?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In the current study, we synthesized an Al-doped TiO2 nanoceramic material using a chemical and solid-state diffusion routes, and the LPG sensing performance of this material was comparatively studied. The structural conformation of the as-synthesized nanoparticles was investigated using X-ray diffraction (XRD) analysis, and the morphology was analysed by scanning electron microscopy (SEM). The optical absorption spectrum of the as-synthesized nanoparticles was recorded by ultraviolet–visible spectroscopy. Thermal study of the samples was performed via thermogravimetric analysis (TGA). Based on a comparison of both samples, the chemically synthesized Al-doped TiO2 nanoparticle exhibited good sensor performance for LPG. The material synthesized by the chemical route exhibited good sensing properties.
1 Introduction
LPG has a wide variety of applications in various fields, such as domestic, agriculture and industry. In addition, LPG can be utilized as a cooking fuel. Because LPG is composed of hydrocarbons, it causes indoor air pollution resulting in detrimental affects on human health (i.e., irritated respiratory tract, nose and eyes). Therefore, its detection becomes necessary at lower concentrations to avoid accidents.
Verma et al. [Citation1] studied the LPG sensing characteristics of iron titanium oxide thin films, which was also employed as an opto-electronic humidity sensor. Parveen et al. [Citation2] synthesized a polyaniline–titanium dioxide nanocomposite for LPG sensing. Yadav et al. [Citation3] reported a solid-state titania for use as a LPG gas sensor at room temperature. Singh et al. reported the LPG sensing characteristics of ZnxCu1−xFe2O4 (0.0 ≤ x ≤ 0.8) nanocomposites. In this study, the effect of the zinc concentration on the magnetic properties was also investigated using a vibrating sample magnetometer. The results of this study indicated that the samples are more selective and sensitive towards LPG than CO2 gas [Citation4]. Singh et al. demonstrated the room temperature sensing application of zinc ferrite nanorods for LPG. In this investigation, more variations in the electrical resistance were observed for LPG than for CO2 gas, which indicates that the samples are more selective and sensitive towards LPG [Citation5]. Jaiswal et al. studied the enhancement of the gas sorption ability of a LPG sensor. This study concluded that mesoporosity plays a crucial role in enhancing the sorption capability of thin films [Citation6]. Tsai et al. [Citation7] prepared Al-doped TiO2 ceramic nanoparticles by a single-step direct combination of vaporized Ti, Al and O2, and these nanoparticles exhibited visible-light photocatalytic activity. Tsai et al. [Citation8] also prepared a high-quality Al-doped TiO2 visible-light photocatalyst by combination of vaporized Ti, Al and O2 using a 6 kW thermal plasma system. Liu et al. [Citation9] demonstrated the synthesis of an aluminium-doped TiO2 mesoporous material using a solid-state reaction with cetyltrimethylammonium bromide as a template agent and tetrabutyl orthotitanate as a precursor. Kumar et al. [Citation10] studied the reflectivity and photostability of Al-doped TiO2 nanoparticles synthesized by a sol–gel method. Li et al. [Citation11] demonstrated p-type hydrogen sensing with an Al-doped TiO2 nanostructure.
In the current study, we synthesized Al-doped TiO2 nanoparticles by two different routes (i.e., chemical and solid-state diffusion) and studied their LPG sensing characteristics. To the best of our knowledge, no studies on the LPG sensing ability of Al-doped TiO2 nanoparticles have been reported. In addition, we investigated the LPG sensing response characteristics at concentration levels up to 400 ppm. The operating temperature was determined to be 448 K, which is much lower than the auto-ignition temperature of LPG.
2 Experimental
2.1 Chemical route
AR grade chemicals were used in this study without further purification. Aluminium nitrate (Al(NO3)3·9H2O) and titanium dioxide (TiO2) were used as starting chemicals. First, 0.2 M Al(NO3)3·9H2O was added to 1 M TiO2 under constant magnetic stirring using an organic media (i.e., methanol). This mixture was maintained at room temperature overnight for complete removal of the methanol. The as-obtained product was sintered at 600 °C.
2.2 Solid state diffusion route
The Al-doped TiO2 nanoparticles were prepared by a solid-state diffusion route using the starting chemicals (i.e., Al(NO3)3·9H2O and TiO2). 0.2 M Al(NO3)3·9H2O was mixed directly with 1 M TiO2 using an agate mortar and pestle. The crushed sample was pre-sintered at 400 °C for 2 h and crushed again in a mortar and pestle. The as-obtained product was sintered at 800 °C for 6 h. Finally, the sample in the crucible was allowed to cool to room temperature.
2.3 Characterization of materials and gas sensing measurements
The structural purity of the as-synthesized nanomaterials was analysed by X-ray diffraction (XRD) analysis carried out on a Rigaku miniflex-II X-ray diffractometer in a 2θ range of 10–70°.
The topography of the samples was observed using scanning electron microscopy (SEM) on a JEOL JSM-7500F. The optical absorption curve for the Al-doped TiO2 nanoparticles was acquired using an Agilent 8453 UV-Visible-NIR Spectrophotometer. The thermal study of the samples was performed using thermogravimetric analysis (TGA) with a Shimadzu DTG-60 h thermal analyser under a nitrogen atmosphere.
The sensing films of the as-prepared samples were prepared by a screen-printing technique on SiO2 substrate with a size of 25 mm × 25 mm using a binding agent (composed of butyl carbitol and ethyl cellulose) [Citation12]. The ohmic contacts were deposited on the sensing film. The gas sensing responses of the as-synthesized materials were analysed by a specially designed sensing unit. The sensing characteristics were studied using air as a background gas. The selected volume of the LPG was inserted into the chamber to maintain the required concentration inside the chamber. The voltage drop method was utilized to measure the resistance change as a function of the LPG concentration [Citation13]. The sensing response of the films is defined as [Citation14]:(1)
(1) where Ra and Rg are the resistances of chemiresistor in air and gas, respectively.
3 Results and discussion
3.1 Structural analysis and surface morphological studies
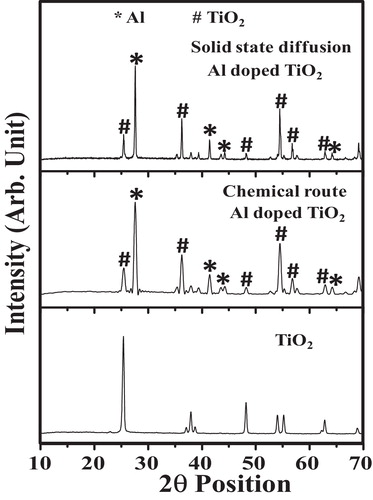
shows the XRD pattern of TiO2 and the Al-doped TiO2 nanoparticles synthesized by the chemical and solid-state diffusion routes. The position of the diffraction peaks in the pattern and the relative intensity data are in agreement with ICDD File card no. 01-084-1285 for anatase TiO2. The characteristics peaks of the Al-doped TiO2 system are indicated in a symbolic manner. The XRD patterns of the Al-doped TiO2 nanoparticles synthesized by the chemical and solid-state diffusion routes are also in excellent agreement with the parent TiO2 with the characteristics peaks of aluminium. Some characteristics peaks of aluminium were suppressed, which indicates that Al is nicely doped in the TiO2 structure. These peaks corresponding to aluminium were indexed to ICDD card no. 01-081-1667. More broadening of the diffraction peaks was observed for the Al-doped TiO2 nanoparticles synthesized by the chemical route. According to Scherrer's equation, the size of the Al-doped TiO2 nanoparticles prepared by the chemical route are smaller than the particles synthesized by the solid-state diffusion route [Citation15]. This increase in the particle size in the solid-state diffusion route is due to the annealing temperature because the increase in temperature may result in merging of the particles [Citation16].
Fig. 1 XRD pattern of the Al-doped TiO2 nanoparticles synthesized by chemical and solid-state diffusion routes.
Because the Al-doped TiO2 belongs to the tetragonal system, the lattice constants are computed using Eq. Equation(2)(2)
(2) .
(2)
(2) where a and c are the lattice constants and d is the lattice spacing. In comparison to anatase TiO2, the doping of Al into TiO2 results in an increased in the a lattice parameter and a decrease in the c lattice parameter. This change in the lattice parameters results in a decrease in the particle size with increasing Al concentration [Citation17]. The lattice parameters estimated for the Al-doped TiO2 system synthesized by both routes are summarized in .
Table 1 Lattice parameters of the Al-doped TiO2 nanoparticles.
Based on the results in , the a lattice parameter is larger than that for the chemically synthesized Al-doped TiO2 nanoparticles. Using Scherrer's equation, the average crystallite sizes were determined to be 21.6 and 24.8 nm for the material prepared by the chemical and solid-state diffusion routes, respectively. This decrease in the size for the particles prepared by the chemical route may be due to a lower annealing temperature and an increase in the lattice parameters. The smaller particle size provides a larger area for the adsorption of gas molecules, which results in improved sensing.
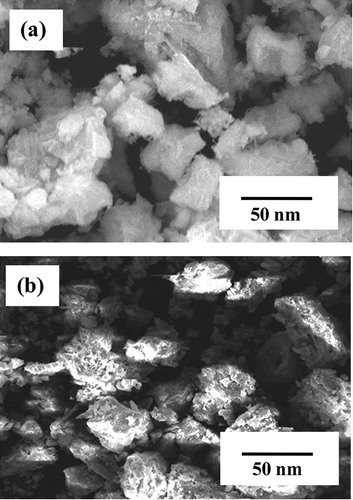
(a) and (b) shows the SEM images of the Al-doped TiO2 nanoparticles synthesized by the chemical and solid-state diffusion routes, respectively. The particles synthesized by the chemical route have a flat surface and exhibit a small amount of aggregation. However, the particles synthesized by the solid-state diffusion route have fluffy surfaces with a rough morphology. The rough morphology is not preferable for gas sensing applications. The particle size calculated using XRD analysis is in good agreement with that obtained in the SEM study.
3.2 Optical and thermal study of Al-doped TiO2 nanoparticles
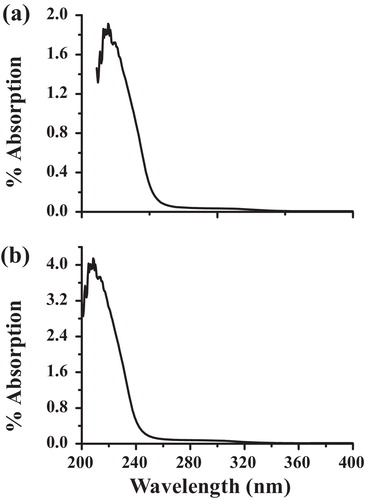
(a) and (b) shows the UV–Vis spectrum of the Al-doped TiO2 nanoparticles synthesized by the chemical and solid-state diffusion routes. The as-synthesized samples exhibit an intense absorption in the UV region at approximately 230–240 nm, which indicates that this material exhibits quantum confinement [Citation18].
Fig. 3 UV–Vis spectrum of the Al-doped TiO2 nanoparticles synthesized by (a) chemical and (b) solid-state diffusion routes.
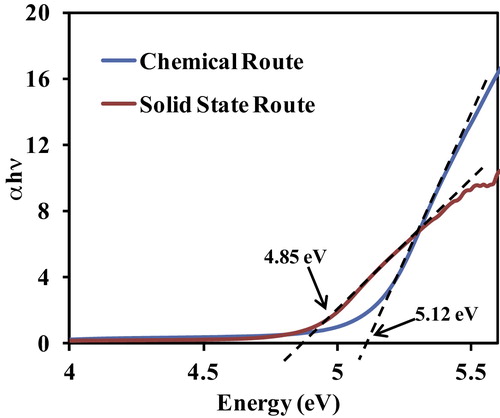
shows the direct band gap plot for the Al-doped TiO2 nanoparticles. The direct band gap of the Al-doped TiO2 nanoparticles synthesized by the chemical route is larger than that for the nanoparticles prepared by the solid-state diffusion route. According to the hyperbolic band model and effective mass approximation model, the band gap and particle size are inversely related to each other [Citation19]. Therefore, the band gap of the Al-doped TiO2 nanoparticles is larger when prepared by the chemical route, which produces a smaller average particle size. This conclusion based on the UV–Vis results is consistent with the previous XRD and SEM analyses.
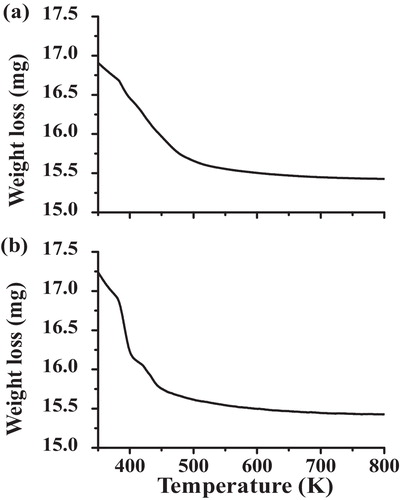
shows the TGA plot for the Al-doped TiO2 nanoparticles synthesized by the chemical and solid-state diffusion routes. The results indicate that significant weight loss was observed up to 450 K. At temperatures higher than 450 K, no weight loss was observed, and the curve plateaus to 800 K. Thermal analysis indicates that the sample acquires thermal stability from 440 to 800 K. Similarly, the operating temperature of the sensors lies in this range, which is favourable for LPG detection.
3.3 LPG sensing response
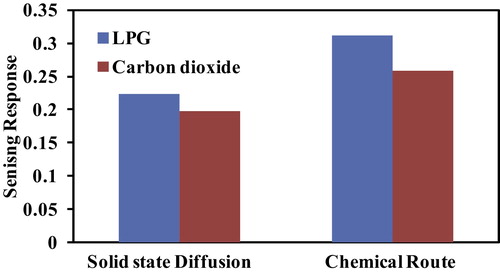
shows the selectivity response of Al-doped TiO2 towards CO2 and LPG at a concentration of 400 ppm and room temperature. Both samples are more selective towards LPG. Therefore, additional investigation of gas sensing is only focused on the LPG response.
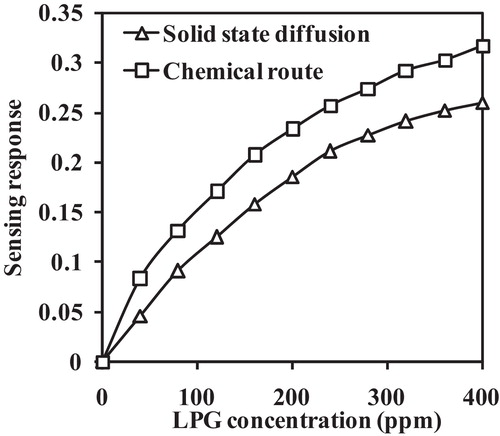
shows the LPG sensing response of the Al-doped TiO2 nanoparticles at room temperature. Based on the plot, the Al-doped TiO2 nanoparticles synthesized by the chemical route exhibit a higher sensing response. When the Al-doped TiO2 nanoparticles are exposed LPG, the sensor resistance decreases. The sensing response curve is nearly linear at lower concentrations of LPG but deviates from linearity at higher concentrations. In the current study, Al was doped into TiO2 as a sensitizer, which result in the formation of highly reactive species. This type of reactive species is useful in efficient gas sensing. The interaction between Al and adsorbed oxygen species can be expressed as
In this surface reaction, the Al atoms weakly interact with atmospheric oxygen. Therefore, the resulting species that form on the surface readily dissociate even at room temperature. In this case, oxygen captures electrons from LPG, which are added to the sensing surface. This addition of electrons into the conduction band of the sensing surface results in a decrease in the resistance [Citation20]. The interaction between LPG and Al-doped TiO2 via adsorbed oxygen can be expressed as
In this surface reaction, electrons are generated due to the formation of H2O and CO2.
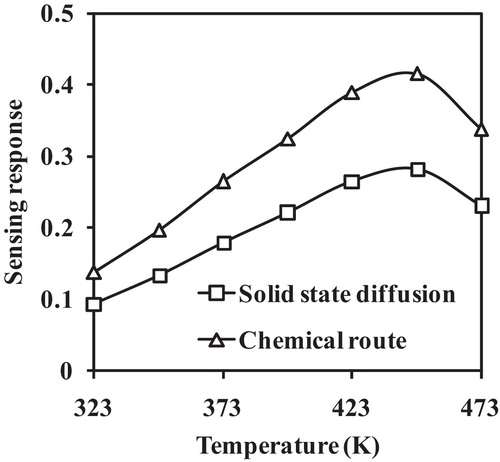
shows the operating temperature response for Al-doped TiO2 towards a fixed concentration (200 ppm) of LPG. The response curve indicates an optimum value at 448 K, which may be due to the large adsorption rate of atmospheric oxygen on the sensing surface resulting in enhancement of the sensing response. Beyond a certain temperature, the response value starts to decrease, which is due to the desorption of adsorbed oxygen molecules from the sensing surface. At a higher temperature, oxygen detaches from the surface due to an increase in thermal vibrations.
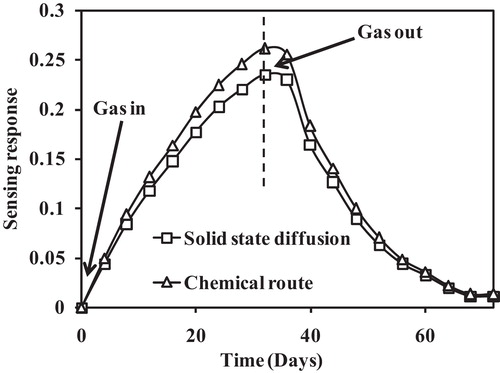
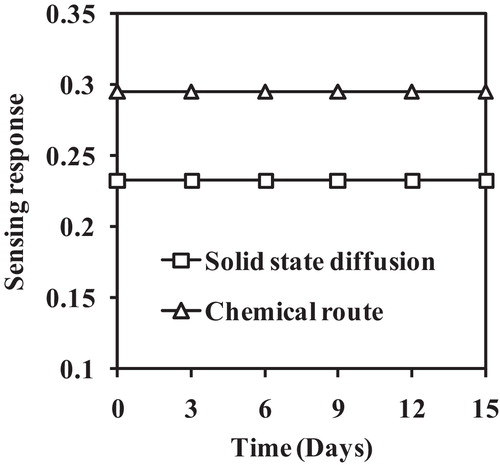
shows the transient response of the Al-doped TiO2 nanoparticles for 200 ppm LPG at room temperature. The rapid response (32 s) and recovery time (40 s) were determined from the plot for both samples. The recovery time is greater than the response time, which may be due to the formation of H2O and CO2 on the sensing surface. These species require longer periods of time to dissociate. Therefore, the recovery time is greater than the response time. shows the stability response for 15 days, which is nearly linear indicating the good stability of the sample to LPG. This good stability may be due to the stable structure of the nanoparticles [Citation21]. Based on these sensing parameters, these sensing films can be effectively used as LPG sensors.
4 Conclusions
In summary, we successfully synthesized Al-doped TiO2 nanoparticles using two different routes (i.e., chemical and solid-state diffusion routes). The XRD pattern confirmed the formation and structural purity of the as-synthesized materials. SEM and UV–Vis analyses indicated that the particle size was smaller for the material obtained by the chemical route. This analysis is in good agreement with the XRD study. The Al-doped TiO2 nanoparticles synthesized by the chemical route exhibit high selectivity, sensitivity and stability towards LPG. The operating temperature of the fabricated sensor was determined to be 448 K, which is much lower than the autoignition temperature of LPG. The good performance of the Al-doped TiO2 nanoparticles synthesized by the chemical route was due to the smaller particle size.
Acknowledgements
The authors wish to thank the Head of the Department of Physics, Sant Gadge Baba Amravati University, Amravati for providing the necessary facilities. One of the authors, Kailash Nemade, wishes to thank Prof. P.B. Maheshwari, Prof. S.M. Bang and Prof. N.H. Patil, Directors, J D College of Engineering and Management, Nagpur.
Notes
Peer review under responsibility of Taibah University.
References
- N.VermaS.SinghR.SrivastavaB.C.YadavFabrication of iron titanium oxide thin film and its application as opto-electronic humidity and liquefied petroleum gas sensorsOpt. Laser Technol.572014181188
- A.ParveenA.KoppalkarA.S.RoyLiquefied petroleum gas sensing of polyaniline–titanium dioxide nanocompositesSens. Lett.112012242248
- B.C.YadavA.YadavT.ShuklaS.SinghSolid-state titania-based gas sensor for liquefied petroleum gas detection at room temperatureBull. Mater. Sci.34201116391644
- A.SinghS.SinghB.D.JoshiA.ShuklaB.C.YadavP.TandonSynthesis, characterization, magnetic properties and gas sensing applications of ZnxCu1−xFe2O4 (0.0 ≤ x ≤ 0.8) nanocompositesMater. Sci. Semicond. Process.272014934949
- A.SinghA.SinghS.SinghP.TandonB.C.YadavR.R.YadavSynthesis, characterization and performance of zinc ferrite nanorods for room temperature sensing applicationsJ. Alloys Compd.6182015475483
- A.K.JaiswalS.SinghA.SinghR.R.YadavP.TandonB.C.YadavFabrication of Cu/Pd bimetallic nanostructures with high gas sorption ability towards development of LPG sensorMater. Chem. Phys.15420151621
- C.TsaiH.HsiH.BaiK.S.FanH.SunSingle-step synthesis of Al-doped TiO2 nanoparticles using non-transferred thermal plasma torchJpn. J. Appl. Phys.512012 01AL01–01AL06
- C.TsaiT.KuoH.HisFabrication of Al-doped TiO2 visible-light photocatalyst for low-concentration mercury removalInt. J. Photoenergy20122012874509874517
- S.LiuG.LiuQ.FengAl-doped TiO2 mesoporous materials: synthesis and photodegradation propertiesJ. Porous Mater.172010197206
- S.KumarN.K.VermaM.L.SinglaStudy on reflectivity and photostability of Al-doped titania nanoparticles and their reflectorsJ. Mater. Res.282013521528
- Z.LiD.DingC.NingNi-doped TiO2 nanotubes for wide-range hydrogen sensingNanoscale Res. Lett.92013118127
- K.R.NemadeS.A.WaghuleyChemiresistive gas sensing by few-layered grapheneJ. Electron. Mater.42201328572866
- K.R.NemadeS.A.WaghuleyLow operable temperature chemiresistive gas sensing by graphene–zinc oxide quantum dots compositesSci. Adv. Mater.62014128134
- K.R.NemadeS.A.WaghuleyLPG sensing application of graphene/Bi2O3 quantum dots compositesSolid State Sci.2220132732
- K.R.NemadeS.A.WaghuleyLPG sensing application of graphene/CeO2 quantum dots compositeAIP Conf. Proc.1536201312581259
- A.GaberM.A.Abdel-RahimA.Y.Abdel-LatiefM.N.Abdel-SalamInfluence of calcination temperature on the structure and porosity of nanocrystalline SnO2 synthesized by a conventional precipitation methodInt. J. Electrochem. Sci.920148195
- L.XuM.P.GarrettB.HuDoping effects on internally coupled seebeck coefficient, electrical, and thermal conductivities in aluminum-doped TiO2J. Phys. Chem. C11620121302013025
- K.R.NemadeS.A.WaghuleyUV–VIS spectroscopic study of one pot synthesized strontium oxide quantum dotsResults Phys.320135254
- K.R.NemadeS.A.WaghuleyLow temperature synthesis of semiconducting α-Al2O3 quantum dotsCeram. Int.40201461096113
- V.R.ShindeT.P.GujarC.D.LokhandeEnhanced response of porous ZnO nanobeads towards LPG: effect of Pd sensitizationSens. Actuators B1232007701706
- K.V.GuravP.R.DeshmukhC.D.LokhandeLPG sensing properties of Pd-sensitized vertically aligned ZnO nanorodsSens. Actuators B1512011365369