Abstract
Heavy-ion beams have unique biophysical and radiobiological properties, such as the inverted depth dose profile compared to photon beams, relative biological effectiveness and oxygen enhancement ratio. These physical and biological properties are much more favourable than those of photon radiotherapy and can be used to treat tumours efficiently. During a long-term stay in space, astronauts will be constantly exposed to low-dose space radiation. Thus, space radiation is one of the major health-related concerns for space exploration. This review summarizes the biophysical and biological properties of charged particles and their advantage in radiotherapy. In addition, we briefly reviewed the importance of the heavy ion during space flight and how to suppress its deleterious effects.
1 Introduction
The principle aim of radiation biophysics is to relate physical properties to observed biological responses to radiations of different qualities and their implications in radiation therapy and radiation protection [Citation1]. The use of proton and heavy ion irradiation in cancer therapy was proposed due to their biophysical and biological superiority compared with photon beams [Citation2]. R. Wilson, from Berkeley, analyzed the depth dose profile of protons and proposed their use in 1946. In 1954, Tobias, Lawrence and Larson began to treat patients with protons and, later on, with He (helium) ions [Citation3]. Now, interest in the biological effects of heavy ions is rapidly growing in the scientific community. Heavy charged particles represent the best tool for an external radiotherapy due to their favourable depth dose distribution, i.e., where the dose increases with penetration depth, allowing irradiation of deep-seated target volumes with optimum precision. Recent results of heavy-ion cancer therapy in Japan and Germany [Citation4] have stimulated the construction of several accelerator facilities for particle therapy.
On the other hand, the greater RBE (relative biological effectiveness) of heavy ion particles is the concern of space-radioprotection because the radiation spectrum of the galactic cosmic radiation (GCR) consists of heavy charged particles, from protons to iron ions. Astronauts and cosmonauts aboard Low Earth Orbit (LEO) spacecraft, such as the NASA Space Shuttle and the International Space Station (ISS), or aboard spacecraft travelling outside the Earth's magnetosphere on missions to and from the Moon or Mars are exposed to levels of ionizing radiation far in excess of those encountered on the ground [Citation5]. The levels of ionizing radiation in deep space are far in excess to levels on the ground, and crew members could be exposed to GCRs at a dose rate of ≈1 mSv/day, compared to an average ≈10 μSv/day on Earth. Moreover, SPEs ranging from less than an hour to several weeks can cause lethal dose rates 100 times higher than those of GCRs. Therefore, space radiation is one of the major health-related concerns for manned space exploration [Citation6].
The radiation environment in space is a complex mixture of particles of solar and galactic origin with a broad range of energies. For radiological protection, the relevant radiation fields are galactic cosmic radiation (GCR), particles ejected from the Sun during solar particle events (SPE) and secondary radiation produced through interaction with the planet's atmospheric nuclei [Citation7].
The distribution of GCRs is believed to be isotropic throughout interstellar space and consists mainly of protons and ions with energies up to several hundred GeV, with their peaks ranging from several hundred MeV to approximately 1 GeV. The GCRs consist of approximately 98% hadrons and approximately 2% leptons (e+ and e−), and the hadron component consists of approximately 87% protons, 12% α particles and 1% heavy ions [Citation8]. In the case of ISS, the GCRs contribute approximately 50% of the total dose equivalent rate received by astronauts/cosmonauts. SPE are predominantly composed of protons, electrons and an even smaller part of heavy ions. Geomagnetically trapped particles consist of protons and electrons, which are trapped in the geomagnetic field layer [Citation9].
2 LET (linear energy transfer) and stopping power
HZE (High charge and energy) particles create ionization immediately and continuously as they penetrate matter. Because of their large mass, they travel in straight trajectories with a relatively well defined stopping point or range. The pattern of HZE energy deposition is characterized by a dense core of ionization that is localized along the trajectory of the particle [Citation10]. LET reflects the rate at which ionization is produced along the track of charged particles and has dimensions of energy per unit length (e.g., keV/μm).
X-ray and γ-ray photons deposit energy in tissue in a highly dispersed manner, characterized as low LET. Linear energy transfer (LET) is the major parameter that characterizes radiation in this field. Also referred to as stopping power, LET represents the mean amount of energy an incident particle transfers to the target medium per unit path length. IR (ionizing radiation) can either be low LET (sparsely ionizing) or high LET (densely ionizing). Photons are low LET radiation, displaying a very broad energy distribution in tissue, and the peak dose is located relatively close to the surface [Citation10] ().
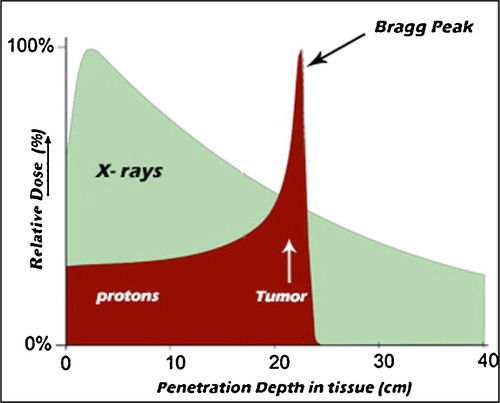
Fig. 1 Comparison of the depth dose profiles of high energetic photons and protons. Protons show the characteristic inverse depth dose profile (Bragg peak).
Electrons have sparse ionizations along the track (0.2 keV/μm) and are classified as low LET radiation. This classification also applies to photons that produce sparsely ionizing electrons, whereas HZE particles can have a LET > 100 keV/μm and are classified as high LET radiations [Citation11].
3 Depth dose distribution (Bragg peak)
In , the depth dose profile of electromagnetic radiation is compared to that of carbon ions. For low-energy X-rays, the stochastic absorption by photoelectric and Compton scattering yield an exponential decay of absorbed dose with penetration. For greater photon energies, the produced Compton electrons are strongly forwardly scattered and transport some of the transferred energy from the surface to greater depths, yielding an increase in dose in the first few centimetres. For photon beams produced as electron Bremsstrahlung in clinical linacs, there is an increase of the dose distribution within the first few centimetres (‘build up’), and after the maximum, the dose drops essentially according to an exponential law. Thus, for deep seated tumours, the dose delivered to the tumour using a single beam is generally lower than the dose to the normal tissue in front of the tumour [Citation12].
Because of their large mass, compared with electrons and photons, heavy charged particles travel in straight trajectories as they penetrate tissue. High energy hadrons incident upon tissues begin to slow down from collisions with electrons in matter and release small amounts of energy along the track. This is referred to as the Bragg plateau. As they approach the end of their range, they begin to decelerate rapidly, depositing a large amount of energy in a very short distance (). This is referred to as the Bragg peak, first described in 1907 [Citation13]. Beyond the Bragg peak, the energy deposition diminishes very rapidly, and thus tissue beyond the Bragg peak receives little or no radiation dose. The inverse dose profile, i.e., the increase of energy deposition with penetration depth up to a sharp maximum at the end of the particle range, is the main reason to use heavy charged particles in tumour therapy instead of photons [Citation11].
Carbon ion beams in the energy range of approximately 100–450 MeV/u offer excellent conditions for tumour therapy, in particular for the treatment of deep-seated, radio-resistant tumours. Their depth-dose distribution is characterized by a low dose in the entrance channel, small lateral beam spread and an elevated biological effectiveness in the Bragg peak region [Citation14].
Clinical experiences have demonstrated that carbon ion radiotherapy is effective in such regions as the head and neck, skull base, lung, liver, prostate, bone and soft tissues, and pelvic recurrence of rectal cancer, as well as for histological types, including adenocarcinoma, adenoid cystic carcinoma, malignant melanoma and various types of sarcomas, against which photon therapy could be less effective [Citation15].
Therefore, the physical properties of charged particle radiation provide several significant benefits in radiotherapy. First, the depth dose profile is inverted compared to photon beams and they exhibit an increased biological effectiveness, in particular, at the end of their range and thus in the target volume. Second, there is little damage to surrounding normal tissue, particularly beyond the distal boundary of the tumour, due to the rapid decrease in energy deposition distal to the Bragg peak [Citation16,Citation17].
4 RBE and OER (Oxygen enhancement ratio)
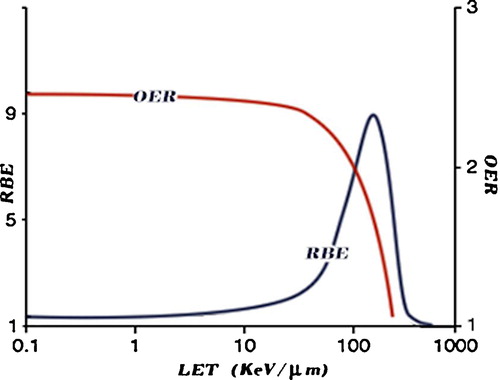
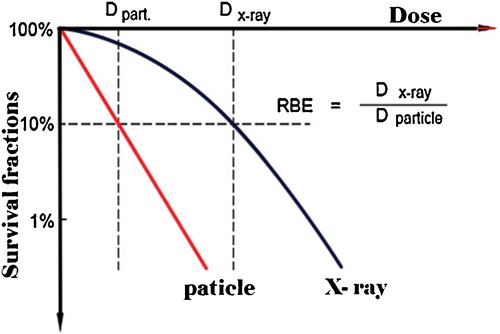
RBE compares the efficiency of different types of radiation to produce a defined biological effect compared to a reference photon radiation. It is defined as a ratio of the dose of the reference radiation to that of the test radiation required to yield the same biological end point, such as cell killing, DNA damage, and chromosome aberrations [Citation2,Citation18]. The average RBE for protons is 1.1 [Citation19], while the average RBE for carbon ions is much higher, estimated to be 2.5–3. demonstrates a schematic dose effect curve for cell inactivation for particles compared to that of X-rays.
Fig. 2 Definition of relative biological effectiveness (RBE), illustrated for cell survival curves.
The RBE varies not only with the type of radiation but also with the type of cell or tissue, biologic effect under investigation, dose, dose rate and fractionation. In general, the RBE increases with the LET to reach a maximum RBE of 3–8 (depending on the level of cell kill) at LET ∼ 200 keV/μm and then decreases because of energy overkill, as shown in .
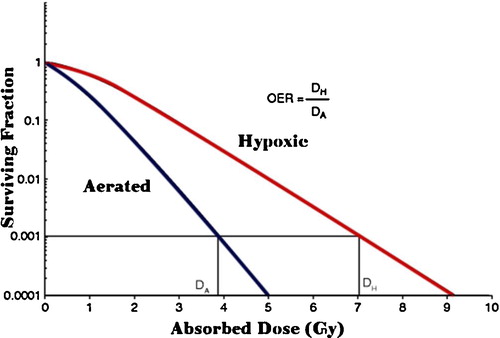
The majority of DNA damage by either high- or low-LET radiation is thought to arise indirectly through the production of reactive oxygen species (ROS). Therefore, the presence or absence of molecular oxygen within a cell influences the biological effects of ionizing radiation. Oxygenated tissues are more sensitive to low LET radiation, reflecting the requirement for ROS production to damage DNA. Unfortunately, many tumours are hypoxic, or have hypoxic regions, and show significant radioresistance to low-LET radiation. Especially for low-LET radiations, the larger the cell oxygenation above anoxia, the larger the biological effects until saturation of the effect of oxygen occurs. One measure of this resistance is the “oxygen enhancement ratio” (OER). OER is defined as the ratio of doses without and with oxygen (hypoxic versus well oxygenated cells) to produce the same biological effect. The OER decreases as the LET increases and approaches OER = 1 at approximately LET = 150 keV/μm, as shown in .
For both photon and proton radiation, the OER is ∼3, meaning that hypoxic cells require three times the dose as normoxic cells to achieve the same level of cell killing. Importantly, carbon ions show a much lower OER; thus, even hypoxic tumours show significant sensitivity to high LET carbon ion radiation. This most likely reflects the fact that the highly charged carbon ions achieve dense ionization along the tracks even in hypoxic conditions, resulting in a high level of clustered DNA damage. Clustered DNA damages are difficult to repair [Citation22,Citation23] ().
Fig. 4 Effect of hypoxia upon cell SF and the definition of the oxygen-enhancement ratio (OER).
Although heavy ion beams and protons exhibit a similar physical profile, carbon ions have a significantly better biological effectiveness. For protons, the RBE is elevated only at the last few micrometres of the ion's range. For carbon ions, the regime of increased RBE extends over the last centimetres of range and coincides with the dose elevation of the Bragg peak. Clinical proton beams in the range of 160–230 MeV are assumed to have an overall clinical RBE of 1.1–1.2, while for the heavier carbon ions, the RBE distribution in the target volume varies between 2 and 5 [Citation24].
5 Nuclear fragmentation
Protons and carbon ions are classified as hadrons and are thus capable of nuclear interactions. When heavy ions pass through a thick absorber, such as the human body or the thick shielding of a space craft, even small cross sections for nuclear reactions produce a significant amount of lighter reaction products. The projectile fragments have the same velocity as the original particle but reduced mass and charge. This results in a mixture of particles with different LETs passing through the tumour.
In radiotherapy, the change in biological efficiency between the primary ions, e.g., carbon, and the lighter secondaries has to be taken into account in treatment planning. Currently, the only technically feasible method for the evaluation of the delivered dose distribution is based on tissue activation measurements by means of positron emission tomography (PET) [Citation25]. This makes use of β+-active nuclei (e.g., 10C, 11C, 13N and 15O) produced in nuclear interactions of the therapeutic beam with the tissue nuclei. This method has been shown to be applicable for the monitoring of both proton and carbon ion beams [Citation26].
For space research, fragmentation represents a major obstacle: to shield against the very numerous low energy particles of a few hundred MeV, very efficient energy absorbers are needed, in which the few high-energy particles produce showers of light low energy particles [Citation27]. The process of nuclear fragmentation can play a key role in reducing both the physical dose and biological effectiveness of the radiation encountered in deep space. Hydrogenous materials and light elements are expected to be more effective shields against the deleterious effects of galactic cosmic rays (GCR) than aluminium, which is used in current spacecraft hulls [Citation28].
6 Direct and indirect radiation effect
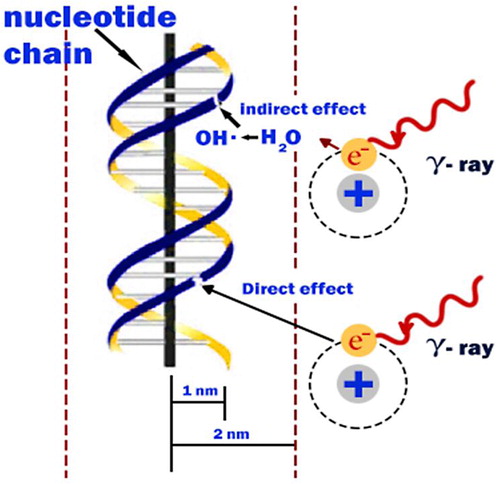
Radiation damage to the cell can be caused by the direct or indirect action of radiation on the DNA molecules. In the direct action, the radiation hits the DNA molecule directly, disrupting the molecular structure. Such structural change leads to cell damage or even cell death. Damaged cells that survive may later induce carcinogenesis or other abnormalities. This process becomes predominant with high-LET radiations, such as α-particles and neutrons, and high radiation doses. In the indirect action, the radiation hits the water molecules, the major constituent of the cell, and other organic molecules in the cell, whereby free radicals such as perhydroxyl (HO2•) and alkoxy (RO2•) are produced.
Free radicals are characterized by an unpaired electron in the structure, which is very reactive and therefore reacts with DNA molecules to cause molecular structural damage. Hydrogen peroxide, H2O2, is also toxic to the DNA molecule. The result of the indirect action of radiation on DNA molecules is the impairment of function or death of the cell. The number of free radicals produced by ionizing radiation depends on the total dose. It has been found that the majority of radiation-induced damage results from the indirect action mechanism because water constitutes nearly 70% of the composition of the cell [Citation29].
Low-LET radiations (photon) show a uniform, sparse spatial distribution of ionization in cells. High-LET particles bring about a dense ionization along their track through energy deposit to the medium, showing distributions called track structures. Charged-particle beams that form the Bragg peak in matter change the ionization density along the travelling direction, showing complexities [Citation30] ().
7 DNA damage and repair
The DNA molecule of a cell is the most sensitive target of radiation. The biological effects of heavy ions with high LET are to induce complex DNA damage, including DNA double-strand breaks (DSBs) and non-DSB clustered DNA damage. Clustered lesions are defined as two or more lesions (base damage, single strand break, abasic site) formed within a ∼10-bp segment by a single radiation track [Citation32]. This type of damage is difficult to repair and requires coordination with more DNA repair factors [Citation33]. Any single unrepaired or mis-repaired DSB (Double strand break) is adequate to induce genome instability and promote tumourigenesis [Citation34]. The dense core of ionization along the trajectory makes heavy ion particles have higher radiobiological effects than electromagnetic radiation. Moreover, the DNA damages induced by its energy deposition are DSB dominant.
When DNA DSBs occur, several DNA damage responses will be triggered, such as cell cycle arrest, DNA repair and apoptosis. DNA damage response incorporates many types of proteins because, according to the gene code in humans, including Mre11-Rad50-Nbs1 (MRN) complex, 53BP1, BRCA1 and H2AX, each type of protein plays different roles in different phases of DNA damage response [Citation35,Citation36].
DNA double-strand breaks (DSBs) are the most dangerous lesions in eukaryotic cells. If unrepairable, they can cause cell death or carcinogenesis. One of the earliest responses to DSBs caused by ionizing radiation (IR) or by chemicals was the phosphorylation of the core variant histone H2AX at 139-serine in megabase chromatin domains around DSB sites with the formation of discrete nuclear γH2AX foci [Citation37]. In human cells, the maximum induction of γH2AX is observed approximately 30–60 min after IR. Next, it is slowly eliminated, and the kinetics of elimination correlate with the kinetics of DSB rejoining [Citation38]. γH2AX is required for the concentration and stabilization of DNA repair proteins and plays a role in both non-homologous end-joining (NHEJ) and homologous recombination (HR) repair pathways. The ratio of DNA DSBs to visible γH2AX foci is approximately 1:1, which forms the basis of a sensitive quantitative method for the detection of DNA DSBs in mammalian cells [Citation39].
8 Radiation protection and mitigation
Highly energetic heavy charged particles known as HZE particles and protons are among the most biologically significant components of space radiation. While there are many different types of cellular and molecular damage induced by HZE particles and protons, these types of ionizing radiations can induce oxidative stress in cells [Citation40] and animals [Citation41].
Oxidative stress is a term that describes the biological damage of DNA, lipids and proteins by either oxygen reactive organic radicals or oxygen radicals, e.g., the hydroxyl radical. Oxidative stress results whenever there is an imbalance between the pro-oxidants and antioxidants. Because the levels of oxidative stress are expected to be higher than normal during space travel due to the higher doses of radiation to which astronauts are exposed, the use of antioxidants could possibly counteract the effects of radiation-induced oxidative stress in astronauts during space flight, thereby preventing the downstream effects of the excessive oxidative stress induced by radiation, such as the development of malignancy. During a long-term stay in space, astronauts will be constantly exposed to low-dose space radiation. Thus, space radiation is one of the major health-related concerns for space exploration [Citation42].
There are three fundamental approaches to pharmacologic intervention (), and a US National Cancer Institute Workshop [Citation43] has recommended using different terminology for the different approaches. Protection or prophylaxis would refer to therapies that must begin before the time of irradiation; a classic example is the use of a free radical scavenger such as amifostine. Mitigation refers to therapies that begin after irradiation but before there is clear evidence of clinical disease [Citation44,Citation45]. Treatment refers to therapies that are effective after clear clinical disease has developed [Citation46].
Fig. 6 Recommended terminology for therapeutic approaches to normal tissue radiation injuries.

The ideal radioprotector would be one that can be taken either before or after radiation exposure and that would result in an increase in radiation protection, especially for late effects. It would have limited toxicity and should be able to decrease the lifetime cancer risks from the radiation exposure. Several different classes of radioprotectors and mitigators are currently being tested for use in space exploration. They can be categorized by their origin as synthetic or natural compounds, their availability as pharmaceutical drugs or nutritional supplements, and, most importantly, according to their molecular mechanism of action. On the molecular and cellular levels, this classification may include (1) direct scavengers of ROS and other free radicals, (2) anti-oxidant agents that induce/alter endogenous levels of ROS-detoxifying enzymes such as MnSOD, (3) agents that enhance or modulate DNA damage signalling and repair, and (4) agents that prevent execution of death pathways in radiation-damaged cells. According to several recent studies, these mechanisms are not mutually exclusive. One radioprotective agent could exert its biological activity through one or more pathways [Citation47].
The effectiveness of antioxidants in space is further complicated by the presence of HZE particles. In principle, antioxidants should provide reduced or no protection against densely ionizing radiation because direct effect is more important than free radical-mediated indirect radiation damage at high-LET. However, recent experiments suggest that an efficient radioprotection by dietary supplements can be achieved even in cases of exposure to high-LET radiation. Vitamin A strongly reduces the induction of fibroma in rats exposed to swift Fe-ions [Citation48]. Following exposure of human cells to accelerated high-LET carbon ions, it has been shown that beer reduces the yield of chromosomal aberrations. Most of the detrimental effects of heavy ions on the CNS of rats are suppressed by dietary supplements of strawberries [Citation49].
9 Outlook
The biophysical and biological properties of heavy charged particles are an attractive topic in radiotherapy and space missions. In particle therapy, the particles offer excellent conditions for tumour therapy, particularly for the treatment of deep-seated radio-resistant tumours. Heavy ion particles are the concern of space-radioprotection because the radiation spectrum of the galactic cosmic radiation (GCR) consists of heavy charged particles, from protons to iron ions. Astronauts and cosmonauts aboard Low Earth Orbit (LEO) spacecraft, such as the International Space Station (ISS), are exposed to levels of ionizing radiation far in excess of those encountered on the ground. Large basic research works are still needed to elucidate indications of most of the advantages of particle therapy and to diminish the radiation hazards present during space exploration.
Acknowledgments
This work was supported by TWAS-UNISCO, the Chinese Academy of Science and Institute of Modern Physics, Institute of modern physics (Lanzhou, China).
Notes
Peer review under responsibility of Taibah University.
References
- H.NikjooS.UeharaD.EmfietzoglouA.BrahmeHeavy charged particles in radiation biology and biophysicsNew J. Phys.102008128
- E.FokasG.KraftH.AnR.Engenhart-CabillicIon beam radiobiology and cancer: time to update ourselvesBiochim. Biophys. Acta17962009216229
- L.D.SkarsgardRadiobiology with heavy charged particles: a historical reviewPhys. Med.141998119
- M.DuranteJ.S.LoefflerCharged particles in radiation oncologyNat. Rev. Clin. Oncol.720103743
- E.R.BentonE.V.BentonSpace radiation dosimetry in low-Earth orbit and beyondNucl. Instrum. Methods Phys. Res. B1842001255294
- L.SihverPhysics and biophysics experiments needed for improved risk assessment in spaceActa Astronaut.632008886898
- ICRPAssessment of radiation exposure of astronauts in space, ICRP Publication 123Ann. ICRP4220131339
- J.W.WilsionM.S.ClowdsleyF.A.CucinottaDeep space environments for human explorationAdv. Space Res.34200412811287
- M.DuranteF.A.CucinottaPhysical basis of radiation protection in space travelRev. Mod. Phys.83201112451278
- U.WeberG.KraftComparison of carbon ions versus protonsCancer J.152009325332
- A.ChristopherB.ThomasT.HirohikoJ.A.NickoloffHeavy charged particle radiobiology: using enhanced biological effectiveness and improved beam focusing to advance cancer therapyMutat. Res.7112011150157
- M.ScholzHeavy ion tumour therapyNucl. Instrum. Methods Phys. Res. B16120007682
- W.BraggR.KleemannOn the α-particles of radium and their loss of range in passing through various atoms and moleculesPhilos. Mag.101905318340
- E.HaettnerH.IwaseM.KramerG.KraftD.SchardtExperimental study of nuclear fragmentation of 200and 400 MeV/u 12C ions in water for applications inparticle therapyPhys. Med. Biol.58201382658279
- T.HirohikoK.TadashiB.MasayukiClinical advantages of carbon-ion radiotherapyNew J. Phys.102008110
- T.DeLaneyH.M.KooyProton and Charged Particle Radiotherapy2007Lippincott, Williams and WilkinsPhiladelphia
- A.J.LomaxCharged particle therapy: the physics of interactionCancer J.152009285291
- U.AkikoA.KoichiK.SachikoComparison of biological effectiveness of carbon-ion beams in Japan and GermanyInt. J. Radiat. Oncol. Biol. Phys.73200915451551
- D.Schulz-ErtnerO.JakelW.SchlegelRadiation therapy with charged particlesSemin. Radiat. Oncol.62006249259
- G.KraftTumor therapy with heavy charged particlesProg. Part. Nucl. Phys.452000S473S544
- J.M.BrianBiological Effects of Ionizing Radiation, Nuclear Medicine Radiation Dosimetry Advanced Theoretical2010Springer-VerlagLondon
- H.PaganettiSignificance and implementation of RBE variations in proton beamtherapyTechnol. Cancer Res. Treat.22003413426
- B.M.SutherlandP.V.BennettH.SchenkO.SidorkinaClustered DNA damages induced by high and low LET radiation, including heavy ionsPhys. Med.172001202204
- M.HadaA.G.GeorgakilasFormation of clustered DNA damage after high-LET irradiation: a reviewJ. Radiat. Res.492008203210
- W.EnghardtP.CrespoF.FiedlerR.HinzK.ParodiJ.PawelkeF.PönischCharged hadron tumour therapy monitoring by means of PETNucl. Instrum. Methods A5252004284288
- K.ParodiT.BortfeldW.EnghardtF.FiedlerA.KnopfH.PaganettiJ.PawelkeG.ShakirinH.ShihPET imaging for treatment verification of ion therapy: implementation and experience at GSI Darmstadt and MGH BostonNucl. Instrum. Methods A5912008282286
- G.KraftRadiobiological effects of highly charged ions. Their relevance for tumor therapy and radioprotection in spaceF.J.CurrellThe Physics of Multiply and Highly Charged Ionsvol. 12003Kluwer Academic Publishers149196
- L.GueterslohC.ZeitlinJ.HeilbronnT.MillerPolyethylene as a radiation shielding standard in simulated cosmic-ray environmentsNucl. Instrum. Methods Phys. Res. B2522006319332
- G.B.SahaRadiation biologyPhysics and Radiobiology of Nuclear Medicine2013Springer Science+Business MediaNew York
- Y.FurusawaHeavy-ion radiobiologyH.Tsujiiet al.Carbon-Ion Radiotherapy: Principles, Practices, and Treatment Planning2014SpringerJapan
- E.J.HallA.J.GiacciaRadiobiology for the Radiologist7th ed.2012LippincottPhiladelphia
- Li.SuiY.WangX.WangClustered DNA damage induced by protons radiation in plasmid DNAChin. Sci. Bull.58201332173223
- A.AsaithambyN.UematsuA.ChatterjeeM.D.StoryS.BurmaD.J.ChenRepair of HZE-particle-induced DNA double-strand breaks in normal human fibroblastsRadiat. Res.1692008437446
- S.P.JacksonSensing and repairing DNA double-strand breaksCarcinogenesis232002687696
- S.GiuntaR.BelotserkovskayaS.P.JacksonDNA damage signaling in response to double-strand breaks during mitosisJ. Cell Biol.1902010197207
- S.T.Al RashidS.M.HardingC.LawC.CoackleyR.G.BristowProtein–protein interactions occur between p53 phosphoforms and ATM and 53BP1 at sites of exogenous DNA damageRadiat. Res.1752011588598
- N.V.TomilinL.V.SolovjevaM.P.SvetlovaN.M.PleskachI.A.ZalenskayaP.M.YauE.M.BradburyVisualization of focal nuclear sites of DNA repair synthesis induced by bleomycin in human cellsRadiat. Res.1562001347354
- M.SvetlovaL.SolovjevaK.NishiI.NazarovJ.SiinoN.TomilinElimination of radiation-induced gamma-H2AX foci in mammalian nucleus can occur by histone exchangeBiochem. Biophys. Res. Commun.3582007650654
- K.RothkammM.LobrichEvidence for a lack of DNA double strand break repair in human cells exposed to very low X-ray dosesProc. Natl. Acad. Sci. U.S.A.100200350575062
- A.R.KennedyJ.H.WareJ.GuanJ.J.DonahueJ.E.BiaglowZ.ZhouJ.StewartM.VasquezX.S.WanSelenomethionine protects against adverse biological effects induced by space radiationFree Radic. Biol. Med.362004259266
- J.GuanJ.StewartJ.H.WareZ.ZhouJ.J.DonahueA.R.KennedyEffects of dietary supplements on the space radiation-induced reduction in total antioxidant status in CBA miceRadiat. Res.1652006373378
- L.TingtingX.DanL.HeP.HailongZ.MingyueW.JufangZ.GuangmingRisk assessment of space radiation during manned space flightsRend. Fis. Acc. Lincei252014S17S21
- H.B.StoneJ.E.MoulderC.N.ColemanModels for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuriesRadiat. Res.1622004711728
- J.E.MoulderB.L.FishE.P.CohenACE, inhibitors and AII receptor antagonists in the treatment and prevention of bone marrow transplant nephropathyCurr. Pharm. Des.92003737749
- J.H.KimS.L.BrownA.KolozsvaryModification of radiation injury by Ramipril, inhibitor of angiotensin converting enzyme, on optic neuropathy in the ratRadiat. Res.1612004137142
- E.P.CohenS.HussainJ.E.MoulderSuccessful treatment of radiation nephropathy with angiotensin II blockadeInt. J. Radiat. Oncol. Biol. Phys.552003190193
- D.JaroslawG.WilfriedJ.E.BaulchHeavy ions radioprotectors and genomic instability: implications for human space explorationRadiat. Environ. Biophys.492010303316
- F.J.BurnsM.S.TangK.FrenkelA.NadasF.WuA.UddinInduction and prevention of carcinogenesis in rat skin exposed to space radiationRadiat. Environ. Biophys.462007195199
- B.M.RabinJ.A.JosephB.Shukitt-HaleEffects of age and diet on the heavy particle-induced disruption of operant responding produced by a ground-based model for exposure to cosmic raysBrain Res.10362005122129