Abstract
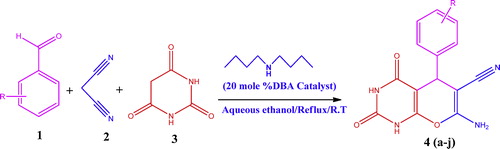
Annulated pyrano[2,3-d]pyrimidine derivatives were synthesized via one-pot, three-component condensation reactions of various aromatic aldehydes, malononitrile and barbituric acid in aqueous ethanol using dibutylamine (DBA) as catalyst. The potential application of DBA in organic synthesis is increasing rapidly due to its reaction simplicity, minimal reaction time, high yields of the desired products (83–94%) and low cost chemicals. All of the synthesized pyrano[2,3-d]pyrimidine derivatives were characterized by IR, 1H NMR, 13C NMR and mass spectra.
1 Introduction
Heterocyclic compounds have received considerable attention in recent years due to their variety of biological activities, especially as inhibitors of PDE5 extracted from human platelets [Citation1], HIV-1 reverse transcriptase [Citation2], human EPK2 [Citation3] and cyclin-dependent kinase [Citation4]. The study of aromatic six membered N-heterocyclic rings is of great importance in the pharmaceutical sector due to bio-isosteric factors, which are of theoretical and practical importance. Pyrimidine rings have significant pharmacological importance as an integral part of DNA and RNA in several biological processes [Citation5–Citation7]. Therefore, the chemotherapeutic efficacy of annulated pyrano[2,3-d]pyrimidines is related to their ability to inhibit enzyme action for DNA biosynthesis, such as dihydrofolate reductase (DHFR), thymidylate synthetase (TSase), thymidine phosphorylase (TPase) and reverse transcriptase (RTase). When pyrano[2,3-d]pyrimidine moieties are annulated into one molecule, the resultant derivative has enhanced pharmaceutical activity, such as, antitumor [Citation8], antihypertensive [Citation9], antibacterial [Citation10] and antileishmanial activity [Citation11]. Therefore, for the preparation of these complex molecules, large efforts have been made towards the synthetic manipulation of annulated uracils, which occupy a distinct and unique place in medicinal chemistry. Annulated pyrano[2,3-d]pyrimidine derivatives are unsaturated N-heterocyclics formed as a fusion of pyran and pyrimidine rings, consisting of one oxygen atom at 8 and two nitrogen atoms at the 1 and 3 positions. They are synthesized by various procedures based on multicomponent reactions, such as Knoevenagel condensation, Michael addition followed by cyclodehydration and finally heterocyclization.
Multicomponent reactions (MCRs) have gained significant interest from modern medicinal and combinatorial chemists [Citation12,Citation13] due to the powerful bond forming efficiency, diversity-oriented synthesis (DOS), simple reaction design, atom-economy, environmental concerns and the possibility to construct target compounds using several assorted elements in a single chemical procedure [Citation14,Citation15]. Pyrano[2,3-d]pyrimidine derivatives have been reported using different catalysts, such as Zn[(l)proline]2 [Citation16], [BMIm]BF4 [Citation17], N-methylmorpholine [Citation18], DAHP [Citation19], SBA-Pr-SO3H [Citation20], H14[NaP5W30O110] [Citation21], 1,4-dioxane [Citation22], l-proline [Citation23] and [K Al(SO4)2] [Citation24]. Et3N was used as a catalyst for the synthesis of pyrano[2,3-d]pyrimidine derivatives under microwave irradiation [Citation25]. A catalyst-free procedure was also examined for the preparation of pyrano[2,3-d]pyrimidine derivatives [Citation26]. In addition, ultrasonic irradiation [Citation27] and ball-milling techniques [Citation28] were used for the synthesis of pyrano[2,3-d]pyrimidine derivatives. The solvent and the inexpensive, mild and reusable catalyst for MCRs play an important role in the synthesis and its selectivity of targeted products. One of these catalysts is dibutylamine (DBA), which has received significant interest as a high reactive, eco-friendly, inexpensive, readily available and non-toxic catalyst to obtain corresponding products in excellent yields with high selectivity [Citation29].
In continuation of the current research from our laboratory to develop efficient multicomponent reactions (MCRs) for the preparation of pyrimidine-annulated bioactive molecules [Citation30], we report here, the dibutylamine (DBA) catalyzed efficient, simple and fast synthesis of pyrano[2,3-d]pyrimidine derivatives via one-pot, three-component domino Knoevenagel–Michael addition reaction in aqueous media ().
2 Experimental
2.1 Apparatus and analysis
All chemicals were obtained from Merck and S.D. Fine Chem. Co. and were used without further purification. Melting points were determined by the open capillary method and were uncorrected. IR spectra were recorded on a Perkin–Elmer 298 spectrophotometer using KBr pellets. 1H and 13C NMR spectra were recorded using a Bruker instrument (1H at 400 MHz and 13C at 100 MHz) in DMSO-d6 solvent with tetramethylsilane as the internal standard. Chemical shifts are reported in ppm. Mass spectra were recorded on a Shimadzu GC-MS-QP-2010 model using the direct injection probe technique. Reactions were monitored by thin-layer chromatography on 0.2-mm precoated plates of silica gel G60 F254 (Merck).
2.2 General procedure for the synthesis of pyrano[2,3-d]pyrimidine derivatives 4(a–j)
Substituted aromatic aldehydes 1 (1 mmol), malononitrile 2 (1 mmol), barbituric acid 3 (1 mmol) and 20 mol% dibutylamine (DBA) were added to an RB flask with 16 ml aqueous media (1:1 ratio) and were stirred for 43–129 min at room temperature. The progress of the reaction was monitored by TLC. The solid product was filtered, washed with cold water and recrystallized from ethanol to obtain pure pyrano[2,3-d]pyrimidine derivatives with excellent yields (83–94%).
2.3 Spectral data for the synthesized pyrano[2,3-d]pyrimidine derivatives 4(a–j)
2.3.1 7-Amino-5-(4-nitrophenyl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4a)
White powder, IR (KBr, ν cm−1): 3411 (NH2), 3209, 3163 (NH), 2989 (C–H), 2206 (C–N), 1732 (C=O), 1461 (C=C). 1H NMR (400 MHz, DMSO-d6): δ = 13.01 (s, 1H, NH), 8.93 (s, 1H, NH), 7.94 (d, J = 7.1 Hz, 2H, Ar-H), 7.47 (d, J = 7.1 Hz, 2H, Ar-H), 6.79 (s, 2H, NH2), 4.96 (s, 1H, CH) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 176.47 (C=O), 170.60 (CNH2), 153.93 (CONH), 151.36 (C=O), 150.32 (C-14), 147.44 (C-11), 128.99 (C-12), 123.19 (C-13), 120.41 (C–N), 104.46 (C-5), 75.53 (C-9), 70.70 (C-10) ppm; MS (m/z): 328.2 [M+H+].
2.3.2 7-Amino-5-(3-nitrophenyl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4b)
White powder, IR (KBr ν cm−1): 3314 (NH2), 3301, 3247 (NH), 2942 (C–H), 2212 (C–N), 1605 (C=O), 1476 (C=C). 1H NMR (400 MHz, DMSO-d6): δ = 12.02 (s, 1H, NH), 10.21 (s, 1H, NH), 8.38 (s, 1H, Ar-H), 8.19 (d, J = 6.3 Hz, 2H, Ar-H), 6.82 (s, 2H, NH2), 3.94 (s, 1H, CH) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 166.12 (C=O), 160.73 (CNH2), 157.01 (CONH), 151.56 (C=O), 150.57 (C-14), 146.11 (C-11), 129.23 (C-12), 123.08 (C-13), 119.45 (C–N), 104.42 (C-5), 79.32 (C-9), 71.10 (C-10) ppm; MS (m/z): 328.2 [M+H+].
2.3.3 7-Amino-5-(2-nitrophenyl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4c)
White powder, IR (KBr ν cm−1): 3302 (NH2), 3331, 3142 (NH), 2938 (C–H), 2172 (C–N), 1645 (C=O), 1527 (C=C). 1H NMR (400 MHz, DMSO-d6): δ = 10.12 (s, 1H, NH), 10.79 (s, 1H, NH), 7.69–7.63 (m, 3H, Ar-H), 6.82 (s, 2H, NH2), 3.94 (s, 1H, CH) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 179.44 (C=O), 170.14 (CNH2), 159.59 (CONH), 156.79 (C=O), 149.87 (C-14), 134.52 (C-11), 132.12 (C-12), 130.05 (C-13), 127.31 (C–N), 91.81 (C-5), 84.24 (C-9), 57.01 (C-10) ppm; MS (m/z): 350.02 [M+Na+].
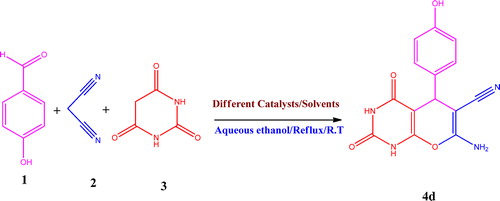
2.3.4 7-Amino-5-(4-hydroxyphenyl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4d)
Yellow powder, IR (KBr, ν cm−1): 3457 (OH), 3260 (NH2), 3131, 3088 (NH), 2293 (C–H), 2242 (C–N), 1729 (C=O), 1678 (C=O), 1555 (C=C). 1H NMR (400 MHz, DMSO-d6): δ = 10.87 (s, 1H, NH), 10.79 (s, 1H, NH), 6.98 (d, J = 7.5 Hz, 2H, Ar-H), 6.82 (s, 2H, NH2), 6.61 (d, J = 7.6 Hz, 2H, Ar-H), 6.05 (s, 1H, OH), 4.31 (s, 1H, CH) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 168.93 (C=O), 157.19 (C-14), 155.61 (CNH2), 153.38 (CONH), 152.72 (C=O), 129.32–129.20 (C-12 & C-16), 118.20 (C–N), 115.16 (C-13), 97.22 (C-5), 58.65 (C-9), 54.87 (C-10) ppm; MS (m/z): 298.06 [M+H+].
2.3.5 7-Amino-5-(3-hydroxyphenyl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4e)
Yellow powder, IR (KBr, ν cm−1): 3439 (OH), 3337 (NH2), 3193, 3028 (NH), 2206 (C–H), 2131 (C–N), 1677 (C=O), 1625 (C=O), 14,741 (C=C); 1H NMR (400 MHz, DMSO-d6): δ = 12.04 (s, 1H, NH), 10.62 (s, 1H, NH), 6.97–6.83 (m, 3H, Ar-H), 6.61 (s, 2H, NH2), 6.09 (s, 1H, OH), 3.94 (s, 1H, CH); 13C NMR (100 MHz, DMSO-d6): δ = 179.43 (C=O), 170.23 (C-14), 159.49 (CNH2), 157.19 (CONH), 155.08 (C=O), 129.31 (C-16), 129.20 (C–N), 115.15 (C-13), 93.41 (C-5), 84.21 (C-9), 52.03 (C-10); MS (m/z): 299.02 [M+H+].
2.3.6 7-Amino-5-(4-bromophenyl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4f)
White powder, IR (KBr, ν cm−1): 3370 (NH2), 3340, 3189 (NH), 3080 (C–H), 2220 (C–N), 1684 (C=O), 1567 (C=C). 1H NMR (400 MHz, DMSO-d6): δ = 14.10 (s, 1H, NH), 13.01 (s, 1H, NH), 7.75 (d, J = 7.1 Hz, 2H, Ar-H), 7.33 (d, J = 7.1 Hz, 2H, Ar-H), 6.82 (s, 2H, NH2), 3.90 (s, 1H, CH) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 173.47 (C=O), 160.34 (CNH2), 153.89 (CONH), 145.91 (C=O), 138.53 (C-11), 131.97 (C-13 & C-15), 129.69 (C-12 & C-16), 124.03 (C-14), 121.28 (C–N), 105.25 (C-5), 69.60 (C-9), 50.10 (C-10) ppm; MS (m/z): 384.12 [M+Na+].
2.3.7 7-Amino-2,4-dioxo-5-phenyl-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4 g)
Yellow powder, IR (KBr, ν cm−1): 3371 (NH2), 3301, 3212 (NH), 3114 (C–H), 2129 (C–N), 1694 (C=O), 1572 (C=C). 1H NMR (400 MHz, DMSO-d6): δ = 10.98 (s, 1H, NH), 10.80 (s, 1H, NH), 7.31 (t, J = 7.3 Hz, 2H, Ar-H), 7.14–7.06 (m, 3H, Ar-H), 6.82 (s, 2H, NH2), 4.29 (s, 1H, CH) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 160.39 (C=O), 155.61 (CNH2), 153.91 (CONH), 151.36 (C=O), 145.92 (C-11), 128.68 (C-12), 128.49 (C-13), 127.61 (C-14), 118.21 (C–N), 93.41 (C-5), 58.66 (C-9), 50.89 (C-10) ppm; MS (m/z): 283.08 [M+H+].
2.3.8 7-Amino-5-(4-methoxyphenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d] pyrimidine-6-carbonitrile (4 h)
Dark yellow powder, IR (KBr, ν cm−1): 3317 (NH2), 3282, 3145 (NH), 3063 (C–H), 2215(C–N), 1743 (C=O), 1668 (C=C). 1H NMR (400 MHz, DMSO-d6): δ = 10.98 (s, 1H, NH), 10.80 (s, 1H, NH), 7.14 (d, J = 7.5 Hz, 2H, Ar-H), 6.86–6.82 (m, 4H, Ar-H & NH2), 4.16 (s, 1H, CH), 3.81 (s, 3H, OCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 161.99 (C=O), 159.11 (C-14), 155.61 (CNH2), 151.97 (CONH), 151.36 (C=O), 130.34 (C-11), 129.50 (C-12), 123.56 (C–N), 113.14 (C-13), 93.41 (C-5), 58.66 (C-9), 57.46 (CH3), 53.46 (C-10) ppm; MS (m/z): 313.01 [M+H+].
2.3.9 7-Amino-5-(2-methoxyphenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d] pyrimidine-6-carbonitrile (4i)
Yellow powder, IR (KBr, ν cm−1): 3419 (NH2), 3202, 3137 (NH), 3016 (C–H), 2272(C–N), 1765 (C=O), 1609 (C=C). 1H NMR (400 MHz, DMSO-d6): δ = 10.93 (s, 1H, NH), 10.71 (s, 1H, NH), 7.44 (d, J = 4.6 Hz, 1H, Ar-H), 7.14–7.7.11 (m, 4H, Ar-H & NH2), 4.16 (s, 1H, CH), 3.73 (s, 3H, OCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 169.87 (C=O), 161.02 (C-14), 158.21 (CNH2), 152.46 (CONH), 151.82 (C=O), 131.15 (C-11), 129.09 (C-12), 124.02 (C–N), 113.18 (C-13), 94.39 (C-5), 58.93 (C-9), 57.41 (CH3), 53.57 (C-10) ppm; MS (m/z): 313.05 [M+H+].
2.3.10 7-Amino-5-(3,4-dimethoxyphenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4j)
Yellow powder, IR (KBr, ν cm−1): 3194 (NH2), 3103, 2223 (NH), 1734 (C–H), 2609 (C–N), 1662 (C=O), 1262 (C=C). 1H NMR (400 MHz, DMSO-d6): δ = 10.98 (s, 1H, NH), 10.80 (s, 1H, NH), 6.83–6.80 (m, 5H, Ar-H & NH2), 4.29 (s, 1H, CH), 3.83 (s, 3H, OCH3), 3.75 (s, 3H, OCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 164.65 (C=O), 155.61 (CNH2), 153.94 (CONH), 151.97 (C=O), 149.25 (C-14), 148.54 (C-13), 131.34 (C-11), 126.92 (C-16), 119.53 (C–N), 116.52 (C-12), 114.27 (C-15), 93.40 (C-5), 58.66 (C-9), 57.91 (CH3), 56.78 (CH3), 51.23 (C-10) ppm; MS (m/z): 343 [M+H+].
3 Results and discussion
Herein, we report the synthesis of pyrano[2,3-d]pyrimidinones (4a–j) through domino Knoevenagel–Michael condensation pathways (). In the initial studies, hydroxybenzaldehyde 1 (1.0 mmol), malononitrile 2 (1.0 mmol) and barbituric acid 3 (1.0 mmol) were refluxed in aqueous ethanol using different catalysts for the synthesis of model product 4d. We found the base catalysts, such as N-methylimidazole, triethylamine, piperidine, morpholine and dibutylamine (), were equally competent in furnishing the desired model product 4d in good yields. Among these basic catalysts, 20 mol% of dibutylamine (DBA) gave the best result in terms of time of completion, and the product yield was 94%. In the absence of catalyst, the reaction was completed under reflux conditions after 189 min, and the yield of product was 37% (). Therefore, dibutylamine (DBA) catalyst is superior to all of the other tested catalysts.
Table 1 Synthesis of pyrano[2,3-d]pyrimidine derivatives 4(a–j).
Table 2 Effect of catalysts for the synthesis of model product 4d using aqueous ethanol.Table Footnotea
Further, we focused on the systematic evaluation of different solvents, such as ethanol:H2O, DMF, CH2Cl2 and 1,4-dioxane (, entry 1–6), for the model reaction 4 using hydroxybenzaldehyde 1 (1.0 mmol), malononitrile 2 (1.0 mmol) and barbituric acid 3 (1.0 mmol) as reactants in presence of 20 mol% dibutylamine (DBA) catalyst (). The results indicated that the solvent has a significant effect on the product yield and reaction time. The best conversion was observed when the reaction proceeded in ethanol:H2O (, entry 1). The use of aqueous ethanol as solvent in the reaction medium exhibits remarkable benefits, such as environmentally safe, devoid of carcinogenic effects, comparatively cheaper to operate and simple work up. shows the comparative results of the different catalysts with the catalytic activity of DBU for the synthesis of pyrano[2,3-d]pyrimidine derivatives.
Table 3 Optimization of different solvents for the model reaction.Table Footnotea
Table 4 DBA comparison with different catalysts for the synthesis of pyrano[2,3-d]pyrimidine derivatives.
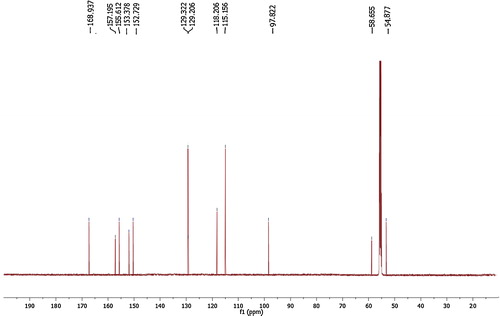
The structural assignment of model product 4d was confirmed by IR, 1HNMR, 13CNMR and mass spectrometric analysis. The IR spectra exhibited sharp absorption bands at 3457, 3260, 3131–3088, 2293, 1729, 1678 and 1555 cm−1, which are attributed to OH, NH2, NH, C–H, C–N, C=O and C=C stretching vibrations, respectively. The 1H NMR spectrum of compound 4d had peaks at δ 10.87 (s, 1H, NH), δ 10.79 (s, 1H, NH), δ 6.98 (d, J = 7.5 Hz, 2H, Ar-H), δ 6.82 (s, 2H, NH2), δ 6.61 (d, J = 7.6 Hz, 2H, Ar-H), δ 6.05 (s, 1H, OH) and δ 4.31 (s, 1H, CH) ppm (). In the 13CNMR spectrum of the compound 4d, 12 significant signals were recorded at 168.93, 157.19, 155.61, 153.38, 152.72, 129.32–129.20, 118.20, 115.16, 97.22, 58.65 and 54.87 ppm (). The molecular ion peak was observed in agreement with the molecular weight of the compound. The results indicated that a series of substituted aromatic aldehydes were successfully employed to prepare the corresponding product in excellent yields (83–94%) and there is no major effect on the yield of product by electron donating/withdrawing substituents.
Fig. 1 1H NMR spectra of 7-amino-5-(4-hydroxyphenyl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitril (4d).
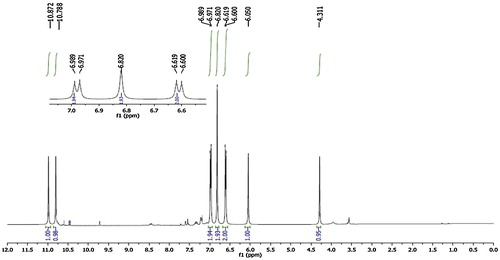
Fig. 2 13C NMR spectra of 7-amino-5-(4-hydroxyphenyl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitril (4d).
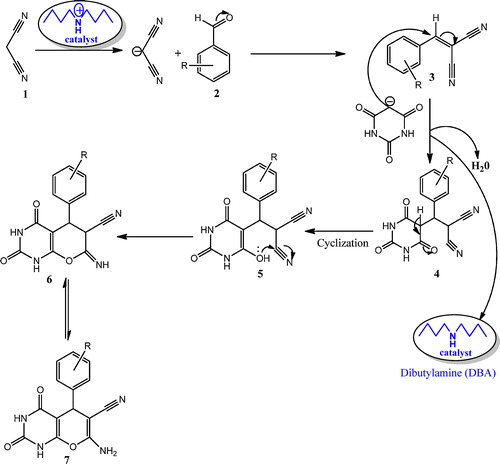
Dibutylamine (DBA) is an amine that is basic in character, so it facilitates proton removal from active methylene compounds, thereby increasing the reaction rate yields of the desired products. Mechanistically, DBA is an effective catalyst for the formation of the higher reactive iminium group, which is utilized to facilitate the Knoevenagel condensation of malononitrile with aromatic aldehydes by loss of a water molecule, followed by Michael addition of barbituric acid on an electron deficient C-atom and an intra-molecular heterocyclization that leads to the formation of the pyrano[2,3-d]pyrimidine derivatives. A plausible mechanism for the synthesis of pyrano[2,3-d]pyrimidines is shown in .
4 Conclusion
In conclusion, we have synthesized pyrano[2,3-d]pyrimidine derivatives via a one-pot, three-component domino Knoevenagel–Michael addition reaction using dibutylamine (DBA) as a new efficient catalyst in aqueous media. The synthetic method is simple because no special apparatus is required, and the compound formed is filtered and purified by simple crystallization. This synthesis is also advantageous in terms of atom economy and is devoid of any hazardous chemicals.
Support information
Download MS Word (841.1 KB)Acknowledgements
We are grateful to the Indian Institute of Integrative Medicine, Jammu & Kashmir, India for providing spectral data, and the Head of the Department of Chemistry, Prof. J.S. Meshram Nagpur University, for allowing synthetic work in the organic research laboratory.
Notes
Peer review under responsibility of Taibah University.
References
- V.D.PiazM.C.CastellanaC.VergelliM.P.GiovannoniA.GavaldaV.SegarraJ.BeletaSynthesis and evaluation of some pyrazolo[3,4-d]pyridazinones and analogues as PDE5 inhibitors potentially useful as peripheral vasodilator agentsJ. Enzyme Inhib. Med. Chem.172002227233
- Z.K.SweeneyS.F.HarrisN.N.AroraH.H.JavanbakhtY.Y.LiJ.J.FretlandDesign of annulated pyrazoles as inhibitors of HIV-1 reverse transcriptaseJ. Med. Chem.51200874497458
- K.TakayoshiW.MasaichiO.MakotoS.KentaroN.MasahiroF.TakashiCrystal structure of human ERK2 complexed with a pyrazolo[3,4-c]pyridazine derivativeBioorg. Med. Chem. Lett.1620065558
- M.F.BranaM.CachoM.L.GarciaE.P.MayoralB.LopezB.D.Pascual-TeresaPyrazolo[3,4-c]pyridazines as novel and selective inhibitors of cyclin-dependent kinasesJ. Med. Chem.48200568436854
- A.A.FayedH.M.HosniE.M.FlefelA.E.AmrSynthesis and pharmacological activities of some new thieno[2,3-d]pyrimidine and pyrimidino pyrazolo thieno pyrimidine derivativesWorld J. Chem.420095865
- A.H.F.Abd El-WahabActivated nitriles in heterocyclic synthesis: synthesis of new [1]benzopyrano[30,40:5,6]pyrano[2,3-d]pyrimidine and [1]benzopyrano[30,40:5,6]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives with promising antibacterial activityActa Pharm.522002269280
- L.V.G.NargundY.S.R.ReddyR.JoseSynthesis and antibacterial activity of pyrido[1,2-a]pyrimidin-4(1H)-onesIndian Drugs2919914546
- E.M.GrivskyS.C.LeeW.SigelD.S.DuchC.A.NicholSynthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidineJ. Med. Chem.231980327329
- L.R.BennettC.J.BlankelyR.W.FlemingR.D.SmithD.K.TessonamAntihypertensive activity of 6-arylpyrido[2,3-d]pyrimidin-7-amine derivativesJ. Med. Chem.241981382389
- R.B.AjmalS.D.RajendraS.S.RupaliPotent in vitro antibacterial and antifungal activities of pyrano[2,3-d]pyrimidine derivatives with quantitative yieldInt. J. Pharm. Biosci.52014422430
- A.R.AgarwalN.AshutoshP.M.S.GoyalS.G.ChauhanDihydropyrido[2,3-d]pyrimidines as a new class of antileishmanial agentsBioorg. Med. Chem.24200566786684
- L.WeberK.IllegelM.AlmstetterDiscovery of new multi component reactions with combinatorial methodSynlett1999366374
- R.W.ArmstrongA.P.CombaP.A.TempstS.BrownT.A.KeatingMultiple-component condensation strategies for combinatorial library synthesisAcc. Chem. Res.291996123131
- I.UgiA.DomlingW.HorlMulticomponent reactions in organic chemistryEndeavour181994115122
- G.H.PosnerMulticomponent one-pot annulations forming three to six bondsChem. Rev.861986831844
- M.M.HeraviA.GhodsK.BakhtiariF.DerikvandZn[(l)proline]2: an efficient catalyst for the synthesis of biologically active pyrano[2,3-d]pyrimidine derivativesSynth. Commun.40201019271931
- J.YuH.WangGreen synthesis of pyrano[2,3-d]pyrimidine derivatives in ionic liquidsSynth. Commun.35200531333140
- A.A.ShestopalovL.A.RodinovskayaA.M.ShestopalovV.P.LitvinovOne-step synthesis of substituted 4,8-dihydropyrano[3,2-b]pyran-4-onesRuss. Chem. Bull.532004724725
- S.BalalaieS.AbdolmohammadiH.R.BijanzadehA.M.AmaniDiammonium hydrogen phosphate as a versatile and efficient catalyst for the one-pot synthesis of pyrano[2,3-d]pyrimidinone derivatives in aqueous mediaMol. Divers.1220088591
- G.M.ZiaraniS.FaramarziS.AsadiA.BadieiR.BazlM.AmanlouThree-component synthesis of pyrano[2,3-d]-pyrimidine dione derivatives facilitated by sulfonic acid nanoporous silica (SBA-Pr-SO3H) and their docking and urease inhibitory activityDARU J. Pharm. Sci.2120133
- M.M.HeraviA.GhodsF.DerikvandK.BakhtiariF.F.BamoharramH14[NaP5W30O110] catalyzed one-pot three-component synthesis of dihydropyrano[2,3-c]pyrazole and pyrano[2,3-d]pyrimidine derivativesJ. Iran. Chem. Soc.72010615620
- H.H.ZoorobM.AbdelhamidM.A.El-ZahabM.Abdel-Mogib1,3-Dimethylpyrimidoheterocycles as antibacterial agentsArzneim. Forsch.471997958962
- M.BararjanianS.BalalaieB.MovassaghA.M.AmaniOne-pot synthesis of pyrano[2,3-d]pyrimidinone derivatives catalyzed by l-proline in aqueous mediaJ. Iran. Chem. Soc.62009436442
- A.MobinikhalediN.ForoughifarM.A.B.FardEco-friendly and efficient synthesis of pyrano[2,3-d]pyrimidinone and tetrahydrobenzo[b]pyran derivatives in waterSynth. React. Inorg. Met. Org. Nano-Met. Chem.402010179185
- I.DeviB.S.D.KumarP.J.BhuyanA novel three-component one-pot synthesis of pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines using microwave heating in the solid stateTetrahedron Lett.44200383078310
- Y.GaoS.TuT.LiX.ZhangS.ZhuF.FangD.ShiEffective synthesis of 7-amino-6-cyano-5-aryl-5H-pyrano[2,3-d]pyrimidine-2,4(1H,3H)-diones under microwave irradiationSynth. Commun.34200412951299
- T.S.JinL.B.LiuS.J.TuY.ZhaoT.S.LiClean one-pot synthesis of 7-amino-5-aryl-6-cyano-1,5-dihydro-2H-pyrano[2,3-d]pyrimidine-2,4(3H)-diones in aqueous media under ultrasonic irradiationJ. Chem. Res.32005162163
- S.MashkouriM.R.Naimi-JamalMechanochemical solvent-free and catalyst-free one-pot synthesis of pyrano[2,3-d]pyrimidine-2,4(1H,3H)-diones with quantitative yieldsMolecules142009474479
- R.M.N.KallaC.Jin-SeokW.Y.JinS.J.ByeonM.S.HeoI.l.KimSynthesis of 2-amino-3-cyano-4H-chromen-4-ylphosphonates and their anticancer propertiesEur. J. Med. Chem.7620146166
- R.B.AjmalH.S.AabidD.S.RajendraSynthesis of new annulated pyrano[2,3-d]pyrimidine derivatives using organo catalyst (DABCO) in aqueous mediaJ. Saudi Chem. Soc.201410.1016/j.jscs.2014.03.008
- T.S.JinL.B.LiuS.J.TuY.ZhaoT.S.LiA clean one-pot synthesis of 7-amino-5-aryl-6-cyano-1,5-dihydro-2H-pyrano[2,3-d]pyrimidine-2,4(3H)-diones in aqueous media under ultrasonic irradiationJ. Chem. Res.32005162163
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jtusci.2015.03.004.