?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Hopeaphenol is naturally occurring plant polyphenol composed of resveratrol units. We examined the antioxidant activity of hopeaphenol using various in vitro assays, including 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging, superoxide anion radical, hydrogen peroxide scavenging and ferric reducing power. Hopeaphenol showed 63.76% of the activity of DPPH, 64.48% of superoxide anion and 54.64% of hydrogen peroxide scavenging activity at a concentration of 100 μg/mL. In addition, hopeaphenol showed effective ferric reducing power. Those antioxidant activities were compared to that of quercetin. The study shows that hopeaphenol is an effective natural antioxidant component that could be used as food preservative or nutraceutical.
1 Introduction
The pharmaceutical properties of natural products are interesting for the development of alternative treatments with little toxicity and few side-effects. Many plant-derived compounds are used in the treatment of human diseases [Citation1]. Some natural products are antioxidants, which quench radicals or postponed the oxidation of other compounds in human [Citation2], thus protecting the body from the effects of free radicals and reactive oxygen species such as O2−•, hydroxyl (OH•), peroxyl (ROO•) and hydrogen peroxide (H2O2•) radicals. Reactive nitrogen species include nitric oxide (NO•) and nitrogen dioxide (NO2•) [Citation3]. The most commonly used antioxidants, such as butylated hydroxyl anisole and butylated hydroxyl toluene, have been reported to have adverse effects in humans, and they are restricted in food products because of negative perceptions of consumers [Citation4,Citation5]. Therefore, potent natural antioxidants have been sought, especially natural ones derived from plants, to counter radical-mediated damage [Citation6]. Phenolic compounds are some of the most important natural antioxidants because of their biological activities [Citation7].
Shorea species are a major sources of hopeaphenol, in which oligomeric resveratrols are the main polyphenols. These have been shown to have important biological activity, such as cytotoxicity and antibacterial and antioxidant activities [Citation8–Citation15]. The aim of this study was to investigate the antioxidant properties of hopeaphenol as 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging, superoxide anion radical scavenging, hydrogen radical scavenging and ferric antioxidant reducing power. Another goal of this investigation was to compare the antioxidative effects of hopeaphenol in vitro with those of the natural antioxidant quercetin.
2 Materials and methods
2.1 Chemicals and plant material
1,1-Diphenyl-2-picrylhydrazyl radical was purchased from Sigma Aldrich, Bangalore, India. Quercetin, potassium ferricyanide, trichloroacetic acid, ferric chloride, hydrogen peroxide, nitroblue tetrazolium and other laboratory chemicals were purchased from Merck, India. All other chemicals and solvents used were of analytical grade.
2.2 Extraction, isolation and characterization
The stem bark of S. roxburghii was identified and collected from Alagar Hills, Madurai, by Professor S. Karupusamy, Madura College, Madurai, India, in March 2010. Powdered stem bark (1.0 kg) was defatted with hexane and sequentially extracted with acetone and methanol. The acetone extract was fractionated with ethyl acetate, and 50 g of the soluble portion were subjected to column chromatography eluted with an n-hexane/ethyl acetate step gradient. The eluted column fractions were monitored by thin-layer chromatography with n-hexane/ethyl acetate, v/v 7:3 and chloroform/methanol, v/v 8:2. Repeated column chromatography gave 1.0 g of the compound. The compound was visualized under ultraviolet light in an iodine chamber. The structure of the isolated compound was established by spectral techniques.
2.3 Identification of compound
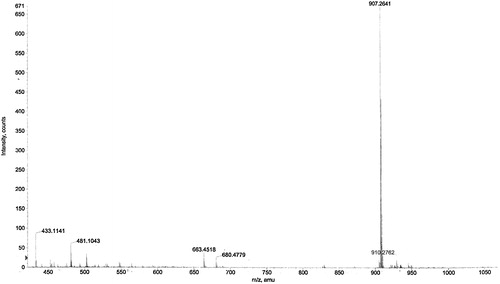
Brown solid: UV (methanol) λmax (log ɛ) 241, 290 and 393, 280 nm (sh); infrared bands (KBr) 2937 cm−1 (characteristic of aliphatic stretching), 1617, 1513, 1456, 1384 and 840 cm−1 (aromatic ring, presence of 1,4-disubstituted benzene); 1H NMR (acetone-d6, 400 MHz) and 13C NMR; 75 MHz, acetone-d6, electrospray ionization time-of-flight mass m/z [M+H]+ 907 (calc. for C56H42O12, 906).
2.4 DPPH radical scavenging activity
The DPPH radical scavenging assay is commonly used to evaluate the ability of antioxidants to scavenge free radicals. When a hydrogen atom or electron is transferred to the odd electron in the DPPH radical, the absorbance at 517 nm decreases proportionally to the increase in non-radical forms of DPPH•. The DPPH radical scavenging effect was estimated according to the established method [Citation16], with slight modifications. Briefly, a 1 mmol/L solution of DPPH• was prepared in methanol, and 1 mL was added to 3 mL of hopeaphenol solution in methanol at concentrations of 20–100 μg/mL. The solutions were vortexed thoroughly and incubated in the dark for 30 min. The absorbance was measured at 517 nm against blank samples without scavenger. DPPH radical-scavenging activity was calculated from:where Ac is the absorbance without samples and As is the absorbance in the presence of the samples. Lower absorbance of the reaction mixture indicates higher DPPH radical-scavenging activity.
2.5 Hydrogen peroxide radical scavenging activity
The hydrogen peroxide scavenging assay was carried out by the procedure of Ruch et al. [Citation17]. A solution of 1 mmol/L hydrogen peroxide was prepared in 50 mmol/L phosphate buffer (pH 7.4). Concentrations of 20–100 μg of the hopeaphenol solution were added to 0.6 mL of hydrogen peroxide in phosphate solution, and absorbance was recorded at 230 nm after 10 min against a blank solution containing phosphate buffer without hydrogen peroxide. Hydrogen peroxide scavenging activity was calculated from:where Ac is the absorbance without samples and As is the absorbance in the presence of the samples.
2.6 Superoxide anion radical scavenging activity
Superoxide anion radical scavenging activity was measured by the method of Arulmozhi et al. [Citation18], with slight modifications. Superoxide radicals are generated in phenazine methosulfate-nicotinamide adenine dinucleotide systems by oxidation of nicotinamide adenine dinucleotide and assayed by the reduction of nitroblue tetrazolium. In this experiment, superoxide radicals were generated in 3 mL of Tris HCl buffer (16 mmol/L, pH 8.0) containing 1 mL of nitroblue tetrazolium (50 μmol/L), 1 mL nicotinamide adenine dinucleotide (78 μmol/L) and a compound (20–100 μg). The reaction was started by adding 1 mL of phenazine methosulfate solution (10 μmol/L) to the mixture, which was incubated at 25 °C for 5 min; absorbance was measured at 560 nm in a spectrophotometer against the blank. Hopeaphenol was added to the reaction mixture in which O2−• was scavenged, thereby inhibiting the nitroblue tetrazolium reduction. The decreased absorbance of the reaction mixture indicates increased superoxide anion scavenging activity. The percentage inhibition of superoxide anion generation was calculated from:
where the value (μg extract/mL) is the effective concentration at which the reducing power, hydrogen peroxide, DPPH and superoxide anion radical scavenging activities were scavenged by 50%. The IC50 was obtained by extrapolation in linear regression analysis.
2.7 Ferric reducing power
The ferric reducing power of hopeaphenol was determined according to the procedure of Barrors et al. [Citation19], with slight modifications. Briefly, 1 mL of hopeaphenol solution was mixed with 2.5 mL of phosphate buffer (0.2 mol/L, pH 6.6) and 2.5 mL of potassium ferricyanide (1%). The mixture was incubated at 50° C for 30 min, and then 2.5 mL of trichloroacetic acid (10%) were added to the mixture, which was then centrifuged at 3000 rpm for 10 min. Then, 2.5 mL of the upper layer were pipetted out and mixed with 2.5 mL of distilled water, and 0.5 mL of ferric chloride (0.1%) was added. Absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicates greater reducing capacity.
2.8 Statistical analysis
All samples were analyzed in triplicate unless otherwise stated, and the results are expressed as an average and standard deviation (±SD). All statistical analyses were performed in the Microsoft Excel 2007 software package.
3 Results and discussion
3.1 Structural elucidation of the compounds
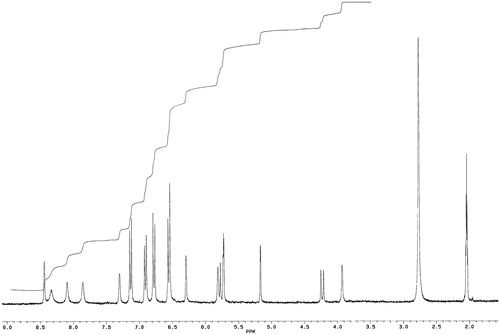
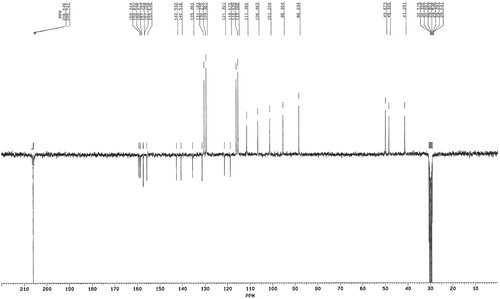
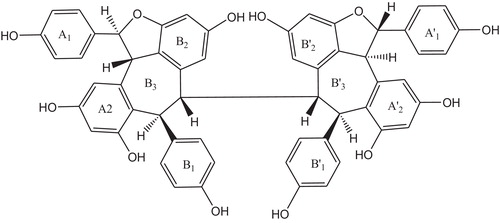
The UV spectrum of the compound showed absorption maxima at 241 and 290 nm and shoulders at 393 and 280 nm, indicating the phenolic chromophore. The infrared spectrum showed an absorption band at 3363 cm−1 attributed to a hydroxyl group. The absorption band at 2937 cm−1 is characteristic of aliphatic stretching, while absorption at 1617, 1513, 1456 and 1384 cm−1 is due to the presence of and aromatic ring, and absorption at 840 cm−1 is typical of the presence of a 1,4-disubstituted benzene. The UV and infrared data are typical and characteristic of oligomeric stilbenes, especially resveratrol tetramer [Citation20,Citation21]. The protons and carbons were assigned by heteronuclear multiple band correlation and rotating frame Overhauser effect. Both the 1H NMR () and 13C NMR () spectra of the compound showed half the number of signals corresponding to the molecular formula C56H42O1, indicating that the compound is a tetrameric stilbene composed of two symmetrical dimmers. This was corroborated by the m/z 906 from mass spectra (). The 1H NMR spectrum showed signals for eight sets of ortho-coupled aromatic protons at δ 7.14 and 6.78 (each 4H, d, J = 8.65 Hz) and 6.79 and 6.75 (each 4H, d, J = 8.62 Hz), suggesting the presence of four 1,4-disubstuituted aromatic rings. The presence of four sets of meta-coupled aromatic protons at δ 6.55 and 6.28 (each 2H, d, J = 2.10 Hz) and δ 5.72, 5.16 (each 2H, d, J = 2.20 Hz) was ascribed to four sets of 1,2,3,5-tetrasubstituted aromatic rings, implying the presence of four additional aromatic rings. The aliphatic hydrogen signals at δ 5.73 and 4.23 (each 2H, d, J = 12.2 Hz) suggested the presence of two dihydrobenzofuran moieties bearing 4-oxyphenyl and 3,5-dioxyphenyl groups characteristic of oligostilbenes derived from the resveratrol molecule [Citation22–Citation24].
The upfield chemical shift value of the two aliphatic methane protons signal at δ 4.23 indicated that it is anti to the two aliphatic methane protons signal at δ 5.73, as this proton is held in the shielding region of the aromatic ring (B1). The coupling constant value (J = 12.20 Hz) of these two sets of aliphatic methane protons suggested the trans stereochemistry between them [Citation22,Citation25]. The other coupled signals, at δ 5.80 and 3.93 (each 2H, d, J = 1.60 Hz), due to the protons attached to the carbons at δ 44.08 (C-7b and 7d) and δ 53.37, suggested the presence of vicinal methines. The presence of 10 hydroxyl groups was deduced from the molecular formula and five broad hydroxyl signals (each two OHs) at δ 8.51, 8.43, 8.18, 7.95 and 7.39 in the 1H NMR spectrum. Comparison of the 1H NMR and 13C NMR () spectra of the compound with (+) hopeaphenol and (−) isohopeaphenol showed that the structure of the compound was similar to that of (+) hopeaphenol. The proposed structure of (+) hopeaphenol, C56H42O12 () was confirmed by comparison with those reported in the literature [Citation25–Citation27].
Table 1 1H NMR and 13C NMR spectral data for (+)-hopeaphenol.
3.2 DPPH radical scavenging activity
Four methods were used to determine the antioxidant property of hopeaphenol, which also allowed us to obtain information about the activity of this compound. The DPPH radical scavenging assay has been widely used to determine the free radical scavenging activities of various natural substances. In this assay, DPPH• is reduced in alcoholic solution in the presence of a hydrogen-donating antioxidant [Citation28]. The radical-scavenging activity of the hopeaphenol was estimated by comparing the percentage inhibition of formation of DPPH radicals by the compound and those of quercetin.
The DPPH radical scavenging activity of hopeaphenol increased with increasing concentration (). The scavenging effects of hopeaphenol and quercetin on DPPH radical at 100 μg were 62.66% and 96.59%. The median inhibitory concentrations (IC50) of DPPH radical scavenging by hopeaphenol and the standard were 66.05 and 46.53 μg/mL, respectively, which were significantly different (p < 0.05). A lower half maximal effective concentration (EC50) value indicates greater free radical scavenging activity. The radical scavenging activity of the compound is probably due to the presence of hydroxyl groups. It is known that compounds such as flavonoids contain hydroxyl functional groups that are responsible for the antioxidant effect of the plants. The ability of polyphenolic compounds to act as antioxidants depends on the redox properties of their phenolic hydroxyl groups and the potential for electron delocalization across the chemical structure [Citation29]. Hopeaphenol has two molecules of ampelopsin B, each built of four resveratrol molecules. Abstraction of a hydrogen atom from the hydroxyl group may occur, and the absorbance decreased when DPPH• was scavenged by an antioxidant through donation of the hydrogen atom to form a stable DPPH radical molecule.
Table 2 Radical scavenging activities of (+)-hopeaphenol.Table Footnoteb
3.3 Superoxide anion radical scavenging activity
The superoxide anion radical scavenging activity of the compound increased with concentration (). Concentrations of 20–100 μg/mL that inhibited superoxide anion scavenging activity were 26.89 ± 0.08%, 38.85 ± 0.38%, 48.58 ± 0.47%, 54.40 ± 0.29% and 64.48 ± 0.82%, respectively. These values were lower that those of the same doses of quercetin. The result implies that the compound is a superoxide scavenger, and its capacity to scavenge the superoxide anion radical may contribute to its antioxidant activity. The IC50 values of superoxide anion (67.53 μg/mL) and DPPH radical scavenging activity (66.05 μg/mL) are similar.
3.4 Hydrogen peroxide scavenging activity
The hydrogen peroxide scavenging activity of hopeaphenol at 100 μg/mL was 54.64 ± 0.24%, while that of quercetin was 76.19 ± 0.70%. Hopeaphenol acid was thus an effective hydrogen peroxide scavenger, although less so than quercetin. The IC50 value was 91.36 μg/mL. The compound had greater hydrogen peroxide-scavenging activity than DPPH radical scavenging. Hydrogen peroxide is a weak oxidizing agent and can penetrate biological membranes. Because of the possible involvement of hydrogen peroxide in the generation of hydroxyl radicals, it causes cytotoxicity rather than chemical reactivity.
3.5 Ferric reducing power
The compound had strong reducing power, with an IC50 value of 47.55 μg/mL, which is significantly lower that that for DPPH radical scavenging (66.05 μg/mL). The compound reduces Fe3+ to Fe2+ by donating an electron or hydrogen, with the formation of Prussian blue complex, and an increase in the absorbance of the reaction mixture indicates greater reducing capacity [Citation30]. The reducing capacity of hopeaphenol may serve as an indicator of its potential antioxidant activity (). There is a direct correlation between the antioxidant activity and reducing power of certain compounds, such as flavonoids [Citation31], and similar results were found in this study. The reducing power of the compound at the concentrations tested was high, demonstrating its ability to donate electrons and neutralize free radicals by forming stable products.
Hopeaphenol is composed of two molecules of ampelopsin B (), each of which is composed of four resveratrol units. The antioxidant activity of hopeaphenol is due to the existence of a phenol ring, the stability of the molecular structure and the existence of the double bonds of the olefinic unit. The phenol ring can freeze hydroxyl, superoxide anion and hydrogen peroxyl radicals by releasing hydrogen [Citation32,Citation33]. Hopeaphenol scavenges hydrogen peroxide due to the presence of phenolic groups that donate electrons to hydrogen peroxide, thereby neutralizing the radicals. Although it has a number of hydroxyl groups, it had less activity than quercetin.
4 Conclusion
We evaluated the antioxidant properties of hopeaphenol isolated from a stem bark extract of S. roxburghii. Hopeaphenol had potent antioxidant properties with all four testing methods. The activity of this compound is attributed to its complex structure, with many hydroxyl groups, which can donate hydrogen or electrons to free radicals, effecting neutralization. Hopeaphenol could therefore be used as a natural antioxidant in foods and pharmaceuticals.
Acknowledgements
We thank Professor D. Gunasekar, Department of Chemistry, Sri Venkatasewara University, for his whole-hearted support in giving spectra and establishing the structure of the compound.
Notes
Peer review under responsibility of Taibah University
References
- T.K.HyunaH.C.KimJ.S.KimAntioxidant and antidiabetic activity of Thymus quinquecostatus CelakInd. Crop. Prod.522014611616
- Y.S.VeliogluG.MazzaL.GaoB.D.OomahAntioxidant activity and total phenolics in selected fruits, vegetables and grain productsJ. Agric. Food Chem.4619984113
- I.GülçinAntioxidant activity of eugenol: a structure–activity relationship studyJ. Med. Food142011975985
- B.SunM.FukuharaEffects of co-administration of butylated hydroxytoluene, butylated hydroxyanisole and flavonoids on the activation of mutagens and drug metabolizing enzymes in miceToxicology122199761
- P.J.ParkW.K.JungK.S.NamF.ShahidiS.K.KimPurification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolkJ. Appl. Chem.782001651656
- G.F.DengX.R.XuY.J.GuoQ.XiaS.LiS.WuF.ChenW.H.LingH.B.LiDetermination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grainsJ. Funct. Foods42012906914
- A.ChandrasekaraF.ShahidiDetermination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC–DAD–ESI–MSnJ. Funct. Foods32011144158
- N.NorizanN.AhmatS.A.Syed MohamadN.A.A.Mohd NazriS.S.Akmar RamliS.N.Mohd KasimW.Z.W.Mohd ZainTotal phenolic content, total flavonoid content, antioxidant and antimicrobial activities of Malaysian ShoreaRes. J. Med. Plants62012489499
- M.ArifullahP.VikramK.K.ChiruvellaM.Miya ShaikI.H.B.Abdullah RipainA review on Malaysian plants used for screening of antimicrobial activityAnn. Res. Rev. Biol.4201420882132
- V.KueteE.J.SeoB.KruscheW.B.OswaldS.SchröderH.J.GretenI.S.LeeT.EfferthCytotoxicity and pharmacogenomics of medicinal plants from traditional Korean medicineEvid. Based Complement. Alternat. Med.2013341724
- M.H.LeeB.Y.ChoiJ.K.KunduJ.K.ShinH.K.NaY.J.SurhResveratrol suppresses growth of human ovarian cancer cells in culture and in a murine xenograft model: eukaryotic elongation factor 1A2 as a potential targetCancer Res.69200974497458
- B.B.AggarwalA.BhardwajR.S.AggarwalN.P.SeeramS.ShishodiaY.TakadaRole of resveratrol in prevention and therapy of cancer: preclinical and clinical studiesAnticancer Res.24200427832840
- S.A.TukiranH.EuisM.LukmanS.KokkiS.KuniyoshiM.YanaOligostilbenoids from Shorea balangeranBiochem. Syst. Ecol.332005631634
- N.S.AminahS.A.AhmadN.AimiE.L.GhisalbertiE.H.HakimM.KitajimaY.M.SyahH.TakayamaDiptoindonesin A, a new C-glucoside of ɛ-viniferin from Shorea seminis (Dipterocarpaceae)Fitoterapia732012501507
- S.HeX.YanFrom resveratrol to its derivatives: new sources of natural antioxidantCurr. Med. Chem.2201310051007
- Q.Y.ZhuR.M.HackmanJ.L.EnsunsaR.R.HoltC.L.KeenAntioxidative activities of Oolong teaJ. Agric. Food Chem.50200269297693
- R.J.RuchS.J.ChengJ.E.KlaunigPrevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green teaCarcinogenesis10198910031008
- S.ArulmozhiM.M.PapiyaPurnimaA. Sathiya NarayananIn vitro antioxidant and free radical scavenging activity of Alstonia scholaris Linn. R.BrIran. J. Pharmacol. Therapeut.62007191196
- L.BarrorsP.BaptissaI.C.F.R.FerreiraEffect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assaysFood Chem. Toxicol.45200717311737
- S. HadiNovianyThe isolation of hopeaphenol, a tetramer stilbene, from Shorea ovalis BlumeAdv. Nat. Appl. Sci.32009107112
- F.MattiviF.ReneiroRelationship between UV spectra and molecular structure of resveratrol oligomersVercauterenJ.C. ChezeM.C.DumonJ.F.WeberPolyphenols Communication1996125126
- J.ItoM.NiwaY.OshimaA new hydroxystilbene tetramer named isohopeaphenol from Vitis vinifera ‘Kyohou’Heterocycles45199718091810
- Y.OshimaY.UenoH.HikinoAmpelopsins A, B and C, new oligostilbenes of Ampelopsis brevipedunculata var. HanceiTetrahedron46199051215126
- K.S.HaungM.LinG.F.ChengAnti-inflammatory tetramers of resveratrol from the roots of Vitis amurensis and the conformations of the seven-membered ring in some oligostilbenesPhytochemistry582001357362
- K.X.YanK.TerashimaY.TakayaM.NiwaA novel oligostilbene named (+)-viniferol A from the stem of Vitis vinifera ‘Kyohou’Tetrahdron57200127112715
- W.PatcharamunJ.SichaemP.SiripongS.KhumkratokJ.Jong-aramruangS.Tip-pyangA new dimeric resveratrol from the roots of Shorea roxburghiiFitoterapia822011489492
- S.RohaizaW.A.YaacobL.B.DinI.NazlinaCytotoxic oligostilbenes from Shorea hopeifoliaAfr. J. Pharm. Pharmacol.5201112721277
- I.GülçinAntioxidant activity of caffeic acid (3,4-dihydroxycinnamicacid)Toxicology2172006213220
- I.GülçinAntioxidant properties of resveratrol: a structure–activity insightInnov. Food Sci. Emerg. Technol.112010210218
- L.G.WoodP.G.GibsonM.L.GargA review of the methodology for assessing in vivo antioxidant capacityJ. Sci. Food Agric.86200620572066
- R.SubramanianP.SubbramaniyanV.RajAntioxidant activity of the stem bark of Shorea roxburghii and its silver reducing powerSpringerPlus2201328
- Y.Z.CaiQ.LuoM.SunH.CorkeAntioxidant activity and phenolic compounds of 112 Chinese medicinal plants associated with anticancerLife Sci.74200421572184
- M.Al-MamunK.YamakiT.MasumizuY.NakaiK.SaitoH.SanoM.TamuraSuperoxide anion radical scavenging activities of herbs and pastures in northern Japan determined using electron spin resonance spectrometryInt. J. Biol. Sci.32007349355