?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nanofluids are tailored suspensions of nanoparticles in a selected base fluid. Nanofluids provide advantages including high thermal conductivity, electrical conductivity and optical absorption compared to the base fluid by itself, even for only a small volume fraction of nanoparticles. The choice of the nanoparticles and base fluid is a challenge in order to achieve maximum absorption. In this work, a method is suggested for selecting the best base fluid for TiO2 nanoparticles to yield the best nanofluid based on the Maxwell-Garnett (MG) effective medium approach.
1 Introduction
Maxwell introduced the idea of microcolloidal suspensions approximately 134 years ago [Citation1]. Since then, researchers have investigated and applied microfluid technologies for heat transfer applications. However, drawbacks such as sedimentation, abrasion, and wear resistance have limited the growth of microfluids as an industry. The birth of nanotechnology gave rise to nanosuspensions or nanofluids. The term nanofluid was coined by Stephen Choi in 1995 [Citation2] at Argonne National Laboratory, USA. Nanofluids are suspensions of nanosized solid particles (1–100 nm) in selected base fluids. Nanofluids can be prepared in two ways: a single-step method [Citation3] or a two-step method [Citation4]. The single-step method is based on the simultaneous synthesis of nanoparticles and nanofluids, and the two-step method involves the synthesis of nanoparticles in the first step followed by their dispersion in a suitable base fluid in the second step. From 1995 to 2008, nanofluids were investigated as a heat transfer fluid, with the best results reported by Eastman and Choi [Citation5]. Nanocopper with ethylene glycol as the base fluid exhibited a 40% increase in its thermal conductivity for a 0.3 vol.% of copper. The increased thermal conductivity of nanofluids can lead to the miniaturization of electronic devices and the ability to provide the required electronic cooling.
Table 1 List of materials investigated in this work.
Table 2 List of samples investigated in this work.
From 2009, nanofluids have been experimentally and theoretically investigated for their electrical properties. Investigations have proven their highly conducting properties compared to their base fluids [Citation6–Citation8]. Magnetic nanofluids with a tunable thermal conductivity and viscosity were investigated by Philip [Citation9]. Nanofluid optical properties were investigated by Taylor [Citation10] for efficient direct absorption solar collectors (DASC). The success of nanofluids for DASC depends on the appropriate choice of both the base fluid and the nanoparticles. The best nanofluids should also have a high stability. In this work, an attempt is made to identify the best base fluid for a given type of nanoparticle to achieve the best optical properties for efficient DASC using the Maxwell model.
2 Experimental description
The selection of best nanofluid for optical absorption depends on the choice of both the nanoparticles and the base fluid. The combination which gives highest dielectric constant for the nanofluid is selected based on the Maxwell-Garnett (MG) effective medium approach.
The Maxwell-Garnett approximation is used when calculating the dielectric properties of inhomogeneous materials or mixtures of materials. It is applicable when one of the components is dispersed within a second component. Nanofluids are solid nanosuspensions in a liquid matrix. It is assumed that the nanoparticles are spherical or ellipsoidal to satisfy the MG model. Electrostatic interactions exist between the different nanoparticles, which can create charged dipoles and lead to distortions. However, the field created by the distortions is assumed to be uniform [Citation11].
To select the best nanofluid for TiO2, water, ethylene glycol, and propylene glycol were used as base fluids. These fluids were chosen as a result of their being used often in heat transfer applications. The volume fractions investigated ranges from 0.1 to 0.2, as low volume fractions yield high stability ( and ).
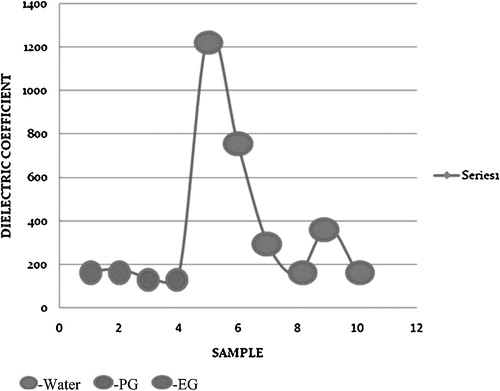
The different combinations of nanofluids and base fluids investigated were grouped into 10 samples. Samples 1–4 include EG and PG as base fluids and were investigated at 104 Hz and 107 Hz, respectively, and at a constant temperature of 20 °C. Samples 5–10 were water-based nanofluids and were investigated at 104 Hz and 107 Hz with increasing temperatures.
The best fluid was theoretically calculated using the Maxwell-Garnett effective medium approach in which:
Eeff – effective dielectric constant of the nanofluid,
Ef – dielectric constant of the base fluid,
fv – volume fraction and
Ep – dielectric constant of the particle.
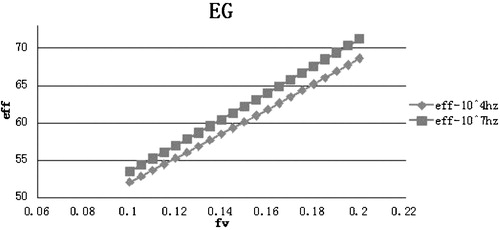
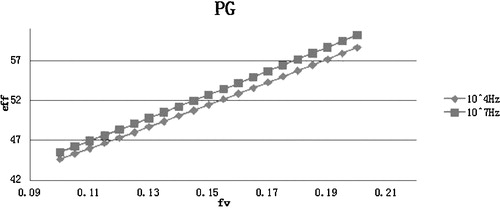
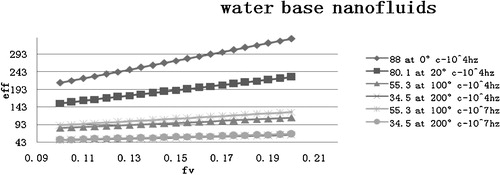
The dielectric constant of materials can change with the temperature and applied frequency. In this investigation, the dielectric constants of the nanofluids are studied at a constant frequency of either 104 Hz or 107 Hz over a temperature range from 0 to 200 °C. The dielectric constants at different temperatures and frequencies are shown in . The investigations of the dielectric constants for nanofluids in ethylene glycol and propylene glycol were performed at the same frequency (104 Hz and 107 Hz), but only at 20 °C due to the limited availability of theoretical data.
Fig. 1 Graph of the dielectric constants for water based nanofluids at different temperatures and frequencies.
2.1 Analysis of the dielectric constant of the nanofluid
The dielectric constants of the ethylene glycol and propylene glycol were assumed to be 37 and 32 at 104 Hz and 107 Hz for a temperature of 20 °C, respectively. The dielectric constant of TiO2 was taken to be 200 and 160 at 104 Hz and 107 Hz, respectively. The volume fraction of all of the nanofluids was varied until reaching 0.2. This is because higher nanoparticle volume fractions can lead to sedimentation and agglomeration, as indicated in previous investigations [Citation12].
2.2 Procedure for analysing the dielectric properties of TiO2
Sample 5 included a TiO2–water nanofluid at 104 Hz and 0 °C. The dielectric properties for this sample were evaluated using the MG equation with the following property values:
Ef = 88 at a temperature of 0 °C,
Ep = 200 at 104 Hz and
fv = varied 0.10–0.20 in increments of 0.05.
The effective dielectric constant was evaluated using the above values and varying the volume fractions. The resulting dielectric constants as functions of the volume fractions are shown in . The same procedure is followed for all of the samples, and the results are depicted in –.
3 Results and discussions
The results indicate an increase in the dielectric constants for all of the nanofluids compared to their base fluids. Furthermore, the dielectric constants of the nanofluids are higher for the low frequency of 104 Hz. This is because the dielectric constant of the solid phase (TiO2) in the nanocollidal suspension is higher at lower frequencies, despite the dielectric constant of the base fluids being the same at 104 Hz and 107 Hz. Thus, the solid phase in the nanofluid is responsible for tuning the dielectric constant of the nanofluid. Among the water, ethylene glycol, and propylene glycol nanofluids, water-based nanofluids exhibited the highest dielectric constant at 20 °C for both the frequencies. Based on the results:
The dielectric constant of the water-based nanofluids decreases from 211 to 82 for a 0.20 volume fraction as the temperature increases from 0 to 200 °C at a frequency of 104 Hz. This indicates a dynamic electric response and is associated with the optical response of nanofluids when they are used as a working fluid in direct absorption solar collectors.
The dielectric constants for all 10 samples are shown in for a fixed volume fraction of 0.2. The water-based nanofluids exhibit the highest dielectric constant at 0 °C. The water-based nanofluids also exhibit high dielectric constants at both frequencies and for all of the volume fractions examined here. The water-based nanofluids at 100 and 200 °C are assumed to be a two phase mixture of solid nanoparticles dispersed in a steam phase.
4 Conclusions
| 1. | Nanofluids exhibit enhanced dielectric properties in the same ways that they show enhanced thermal and electrical conductivities. | ||||
| 2. | Water-based nanofluids have higher dielectric constants than EG and PG for the same frequencies and temperatures. | ||||
| 3. | The dielectric constants of water-based nanofluids exhibit a dynamic variation with temperature and frequency, even when the base fluid exhibits no variations with the frequency. | ||||
| 4. | From these results, we conclude that dielectric constant of the nanofluid is tunable by adjusting the volume fraction of the solid nanoparticles at a given frequency or due to the solid nanoparticles themselves at a given volume fraction. | ||||
Notes
Peer review under responsibility of Taibah University.
References
- J.C.Maxwell2nd ed.A Treatise on Electricity and Magnetismvol. 11881Clarendon PressOxford, UK440
- S.U.S. Choi FED, vol. 231/MD, vol. 66 (1995), 99–105.
- H.ZhuY.LinY.YinJ. Colloid Interfacial Sci.2272004100103
- J.A.EastmanS.U.S.ChoiL.J.ThompsonS.LeeMaterials Research Society Symposium Proceedings, No. 4571997311
- J.A.EastmanS.U.S.ChoiS.LiW.YuL.J.ThompsonAppl. Phys. Lett.7862001718720
- L.P.ShenH.WangM.DongZ.C.MaH.B.WangPhys. Lett.A376201210531057
- S.B.WhiteA.J.-M.ShihK.P.PipeNanoscale Res. Lett.62011346
- S.GangulyS.SikdarS.BasuPowder Technol.1962009326330
- P.D.ShimaJ.PhilipB.RajAppl. Phys. Lett.952009133112
- R.A.TaylorP.E.PhelanT.P.OtanicarR.ArvindR.PrasherNanoscale Res. Lett.62011255
- D.StroudO.LevyPhys. Rev. B56199713
- W.YuH.XieJ. Nanomater.20122012 (Article ID 435873)