?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Two structurally similar biphenyl compounds, biphenyl-2-carboxylic acid (B2C) and biphenyl-4-carboxylic acid (B4C), were selected to assess their cellular cytotoxic and antimitotic behaviour in the quest of a potent anticancer compound. The HeLa and MCF-7 cell lines were used to determine the cytotoxic effect of the two biphenyl compounds using the MTT assay. Confocal microscopy was performed to analyze the degree of nuclear condensation and fragmentation associated with depolymerized microtubules that result from inhibiting in vitro tubulin polymerization. Circular dichroism spectroscopy along with DTNB kinetics were conducted to predict alterations in the tubulin secondary structure and global conformational changes in the tertiary structure of the protein, respectively. Finally, a wound healing assay was employed to assess whether cellular migration was inhibited in the treated HeLa cells. B4C imparted more cellular cytotoxicity in the HeLa and MCF-7 cell lines (IC50 ∼ 4 μM). B4C inhibited in vitro tubulin polymerization into microtubules with an IC50 of 50 μM. Confocal microscopy of treated cell indicated presumptive apoptosis, exhibiting fragmented nuclei with significant microtubular disruption, which was confirmed by Western blot analysis. Circular dichroism revealed a significant reduction in the α-helix content of treated tubulin. DTNB kinetics showed that approximately six –SH groups were buried in the structure. The wound healing assay revealed that B4C prevented cellular invasion. B4C had greater cytotoxic and antimitotic effects against HeLa cells than B2C. To delineate the specific mechanism of action of B4C and its derivatives, further research is warranted.
1 Introduction
Over the past several decades, research has targeted the cytoskeleton protein tubulin [Citation1] to combat abnormal cell proliferation during various types of cancer. In these processes, small molecules have evolved in targeted cancer therapies [Citation2], which have made the treatment more successful and less toxic [Citation2]. These molecules block the growth and spread of cancer cells by interfering with the specific molecular pathways involved in tumour progression. Small molecules have been used for decades in anticancer research to disrupt the molecular dynamics of the tubulin–microtubule system [Citation3]. Antimitotic agents, such as colchicine, vinblastine, paclitaxel and their various derivatives, bind to tubulin and inhibit the dynamics, causing mitotic arrest and apoptosis [Citation4–Citation6]. Many small molecules, such as sulphonamide ER68384 [Citation7], indanocine [Citation3], plumbagin [Citation6], 2-methoxyestradiol [Citation8], combrestatin, and podophyllotoxin [Citation1], were discovered more recently and appear to play pivotal role, as manifested from their application on a range of cancer cell lines. Although many of these compounds are in clinical trials, some limitations on their use remain, including mutations in the tubulin genes, expression of different tubulin isotypes with varied affinity for drugs [Citation3,Citation9], mutations in cell death pathway [Citation10] and over expression of drug efflux pumps, such as the multidrug-resistant P-glycoprotein (MDR/Pgp) [Citation3,Citation11]. These limitations impose restrictions on the use of many anticancer agents, and, therefore, the search for new drugs or lead compounds is ongoing. In an effort to identify small molecules with cytotoxic as well as antimitotic properties, the present study was initiated with two biphenyl compounds, Biphenyl-2-carboxylic acid (B2C) and biphenyl-4-carboxylic acid (B4C). Both biphenyl compounds have two phenyl rings joined by a single bond that rotates freely. It shares partial structural similarity with methoxy-5-(2′,3′,4′-trimethoxyphenyl) tropone (AC) (A), which binds the colchicine binding site on tubulin and inhibits its polymerization [Citation12].
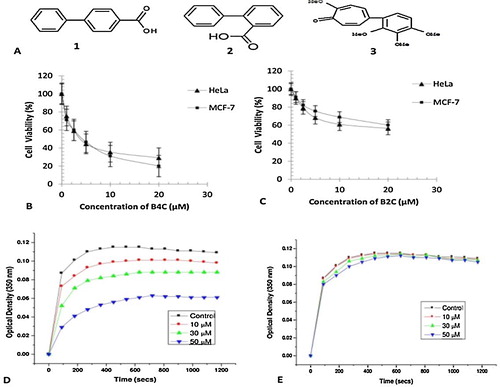
Fig. 1 (A) Structures of the two biphenyl compounds, B4C (1) and B2C (2), and AC (3). Concentration-dependent inhibition of HeLa (▴) and MCF-7 (■) cell proliferation upon exposure to the biphenyl compounds, B4C (B) and B2C (C) using the MTT assay. Inhibition of in vitro polymerization of purified tubulin in the presence of 0(■), 10 (![]()
Simple small molecules, such as phenstatin, aminobenzophenone and their derivatives, were discovered during the process of improving the in vivo activity of combrestatin [Citation13–Citation15]. Phenstatin, with an IC50 of 3.43 μM [Citation13], affects cell viability by inhibiting tubulin polymerization [Citation13]. Similar to phenstatin, 3-aminobenzophenone shows strong inhibition towards in vitro tubulin polymerization (IC50 = 0.3 μM) [Citation16] and arrests cells at the G2/M phase of the cell cycle [Citation15].
An earlier study with 2′-acetoxybiphenyl-2-carboxylic acid, a derivative of biphenyl-2-carboxylic acid (B2C), reported its anti-inflammatory and analgesic properties [Citation17]. Tetrazole derivatives of B2C, after being subjected to benzylic bromination followed by condensation with N-methyl piperazine, induced significant antimicrobial activity against two gram-positive (Staphylococcus aureus, Bacillus subtilis) and two gram-negative (Escherichia coli, Salmonella typhi) strains, comparable to gentamycin [Citation18].
Biphenyl-4-carboxylic acid (B4C) and its hydrazide hydrazone derivatives were previously shown to have antimicrobial properties against gram-positive and gram-negative strains [Citation19]. The nitro derivative (2-NO2C6H4) possesses maximal antifungal activity (against two fungal strains Candida albicans and Aspergillus niger) compared to its chloro derivative (2-ClC6H4), which exhibits the highest antibacterial potential [Citation19] against two gram-positive (S. aureus, B. subtilis) and gram-negative strains (E. coli, Pseudomonas aeruginosa), respectively. There is also a report by the same group describing the anti-inflammatory activity of biphenyl-4-carboxylic acid 2-(aryl)-4-oxothiazolidin-3-yl amides in albino rats [Citation20]. Although a plethora of evidence exists in support of the antimicrobial potential of biphenyl compounds, there are no reports on the B2C and B4C compounds in cytotoxicity tests using cancer cell lines.
Owing to its structural simplicity that is analogous to AC (A (3)) and has previously been reported as an antimitotic agent, the present work was initiated with both B2C and B4C to study their potential as cellular cytotoxic agents by inhibiting in vitro tubulin polymerization. Altered HeLa cell morphology was analyzed in presence of the biphenyl compounds using confocal microscopy and supported by Western blotting. The wound healing assay was used to analyze the compounds’ ability to inhibit cell migration. Alterations in the tubulin secondary structure in presence of biphenyl compounds were monitored with the aid of CD spectroscopy. Thiol group reactivity of the cysteines in tubulin was performed using DTNB kinetics to monitor the global conformational change of protein upon binding with the biphenyl compounds. However, this report is the first indicating that B4C is a cytotoxic and antimitotic agent against cancer cell lines by affecting the tubulin–microtubule system.
2 Materials and methods
2.1 Materials
B4C, B2C, PIPES, EGTA, MgCl2, GTP, l-glutamic acid, DTNB, Trypsin–EDTA and Hoechst 33258 were all purchased from Sigma, St. Louis, MO, USA. DMSO and glycerol were purchased from Merck, Mumbai, India. DMEM, FBS and penicillin–streptomycin were supplied by GIBCO, Bangalore, India. DTT, SDS and bromophenol blue were purchased from SRL, India. Tris, MTT and DPBS were purchased from HIMEDIA, India. Mouse anti-α tubulin was purchased from Invitrogen, India and Alexa 568 from Life Technologies, India.
2.2 Cell culture
HeLa and MCF-7 cells were cultured in DMEM supplemented with 10% FBS and 2% penicillin–streptomycin [Citation5,Citation21,Citation22]. The cells were passaged after every third day (80–90% confluency). The cells grew exponentially at 37 °C in an atmosphere maintained with 5% CO2 [Citation7,Citation22].
2.3 MTT [3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide] assay for cell viability
HeLa and MCF-7 cells (1 × 104) were seeded in 96-well plate and allowed to grow for 24 h [Citation6]. The cells were subsequently treated with different concentrations of B2C and B4C (0, 1, 2.5, 5, 10 and 20 μM) and incubated for 12 h [Citation6]. MTT (20 μl of 5 mg/ml, prepared in DPBS) was added to the treated cells and incubated until a purple precipitate appeared. Triton-X (100 μl) was added and incubated in the dark for 2 h at room temperature. The absorbance was measured on an ELISA plate reader at 650 nm. Data were calculated as the percent of inhibition by the following formula:where At and As indicate the absorbance of the test substances and solvent control, respectively [Citation6].
2.4 Confocal microscopy
The effect of the biphenyl compounds on microtubules and nuclei were studied by confocal microscopy. HeLa cells were grown on poly-l-lysine-coated coverslips [Citation23] at a density of 1 × 105 cells/ml and incubated with different concentrations of the biphenyl compounds (B2C – 4 and 8 μM; B4C – 4 μM [IC50 concentration determined using MTT assay on HeLa cell line]) for one cell cycle (24 h) [Citation6]. The cells were fixed using 3.7% formaldehyde and permeabilized with ice-cold methanol at −20 °C [Citation24]. Nonspecific antibody binding sites were blocked by incubating the cells in 2% BSA/DPBS at 37 °C for 30 min [Citation7]. The cells were incubated with a mouse monoclonal anti-α-tubulin antibody (1:300 dilutions in 2% BSA/DPBS) for 2 h at 37 °C followed by incubation with an Alexa-568-conjugated secondary antibody (1:350 dilution in 2% BSA/DPBS) for 1 h at 37 °C. The cells were incubated with Hoechst 33258 (1 μg/ml) for 10 min in the dark [Citation7,Citation25] and observed under Zeiss LSM 510 Meta confocal microscope [Citation6] to visualize the nuclei.
2.5 Western blot analysis
The HeLa cells were cultured in a petri dish and treated with the test compounds for 24 h. The cells were then lysed directly in the dish by adding lysis buffer (160 mM Tris, pH 6.9, 200 mM DTT, 4% SDS, 20% glycerol, 0.004% bromophenol blue) along with a protease inhibitor cocktail (Sigma, St. Louis, MO, USA). The lysates were collected in Eppendorf tubes, boiled for 10 min and the cellular debris was pelleted at 13,000 × g. The extracts were immunoblotted with Bcl-2, caspase 3 and β-actin antibody (Santa Cruz, CA). The resulting signals were detected using an alkaline phosphatase-conjugated secondary antibody, which reacted with BCIP/NBT (Genei, Bangalore, India). The densities of the visualized bands were quantified using ImageJ Software.
2.6 Wound healing assay
This assay was performed to assess HeLa cell migration in the presence of the two biphenyl compounds. Passaged cells were re-plated on 24-well plates and grown to saturating confluence. A “scratch” was created on the cell monolayer using a p200 pipette tip. The cells were later washed with DPBS and replaced with fresh DMEM supplemented with 10% FBS. The cells were subsequently treated with different concentrations of B2C and B4C and incubated for 36 h. Images of cell migration were captured using a phase-contrast microscope with a 10× objective at 0, 12, 24 and 36 h of incubation [Citation26,Citation27]. The obtained images were analyzed using ImageJ software. At 0 h of incubation, the respective area formed by the wound was calculated at 100% based on the densitometric analysis of the selected area. The subsequent cell migration was considered to be 0% for both B4C- and B2C-treated cell plates. The area created after wound formation was calculated at 12, 24 and 36 h of incubation using ImageJ software. In the control cells without treatment, the gap created by the wound was completely filled after 36 h and the respective % of cell migration was considered to be 100%. The residual unfilled area in the B4C- and B2C-treated cells was calculated at successive periods of incubation (12, 24 and 36 h) and was extrapolated to the corresponding % of cell migration. After comparison with the control plate area (i.e., in absence of B4C and B2C after 36 h) where cell migration is 100% after total gap filling, the % of cell migration in all treated plates was plotted along the Y-axis against the time of incubation (hours) on the X-axis.
2.7 Tubulin isolation and purification
The microtubule protein tubulin was isolated and purified from goat brain using two cycles of temperature-dependent polymerization and depolymerization with 4 M glycerol in PEM (50 mM PIPES, 1 mM EGTA and 0.5 mM MgCl2, pH 6.9) buffer in the presence of 1 mM GTP. It was then followed by two more cycles of polymerization and depolymerization in 1 mM glutamate buffer, pH 6.9, containing 1 mM EGTA and 0.5 mM MgCl2 [Citation7,Citation21]. The protein concentration was determined using the Bradford method and BSA as the standard [Citation21]. The isolated protein was stored at −80 °C.
2.8 Inhibition of in vitro tubulin polymerization
Tubulin (15 μM) was mixed with the different desired concentrations of the biphenyl compounds and allowed to polymerize in PEM buffer, pH 6.9, in presence of 1 mM GTP at 37 °C for 20 min. The 50% inhibition constant (IC50) for polymerization was identified by plotting the data points for polymerization inhibition vs. the concentration of the biphenyl compounds [Citation7].
2.9 Spectral measurements
Circular dichroism (CD) spectra in the far-UV range was recorded using a 0.2 cm path length cuvette in a JASCO J-815 spectrophotometer. Tubulin (1 μM) was treated with different concentrations of B2C and B4C [Citation21,Citation24] in sodium phosphate buffer, pH 7.
2.10 Titration of sulphhydryl groups
Tubulin (1 μM) was incubated with 10 μM of B2C and B4C at 37 °C for 30 min in PEM buffer, pH 6.9. Immediately after incubation, is the solution was brought to 25 °C and mixed with DTNB at a final concentration of 400 μM. Absorbance was measured continuously at 412 nm for 60 min. The molar extinction coefficient (12,000 M−1 cm−1) of TNB was used to calculate the number of sulphhydryl groups that took part in the reaction [Citation21,Citation24].
2.11 Statistical evaluation
All of the data obtained from the MTT assay for cell viability and the wound healing assay were confirmed by performing independent experiments in duplicate and are presented as the mean ± SD [Citation28].
3 Results and discussion
3.1 Effect of the biphenyl compounds on cell viability and inhibition of tubulin polymerization in vitro
From the cellular cytotoxicity studies performed on HeLa and MCF-7 cell lines, it was observed that B4C (B) inhibited both cell lines with IC50 values of 4.1 ± 0.3 μM and 4.3 ± 0.2 μM, respectively. B4C inhibited in vitro polymerization of purified tubulin in a concentration-dependent manner, with an IC50 value of approximately 50 μM (D). In contrast, B2C has an IC50 value of more than 20 μM (not determined) (C) for both cell lines and was not able to inhibit in vitro polymerization of purified tubulin (E) as effectively as B4C. The IC50 values displayed by B4C are in agreement with the IC50 values of other potent small molecules such as phenstatin (IC50 = 3.43 ± 0.7 μM) [Citation13,Citation14] and aminobenzophenone (0.3 μM) [Citation16], which exhibit cytotoxicity against a range of cell lines. Comparing the IC50 values of different similar small, antimitotic compounds () that target the colchicine binding site on tubulin, it is quite clear that although B4C has cytotoxicity and antimitotic properties, its analogue B2C is not a sufficiently promising candidate.
Table 1 IC50 value for the inhibition of in vitro tubulin polymerization and in vitro cell cytotoxicity.
3.2 Biphenyl-4-carboxylic acid alters HeLa cell morphology
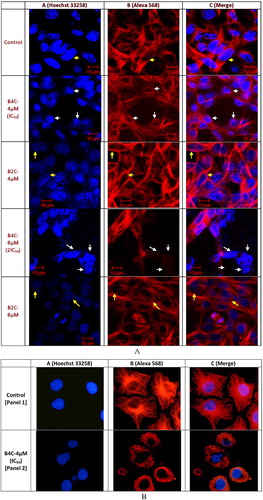
Confocal microscopy was performed to study the alterations in microtubule network and the nuclear morphology of HeLa cells upon treatment with biphenyl compounds [Citation6]. As shown in A, the microtubule network displays abnormal organization upon treatment with 4 μM B4C (shown in white arrows, panel B) when compared to the control cells that were treated with vehicle (0.5% DMSO) (shown in yellow arrows) [Citation7]. Nuclear condensation and a ball-shaped appearance of the nuclei were observed in cells treated with B4C (IC50 – 4 μM [determined by the MTT assay on HeLa cells]) (shown in white arrows, panel A), whereas in the control cells, normal elongated nuclei (shown in yellow arrows) were observed. The effect was more drastic with 8 μM B4C (2IC50), which considerably decreased the cytoskeletal network (shown in white arrows, panel B) and was accompanied by nuclear condensation and fragmentation. This supported the hypothesis that B4C induced apoptotic cell death [Citation7]. Similar observations are shown in earlier studies by Renu Mohan et al. in HeLa cells treated with Compound 4 (a sulphonamide representative). In contrast, there were no significant alterations in the microtubule network or the nuclear structure in cells treated with 4 μM or 8 μM B2C, resembling the control. This revealed a reduced potency of B2C compared to B4C when used in equivalent amounts in HeLa cells. However, treatment with 8 μM B2C resulted in very few cells with an altered microtubule network and it induced the formation of apoptotic bodies, although they were much fewer in number compared to the normal cells. For a better perception of the abnormal microtubular organization, this same was performed with higher resolution using control and 4 μM B4C-treated HeLa cells. The microtubules in control (vehicle-treated) HeLa cells were well-spread with distinctly visible peripheral microtubules (B, panel 1). In the presence of 4 μM (IC50) B4C, the microtubules were markedly depolymerized and appeared to be strongly diminished (B, panel 2) compared to the control cells (B, panel 1). B2C-treated cells, conversely, were unable to produce any significant change in the cytoskeletal structure and had a similar morphology to control cells (data not shown).
Fig. 2 Effect of the biphenyl compounds on HeLa cells. (A) The cells were incubated with 0, 4 and 8 μM B4C and 0, 4 and 8 μM B2C for 24 h. The cells were then processed for Alexa/Hoechst staining. Hoechst-stained nuclei are in blue (panel A) and Alexa 568-stained microtubules are in red (panel B). The yellow arrows indicate normal nuclei and microtubule network. The white arrows indicate the apoptotic nuclei and damaged microtubule network. (B) The cells were incubated with 0 and 4 μM B4C for 24 h. The higher magnification images clearly showed apoptotic nuclei and the damaged microtubule network at the IC50 concentration of B4C in comparison to the control cells.
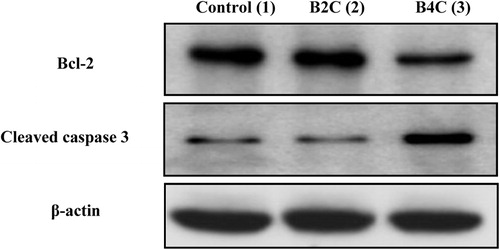
3.3 Biphenyl-4-carboxylic acid induces apoptosis in HeLa cells
Bcl-2 family proteins are the key regulators of mitochondrial outer membrane permeabilization (MOMP), which governs the release of pro-apoptotic molecules, including cytochrome c, to promote caspase activation and apoptosis [Citation29]. To explore whether both compounds induce apoptosis, the effect of B4C and B2C (0 and 4 μM, respectively) on Bcl-2 protein expression was examined (). Exposure of the HeLa cell line to B2C increased the basal expression of the Bcl-2 protein. This, in turn, down-regulated caspase 3 expression, suggesting that Bcl-2 mediated the inhibition of B2C-induced apoptosis. In contrast, B4C treatment down-regulated Bcl-2 expression and augmented the expression of cleaved caspase 3, indicating that the cells were destined for apoptosis. These findings were supported by the changes in HeLa cell morphology as observed from confocal microscopy. Finally, these results indicate Bcl-2 mediated the effect of B4C and B2C on apoptosis induction in HeLa cells.
3.4 Effect of the biphenyl compounds on inhibiting in vitro cell migration using a wound healing assay
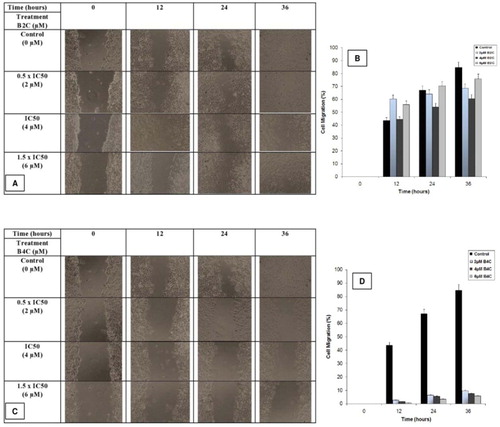
The fundamental step in the malignant transition of a tumour is angiogenesis, which requires the formation of new blood vessels from pre-existing ones and involves the dissolution of the basement membrane underlying the endothelial cells, the migration of endothelial cells, adhesion, proliferation as well as tube formation [Citation30]. It can be studied in vitro by measuring cell migration. To determine whether the biphenyl compounds were inhibitors, a scratch wound was created completely confluent HeLa cells that were then treated with 2, 4 and 6 μM of B2C and B4C, respectively. Migration images of HeLa cells were analyzed over a period of 36 h (A and C). The treated cells showed 60.35%, 64.29% and 68.82% cell migration with 2 μM B2C (B), and 2.83%, 6.6% and 9.93% cell migration with 2 μM B4C (D). Cell migration was decreased to 1.78%, 5.62% and 7.37% for 4 μM B4C and 44.39%, 54.06% and 60.55% for 4 μM B2C compared to 43.74%, 67.12% and 84.72% for untreated cells at 12, 24 and 36 h, respectively. Finally, cells treated with 6 μM B4C showed 0.69%, 3.55% and 5.89% cell migration, while and cells treated with 6 μM B2C showed 38.12%, 51.74% and 58.5% cell migration at the respective time points. The above data indicates that B2C exhibited weaker potency than B4C as an inhibitor of cell migration, which is important for their future prospects.
Fig. 4 Wound healing assay of HeLa cells in the presence of the biphenyl compounds. Altered cell migration after treatment with 0 (control), 2, 4 and 6 μM B2C (A) and B4C (C) was imaged at 0, 12, 24 and 36 h. The percentage of wound area filling due to cell migration after treatment with 0 (■), 2 (![]()
3.5 Alteration of the tubulin secondary structure in the presence of biphenyl-4-carboxylic acid
CD spectral analysis was performed to discern the structural changes in purified tubulin upon binding to the biphenyl compounds. B4C significantly reduced the molar ellipticity of tubulin in the far-UV range, indicating alterations in the secondary structure, especially the α-helix content of bound tubulin compared to unbound tubulin (A). This is in accord with previous results from Gireesh et al. using tubulin and a dose-dependent range of CIL-102 [Citation21]. However, B2C-bound tubulin did not show any significant reduction in the molar ellipticity compared to unbound tubulin at low concentrations (2 μM) but did show some reduction in molar ellipticity at high concentrations (20 μM) (B), although it was not significant when compared to that of B4C-bound tubulin at the same concentrations.
Fig. 5 Global effects of biphenyl compound binding to purified tubulin. The far-UV spectra of 1 μM tubulin in the absence (––) and presence of 2 (![]()
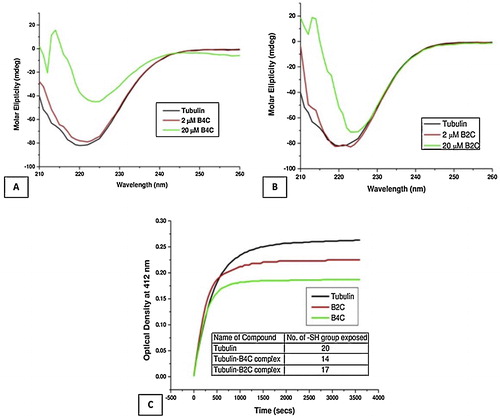
3.6 Biphenyl-4-carboxylic acid changes the global conformation of tubulin upon binding
DTNB kinetics is frequently used to evaluate protein conformation during folding and unfolding due to compound binding. DTNB kinetics is used to analyze the relationship between the loss of drug binding activity towards a target protein due to ageing [Citation24]. DTNB kinetics measures the optical density (412 nm) obtained due to release of TNB− over a period of time after proteins are treated with DTNB. In this case, DTNB kinetics were performed to verify the global conformational changes upon the binding of the biphenyl compounds to purified tubulin (C). The tubulin–B4C complex was treated with DTNB with the objective of identifying the number of exposed and buried cysteine (–SH) groups upon binding of the biphenyl compounds. B4C-bound tubulin had approximately 14 thiol groups exposed compared to 20 in unbound tubulin, whereas the tubulin–B2C complex had approximately 17 thiol groups exposed. This clearly indicated that only 3 cysteine residues were protected due to structural changes upon the binding of B2C to tubulin, whereas B4C protected 6 cysteine residues. The observations confirmed earlier results that showed complete protection of 1.6 –SH groups of cysteine residues in tubulin upon binding to colchicine [Citation24]. Earlier studies by Gireesh et al. showed that 1.6 and 2.8 cysteine thiol group in tubulin were buried in the presence of 15 and 30 μM CIL-102, respectively [Citation21].
4 Conclusions
Biphenyl-2-carboxylic acid and biphenyl-4-carboxylic acid exhibited differential activity in the cellular cytotoxicity assay and in inhibiting in vitro tubulin polymerization. B4C affects cellular viability in both HeLa and MCF-7 cells, whereas B2C appeared to be much less efficient. Similarly, B2C exhibited lower potency in inhibiting in vitro tubulin polymerization, whereas B4C decreased tubulin polymerization in a concentration-dependent manner. B4C altered the microtubule structure as evident from confocal microscopy, which showed nuclear condensation and fragmentation, indicative of apoptosis, and was supported by the Western blot analysis. Compared to B4C, B2C was not potent enough to produce similar changes. While B4C and B2C were tested in the wound healing assay, the results showed that B4C was better able to inhibit cell migration than B2C. CD spectral analysis of the B4C–tubulin complex demonstrated a reduction in the alpha helix content of tubulin that was also accompanied by buried thiol groups of cysteines in DTNB kinetics. On the other hand, B2C affects the tubulin secondary structure to a lesser extent and exposes more cysteine residues in DTNB kinetics, indicating a reduced interaction.
Structural analysis demonstrated that B4C (A (1)) has two phenyl rings joined with a single bond having a carboxyl group at para position, which allows free rotation of the two phenyl rings across the single bond. Conversely, B2C (A (2)) possesses a similar structure, with the carboxyl group at ortho position with respect to the single bond, which may induce steric constraints that hinder the free rotation of the phenyl rings across the single bond. Hypothetically, the restriction in free rotation of the two phenyl rings of B2C may be the reason behind its reduced potency compared to B4C, which needs to be explored further with different derivatives of these compounds. However, the present finding is the first report on the cytotoxic properties and tubulin polymerization inhibition of B4C, which is to be explored further in the future to delineate its mechanism of action.
Acknowledgments
The work was performed with financial assistance from DBT-BioCare (BT/Bio-CARe/01/184/2010-11). NCCS Pune is acknowledged for providing the HeLa and MCF-7 cell lines.
Notes
Peer review under responsibility of Taibah University.
References
- Y.LuJ.ChenM.XiaoW.LiD.D.MillerAn overview of tubulin inhibitors that interact with the colchicine binding sitePharm. Res.29201229432971
- D.E.GerberTargeted therapies: a new generation of cancer treatmentsAm. Fam. Physician772008311319
- L.DasS.GuptaD.DasguptaA.PoddarM.E.JanikB.BhattacharyyaBinding of indanocine to the colchicine site on tubulin promotes fluorescence, and its binding parameters resemble those of the colchicine analogue ACBiochemistry48200916281635
- M.A.JordanL.WilsonMicrotubules as a target for anticancer drugsNat. Rev. Cancer42004253265
- A.RaiA.SuroliaD.PandaAn antitubulin agent BCFMT inhibits proliferation of cancer cells and induces cell death by inhibiting microtubule dynamicsPLoS ONE72012112
- B.R.AcharyaB.BhattacharyyaG.ChakrabartiThe natural naphthoquinone plumbagin exhibits antiproliferative activity and disrupts the microtubule network through tubulin bindingBiochemistry47200878387845
- R.MohanM.BanerjeeA.RayT.MannaL.WilsonT.OwaB.BhattacharyyaD.PandaAntimitotic sulfonamides inhibit microtubule assembly dynamics and cancer cell proliferationBiochemistry45200654405449
- K.KamathT.OkounevaG.LarsonD.PandaL.WilsonM.A.Jordan2-Methoxyestradiol suppresses microtubule dynamics and arrests mitosis without depolymerizing microtubulesMol. Cancer Ther.5200622252233
- S.ChakrabortiD.ChakravartyS.GuptaB.P.ChatterjiG.DharA.PoddarD.PandaP.ChakrabartiS.G.DastidarB.BhattacharyyaDiscrimination of ligands with different flexibilities resulting from the plasticity of the binding site in tubulinBiochemistry51201271387148
- H.C.WuD.K.ChangC.T.HuangTargeted therapy for cancerJ. Cancer Mol.220065766
- J.AvilaMicrotubule dynamicsFASEB J.4199032843290
- B.BhattacharyyaR.HowardS.N.MaityA.BrossiP.N.SharmaJ.WolffB ring regulation of colchicine binding kinetics and fluorescenceBiochemistry83198620522055
- C.M.AbuhaieE.BicuB.RigoP.GautretD.BeleiA.FarceJ.DuboisA.GhinetSynthesis and anticancer activity of analogues of phenstatin, with a phenothiazine A-ring, as a new class of microtubule-targeting agentsBioorg. Med. Chem. Lett.232013147152
- A.GhinetA.TourteauB.RigoV.StockerM.LemanA.FarceJ.DuboisP.GautretSynthesis and biological evaluation of fluoro analogues of antimitotic phenstatinBioorg. Med. Chem.21201329322940
- J.P.LiouC.W.ChangJ.S.SongY.N.YangC.F.YehH.Y.TsengY.K.LoY.L.ChangC.M.ChangH.P.HsiehSynthesis and structure–activity relationship of 2-aminobenzophenone derivatives as antimitotic agentsJ. Med. Chem.45200225562562
- J.P.LiouJ.Y.ChangC.W.ChangC.Y.ChangN.MahindrooF.M.KuoH.P.HsiehSynthesis and structure–activity relationships of 3-aminobenzophenones as antimitotic agentsJ. Med. Chem.47200428972905
- A.GringauzSynthesis of 2′-acetoxybiphenyl-2-carboxylic acid and its derivatives as potential anti-inflammatory and analgesic agentsJ. Pharm. Sci.651976291294
- S.N.RaoT.RavisankarJ.LathaK.S.BabuSynthesis, characterization and antimicrobial activity of novel biphenyl tetrazolesDer Pharma Chem.4201210931103
- A.DeepS.JainP.C.SharmaP.VermaM.KumarC.P.DoraDesign and biological evaluation of biphenyl-4-carboxylic acid hydrazide–hydrazone for antimicrobial activityActa Pol. Pharm.672010255259
- A.DeepS.JainP.C.SharmaSynthesis and anti-inflammatory activity of some novel biphenyl-4-carboxylic acid 5-(arylidene)-2-(aryl)-4-oxothiazolidin-3-yl amidesActa Pol. Pharm.6720106367
- K.K.GireeshA.RashidS.ChakrabortiD.PandaT.MannaCIL-102 binds to tubulin at colchicine binding site and triggers apoptosis in MCF-7 cells by inducing monopolar and multinucleated cellsBiochem. Pharmacol.842012633645
- D.PandaK.RathinasamyM.K.SantraL.WilsonKinetic suppression of microtubule dynamic instability by griseofulvin: implications for its possible use in the treatment of cancerProc. Natl. Acad. Sci. U. S. A.102200598789883
- J.ZhouD.PandaJ.W.LandenL.WilsonH.C.JoshiMinor alteration of microtubule dynamics causes loss of tension across kinetochore pairs and activates the spindle checkpointJ. Biol. Chem.27720021720017208
- M.RoychowdhuryN.SarkarT.MannaS.BhattacharyyaT.SarkarP.B.SarkarS.RoyB.BhattacharyyaSulfhydryls of tubulin – a probe to detect conformational changes of tubulinEur. J. Biochem.267200034693476
- K.RathinasamyD.PandaSuppression of microtubule dynamics by benomyl decreases tension across kinetochore pairs and induces apoptosis in cancer cellsFEBS J.273200641144128
- C.Y.BaiM.OhsugiY.AbeT.YamamotoZRP-1 controls Rho GTPase-mediated actin reorganization by localizing at cell–matrix and cell–cell adhesionsJ. Cell Sci.120200728282837
- C.C.LiangA.Y.ParkJ.L.GuanIn vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitroNat. Protoc.22007329333
- M.O.ChanekL.MazurM.StozakIn vitro cytotoxicity testing of new generation oxazaphosphorines against human histiocytic lymphoma cells, IndiaJ. Exp. Biol.512013615622
- D.D.NewmeyerS.Ferguson-MillerMitochondria: releasing power for life and unleashing the machineries of deathCell1122003481490
- P.C.LeeA.N.SalyapongseG.A.BragdonL.L.ShearsS.C.WatkinsH.D.J.EdingtonT.R.BilliarImpaired wound healing and angiogenesis in eNOS-deficient miceAm. J. Physiol.277199916001608
- R.J.D’AmatoC.M.LinE.FlynnJ.FolkmanE.Hamel2-Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine siteProc. Natl. Acad. Sci. U. S. A.91199439643968
- K.YoshimatsuA.YamaguchiH.YoshinoN.KoyanagiK.KitohMechanism of action of E7010, an orally active sulfonamide antitumor agent: inhibition of mitosis by binding to the colchicine site of tubulinCancer Res.57199732083213