of moist soil with Sorensen's buffer (0.06 mol/L, pH 5.5) and substrate solution at 30 °C for 24 h, the supernatant was reacted with 3,5-dinitrosalicylic acid and heated in a boiling water bath. Optical density was read at λ = 540 nm and the result compared with the standard curve for d-glucose. The results are expressed in micrograms of glucose equivalent per gram soil per hour.
The activity of urease (EC 3.5.1.5) was determined by estimating ammonium nitrogen released on incubation of soil with buffered urea solution [Citation35]. After incubation of 5 g of fresh powdered soil for 2 h at 37 °C with Tris–HCl buffer mixed with 0.2 mol/L urea, the reaction was stopped by the addition of KCl–Ag2SO4 solution and the supernatant was taken after centrifugation for estimation of ammonia. Alkaline hypochlorite solution was added to 1 mL of supernatant, and the colour was developed by adding phenate solution and leaving the mixture for 5 min at 37 °C. Enzyme activity was measured at 625 nm and expressed in micrograms of urea equivalent per gram soil per hour.
2.5 Enumeration of bacterial isolates
Immediately after collection, soil samples were diluted to 10−3, and 100 μL were mixed with 100 mL nutrient agar (Himedia-M1269) and plated. The Petri plates were incubated at 31 ± 0.1 °C in a biological oxygen demand incubator for 24 h, when the colonies were counted manually.
2.6 Statistical analysis
Regression analysis, two-tailed t-tests and Pearson correlations of the soil analysis data were performed with MINITAB 16 software (State College, Pennsylvania, USA, 2013).
3 Results and discussion
3.1 Influence of season on enzyme activity
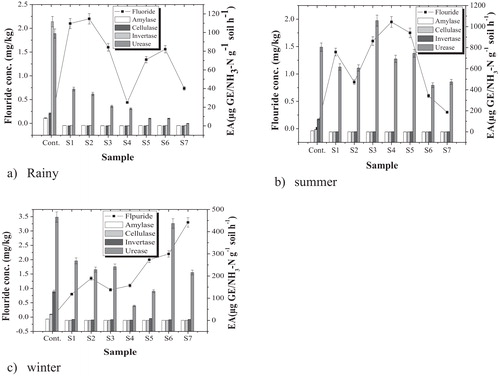
Soil enzymes originate mainly from plant residues [Citation11] and are found as both intra- and extracellular enzymes [Citation12]. The activities of the four enzymes varied significantly (p < 0.0001) by season (). Similar observations were made when the enzyme activities were compared with those in the control area in different seasons (). Similar seasonal variation in soil enzyme activity was reported previously [Citation23,Citation37]. Urease activity was highest in the summer, as also reported by Joachin et al. [Citation38]. Sahrawat [Citation39] reported that urease activity increases with increasing temperature from 10 °C to a maximum at 60 °C for vertisol and 70 °C for alfisol. A further increase in temperature decreased urease activity, which was virtually inhibited at 100 °C. As the temperature changes with season, seasonality in enzyme activity is justified. Seasonal changes in the microbes that produce these enzymes may also give rise to seasonality [Citation40].
Table 2 Seasonal variation of soil enzymes activity (urease, invertase, cellulase and amylase) in polluted and control area.
3.2 Influence of soil fluoride on enzyme activity
The fluoride level in the control area was <0.5 mg/kg soil in all seasons (). In the contaminated area, however, the fluoride concentration ranged from 0.44 to 2.2 mg/kg in the rainy season, from 0.8 to 3.3 mg/kg in winter and from 0.30 to 1.6 mg/kg in summer (). Of the four enzymes studied (amylase, cellulase, invertase and urease), only urease fluctuated with fluoride level. The sensitivity of the enzymes towards fluoride is therefore season-dependent. Inhibition of enzyme activity by fluoride has been reported previously [Citation41,Citation42].
Pearson correlation of urease with fluoride gave low values (0.06–0.17) in all seasons (). Amylase showed a slightly higher but nonsignificant correlation in all seasons. Urease thus appears to be more sensitive to fluoride than the other enzymes.
3.3 Influence of soil physicochemical parameters on enzyme activity
In the rainy season, urease activity was nonsignificantly correlated with pH, organic carbon, available nitrogen and available phosphorus, but electrical conductivity showed a positive correlation (). Nonsignificant correlations with available nitrogen and available phosphorus were also recorded for invertase, cellulase and amylase. Invertase showed a significant (p < 0.01) correlation with organic matter, as also reported by Shi et al. [Citation43]. In summer, invertase, cellulase and amylase showed nonsignificant correlations with organic carbon and organic matter; urease showed a significant negative correlation (p < 0.05) with electrical conductivity (). In winter, however, none of the enzymes showed significant correlations with pH, electrical conductivity, organic carbon, organic matter, available nitrogen or available phosphorus. Nevertheless, all four enzymes showed nonsignificant negative correlations with pH (). During the rainy season, urease activity was correlated with pH and available nitrogen, probably due to the hydrolysis by urease of urea fertilizer applied to the soil into NH3 and CO2, which would increase soil pH and nitrogen [Citation44,Citation45]. Soil pH had a negative impact on urease activity in winter and summer, as also reported by Shi et al. [Citation43]. Amylase activity, which is responsible for hydrolysing starch, increases the organic matter and organic carbon in soil [Citation14,Citation15]. Stepwise regression analysis indicated that available nitrogen and available phosphorus to have minute, nonsignificant negative and positive effects on urease activity, respectively (). The organic matter and organic carbon content of soil are accelerated by cellulase, which degrades cellulose in plant debris to glucose, cellobiose and high-molecular-mass oligosaccharides [Citation46].
Table 1 Summary of stepwise multiple regression analysis of soil enzymes.
Table 3 Pearson correlations of soil enzymes, soil chemical parameters and fluoride concentration in rainy season.
Table 4 Pearson correlations of soil enzymes, soil chemical parameters and fluoride concentration in winter season.
Table 5 Pearson correlations of soil enzymes, soil chemical parameters and fluoride concentration in summer season.
Table 6 Variation of number of soil bacteria in three major seasons (rainy, winter and summer) within the fluoride contaminated area of different study site.
3.4 Influence of fluoride and season on soil microbial population
The bacterial population was much larger in the control area than in the polluted area in all three seasons (). As fluoride is a protoplasmic poison and a minute amount can change biochemistry, the high fluoride level in the polluted area might present an adverse environment for soil bacteria, decreasing the population [Citation47]. Tscherko and Kandelar [Citation48], working on the influence of atmospheric fluorine deposits on soil microorganisms, reported that fluoride contamination can decrease microbial biomass by up to 80%.
The impact of fluoride on the population of soil microbial populations, in relative proportions and absolute numbers, varies spatially and seasonally [Citation49]. shows that the reduction in the bacterial population with soil fluoride level was highest in the rainy season, followed by winter, and lowest in summer. The temperature and conductivity of soil change with the season, with a strong effect on the microbial community, which varies as a function of rainfall during the year [Citation49]. Seasonal variation in soil microbes has been recorded previously [Citation23,Citation37,Citation50–Citation52].
4 Conclusion
This comparative study of fluoride-contaminated soil shows the stress effect of fluoride on soil enzymes, other soil parameters and the availability of microbes, with strong seasonal variation. The findings concern enzyme activities related to soil fertility. Urease was found to be highly sensitive to the soil fluoride level. All the enzymes studied showed seasonal variation as a consequence of changes in rainfall and temperature. The combined effects of fluoride, soil parameters and season on soil enzymes can change the soil profile, with a possible change in crop production. The effect of fluoride on bacteria should be studied further study to determine the effects on crop production.
Acknowledgements
The authors express their sincere thanks to all faculty members of the Department of Environmental Science, University of Burdwan, Burdwan.
Notes
Peer review under responsibility of Taibah University.
References
- P.PatelS.A.BhattFluoride: a major polluting component of ground water in north Gujarat region, IndiaProceedings of the 12th World Lake Conference, Jaipur, 2007, Shiga, International Lake Environment Committee Foundation, B122008245249
- K.BrindhaR.RajeshR.MuruganL.ElangoFluoride contamination in ground water in parts of Nalgonda District, Andhra Pradesh, IndiaEnviron. Monit. Assess.1722011481492
- R.GautamN.BhardwajGroundwater quality assessment of Nawa Tehsil in Nagaur district (Rajasthan) with special reference to fluorideEnvironmentalist302010219227
- N.K.MondalK.C.PalS.KabiPrevalence and severity of dental fluorosis in relation to fluoride in ground water in the villages of Birbhum District, West Bengal, IndiaEnvironmentalist320127084
- K.C.PalN.K.MondalR.BhaumikA.BanerjeeJ.K.DattaIncorporation of fluoride in vegetation and associated biochemical changes due to fluoride contamination in water and soil: a comparative field studyAnn. Environ. Sci.62012123139
- T.N.MorshinaFluorine adsorption by soilsSov. Soil Sci.121980413416
- T.BeckMethods and application domain of soil microbiological analysis at the Landesanstallt für Bodenkulturund Pflanzenbau (LBP) in Munich for the determination of some aspects of soil fertilityM.P.NemesS.KissP.PapacosteaG.StefanicM.RussauProceedings of the Fifth Symposium on Soil Biology1984Romanian National Society of Soil ScienceBucharest1320
- G.StefanicG.EliadeI.ChirnogeanuResearches concerning a biological index of soil fertilityM.P.NemesS.KissP.PapacosteaG.StefanicM.RussauProceedings of the Fifth Symposium on Soil Biology1984Romanian National Society of Soil ScienceBucharest3545
- J.A.PascualC.GarcíaT.HernándezJ.L.MorenoM.RosSoil microbial activity as a biomarker of degradation and remediation processesSoil Biol. Biochem.32200018771883
- B.A.CaldwellEnzyme activities as a component of soil biodiversity: a reviewPedobiologia492005637644
- J.C.PolaccoIs nickel a universal component of plant ureases?Plant Sci. Lett.101977249255
- R.G.BurnsInteraction of enzymes with soil mineral and organic colloidsP.M.HuangM.SchnitzerInteractions of Soil Minerals with Natural Organics and Microbes1986Soil Science Society of America Inc.Madison, WI429452
- H.L.T.MobleyR.P.HausingerMicrobial urease: significance, regulation and molecular characterizationMicrobiol. Rev.53198985108
- R.G.BurnsExtracellular enzymes substrate interactions in soilJ.H.SlaterR.WittenburyJ.W.T.WimpennyMicrobes in their Natural Environment1983Cambridge University PressLondon249298
- R.L.SinsabaughR.K.AntibusA.E.LinkinsAn enzymic approach to the analysis of microbial activity during plant litter decompositionAgric. Ecosyst. Environ.3419914354
- A.C.KennedyK.L.SmithSoil microbial diversity and the sustainability of agricultural soilsPlant Soil17019957586
- O.N.BelyaevaR.J.HaynesO.A.BirukovaBarley yield and soil microbial and enzyme activities as affected by contamination of two soils with lead, zinc or copperBiol. Fertil. Soils4120058594
- G.D.BendingM.K.TurnerF.RaynsM.C.MarxM.WoodMicrobial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimesSoil Biol. Biochem.36200417851792
- P.SollinsP.HomannB.A.CaldwellStabilization and destabilization of soil organic matter: mechanisms and controlsGeoderma74199665105
- J.W.DoranD.C.ColemanD.E.BezdicekB.A.StewartDefining Soil Quality for a Sustainable Environment (Soil Science Society of America Special Publication No. 35)1994Soil Science Society of America and American Society of AgronomyMadison, WI
- E.G.GregorichM.R.CarterD.A.AngersC.M.MonrealB.H.EllertTowards a minimum data set to assess soil organic matter quality in agricultural soilsCan. J. Soil Sci.741994367385
- D.JordanR.J.KremerW.A.BergfieldK.Y.KimV.N.CacnioEvaluation of microbial methods as potential indicators of soil quality in historical agricultural fieldsBiol. Fertil. Soils191995297302
- R.E.J.BoernerJ.A.BrinkmanA.SmithSeasonal variations in enzyme activity and organic carbon in soil of a burned and unburned hardwood forestSoil Biol. Biochem.37200514191426
- J.NowakK.KaklewskiD.KlodkaInfluence of various concentrations of selenic acid (IV) on the activity of soil enzymesSci. Total Environ.2912005105110
- R.E.MarquisS.A.ClockM.Mota-MeiraFluoride and organic weak acids as modulators of microbial physiologyFEMS Microbiol. Rev.262003493510
- U.LangerT.GüntherEffects of alkaline dust deposits from phosphate fertilizer production on microbial biomass and enzyme activities in grassland soilsEnviron. Pollut.1122001321327
- J.C.García-GilJ.KobzaP.Soler-RoviraS.JavorekovaSoil microbial and enzyme activities response to pollution near an aluminium smelterClean Soil Air Water412013485492
- A.K.GhoshP.BhattacharyyaR.PalEffect of arsenic contamination on microbial biomass and its activities in arsenic contaminated soils of Gangetic West Bengal, IndiaEnviron. Int.302004491499
- S.GuptaS.BanerjeeR.SahaJ.K.DattaN.K.MondalFluoride geochemistry of groundwater in Nalhati-1 block of the Birbhum district, West Bengal, IndiaFluoride392006318320
- A.WalkleyI.A.BlackAn examination of Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration methodSoil Sci.3719342938
- B.V.SubbiahG.L.AsijaA rapid procedure for the determination of available nitrogen in soilsCurr. Sci.251956259260
- S.R.OlsenC.V.ColeF.S.WatnabeL.A.DeanEstimation of available phosphorus in soils by extraction with sodium bicarbonate. U.S. Dept. of Agriculture Circular 9391954119
- P.C.JaiswalSoil, Plant and Water Analysis2004Kalyani PublishersLudhiana
- P.C.MishraR.K.MohantyM.C.DashEnzyme activity in subtropical surface soils under pastureIndian J. Agric. Chem.1219791924
- P.K.BeheraSoil and Soil Waste Analysis: A Laboratory Manual2006Dominant Publishers and Distributors (P) LtdDelhi
- R.A.GuicharnaudO.ArnaldsG.I.PatonThe effect of season and management practices on soil microbial activities undergoing nitrogen treatments: interpretation from microcosm to field scaleIcel. Agric. Sci.232010123134
- H.J.R.M.JoachimA.N.PatrickSelected soil enzymes: examples of their potential roles in the ecosystemAfr. J. Biotechnol.72008181191
- K.L.SahrawatEffect of temperature and moisture on urease activity in semi-arid tropical soilsPlant Soil781984401408
- M.D.WallensteinM.N.WeintraubEmerging tools for measuring and modeling the in situ activity of soil extracellular enzymesSoil Biol. Biochem.40200820982106
- G.M.ChristensenD.OlsonB.RiedelChemical effects on the activity of eight enzymes. A review and a discussion relevant to environmental monitoringEnviron. Res.291982247255
- G.A.EvdokimovaFluoride in the soils of the sea basin and bioindication of pollutionChemosphere4220013543
- Z.J.ShiY.LuZ.G.XuS.L.FuEnzyme activities of urban soils under different land use in the Shenzhen city, ChinaPlant Soil Environ.542010341346
- R.K.AndrewsR.L.BlakeleyB.ZernerUrease: a Ni (II) metalloenzymeJ.R.LancasterThe Bioinorganic Chemistry of Nickel1989VCH PublishersNew York146166
- B.H.ByrnesA.AmbergerFate of broadcast urea in a flooded soil when treated with N-(n-butyl) thiophospheric tramide, a urease inhibitorFertil. Res.181989221231
- A.R.WhiteVisualization of cellulases and cellulose degradationR.M.BrownCellulose and Other Natural Polymer Systems: Biogenesis, Structure, and Degradation1982Plenum PressNew York489509
- E.ErenM.OzturkE.F.MumcuD.CanatanFluorosis and its hematological effectsToxicol. Ind. Health212005255258
- D.TscherkoE.KandelerEcotoxicological effects of fluorine deposits on microbial biomass and enzyme activities in grasslandsEur. J. Soil Sci.481997329335
- K.J.EdwardsT.M.GihringJ.F.BanfieldSeasonal variations in microbial populations and environmental conditions in an extreme acid mine drainage environmentAppl. Environ. Microbiol.65199936273632
- C.DasP.AdityaJ.K.DattaN.K.MondalSoil carbon transformation by the influence of exocellular enzyme by litter types of a social forest in BurdwanArch. Agron. Soil Sci.201310.1080/03650340.2013.789869
- S.MukhopadhyayV.C.JoyInfluence leaf types on microbial functions and nutrient status of soil: ecological suitability of forest trees for afforestation in tropical laterite wastelandsSoil Biochem.42201023062315
- M.KerstinM.EgbertResponse of enzyme activities to nitrogen in forest floors of different C/N ratiosBiol. Fertil. Soils382003102109