?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The oxidation of benzaldehyde (BA) as well as 4-methoxy (4-MBA), 3-methoxy (3-MBA), 2-methoxy (2-MBA), 2,6-dimethoxy (2,6-DMBA), 2,4-dimethoxy (2,4-DMBA), 3,4-dimethoxy (3,4-DMBA), 2,4,6-trimethoxy (2,4,6-TMBA), 3,4,5-trimethoxy (3,4,5-TMBA) and 2,3,4-trimethoxy (2,3,4-TMBA) benzaldehydes by benzimidazolium fluorochromate (BIFC) have been studied in a 50% acetic acid–50% water medium. The oxidation leads to the formation of the corresponding carboxylic acids. The reaction is first order with respect to [BIFC], [S] and [H+]. The reaction was catalysed by H+ ions. The reaction was studied at four different temperatures, and the thermodynamic parameters were calculated. The Exner plot indicated that all of the methoxy-substituted benzaldehydes were oxidized via the same mechanism. Based on the observed kinetics, a suitable mechanism has been proposed.
1 Introduction
The oxidation of organic substrates is an important aspect in modern organic synthesis. Therefore, the search for new oxidizing agents is of interest to synthetic organic chemists. In recent years, development of new Cr(VI) reagents for the effective and selective oxidation of organic substrates under mild conditions has been studied because Cr(VI) is a versatile oxidant for the oxidation of various organic compounds [Citation1]. The development of new chromium (VI) reagents for the oxidation of organic substrates continues to be of interest. Many such reagents have been developed in recent years with some success, and some of these reagents include tetrahexylammonium fluorochromate [Citation2], morpholinium chlorochromate [Citation3], nicotinium dichromate [Citation4], triethylammonium chlorochromate [Citation5], tetraethylammonium chlorochromate [Citation6], tetrabutylammonium bromochromate [Citation7], tetraheptylammonium bromochromate [Citation8] and caffeinilium chlorochromate [Citation9].
Benzimidazolium fluorochromate is also an oxidant that was recently developed [Citation10]. This reagent is more efficient and a stronger oxidizing agent. The oxidation kinetics of substituted benzaldehydes by various oxidizing agents have been extensively studied [Citation11–Citation15]. As a part of our continuing investigations of the oxidation of organic substrates by Cr(VI) [Citation16–Citation20], we report the kinetic features of the oxidation of benzaldehyde and various methoxy substituted benzaldehydes by BIFC in an aqueous acetic acid medium. In addition, the mechanistic results are discussed.
2 Experimental
2.1 Materials
Benzimidazole and chromium trioxide were obtained from Fluka (Buchs, Switzerland). BIFC was prepared according to a previously reported method [Citation10], and its purity was confirmed using an iodometric method. Benzaldehyde as well as 4-methoxy, 3-methoxy, 2-methoxy, 2,6-dimethoxy, 2,4-dimethoxy, 3,4-dimethoxy, 2,4,6-trimethoxy, 3,4,5-trimethoxy and 2,3,4-trimethoxy benzaldehydes were used as substrates. Acetic acid was purified using a standard method, and the fraction at 118 °C was collected.
2.2 Kinetic measurements
The pseudo-first-order conditions were achieved by maintaining a large excess (×15 or more) of the aldehydes compared to BIFC. The solvent consisted of 50% acetic acid and 50% water (v/v) unless specified otherwise. The reactions were followed at constant temperatures (±0.01 K) by the spectrophotometric monitoring of the decrease in [BIFC] at 368 nm using a UV–Vis spectrophotometer (Shimadzu UV-1800 model). The pseudo-first-order rate constant (k1) was evaluated from the linear (r = 0.990–0.999) plots of log [BIFC] as a function of time for up to 80% reaction. The second order rate constant (k2) was obtained from the following relationship: k2 = k1/[Substrate].
2.3 Data analysis
Correlation analysis was carried out using Microcal origin (version 6) computer software. The goodness of the fit is discussed using the correlation coefficient (r in the case of simple linear regression and R in the case of multiple linear regressions) and standard deviation (SD).
2.4 Product analysis
Product analysis was performed under mineral acid catalysed conditions in benzaldehyde. Maintaining an excess concentration of BIFC, the two solutions were mixed, and perchloric acid was also added in a 50% acetic acid–50% water mixture. The reaction mixture was allowed to stand for approximately 24 h to ensure that the reaction reached completion. Next, the reaction mixture was evaporated and extracted with ether. The ether layer was washed with water many times. The ether layer was maintained in a water bath to evaporate the ether followed by cooling in an ice bath to yield the product (m.p. 121 °C). The product was dissolved in benzene, and a careful TLC analysis was performed with benzoic acid and benzaldehyde as references. Only one spot corresponding to benzoic acid was observed. The formation of benzoic acid was further confirmed by mixing the product with pure benzoic acid, which did not change the melting point.
2.5 Stoichiometric studies
Stoichiometric analysis indicated that 3 mol of aldehyde consumed 2 mol of BIFC according to Eq. Equation(1)(1)
(1) to yield the corresponding carboxylic acid.
(1)
(1)
3 Results and discussion
The reaction was studied in 50% acetic acid–50% water medium at 303 K under pseudo-first-order conditions. The rate and other experimental data were obtained for benzaldehyde as well as 4-methoxy, 3-methoxy, 2-methoxy, 2,6-dimethoxy, 2,4-dimethoxy, 3,4-dimethoxy, 2,4,6-trimethoxy, 3,4,5-trimethoxy and 2,3,4-trimethoxy benzaldehydes. The pseudo-first-order rate constants are given in .
Table 1 Effect of variation of [S], [BIFC] and [H+] on the rate of reaction at 303 K.Table Footnotea
The results are summarized here.
3.1 Order of reaction
The concentration of BIFC was varied from 0.5 × 10−3 to 2.5 × 10−3 mol dm−3 while keeping all of the other reactant concentrations constant, and the rates were measured (). The nearly constant k1 value irrespective of the concentration of BIFC confirms the first order dependence on BIFC.
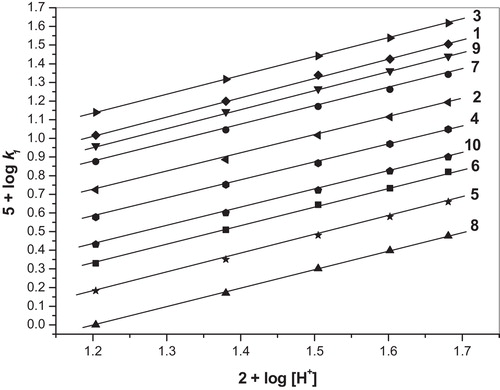
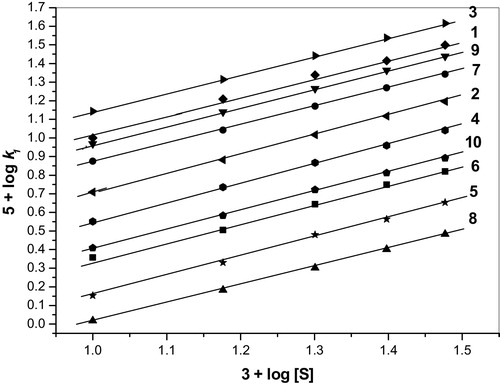
The concentration of the BA, 4-MBA, 3-MBA, 2-MBA, 2,6-DMBA, 2,4-DMBA, 3,4-DMBA, 2,4,6-TMBA, 3,4,5-TMBA and 2,3,4-TMBA substrates were varied from 1.0 × 10−2 to 3.0 × 10−2 mol dm−3 at 303 K while keeping all of the other reactant concentrations constant, and the rates were measured (). The rate of oxidation increased as the concentration of the substrate increased, indicating a first order dependence on the substrate. The linear plots of log k1 as a function of log [S] () (BA: slope = 1.04; 4-MBA: slope = 1.03; 3-MBA: slope = 0.99; 2-MBA: slope = 1.03; 2,6-DMBA: slope = 1.05; 2,4-DMBA: slope = 0.98; 3,4-DMBA: slope = 0.99; 2,4,6-TMBA: slope = 0.97; 3,4,5-TMBA: slope = 0.99; 2,3,4-TMBA: slope = 1.02) demonstrate the first order dependence on [S] (). The k1 values at different [S] are listed in .
Fig. 1 Order plot for the substrate for the oxidation of BA (1), 4-MBA (2), 3-MBA (3), 2-MBA (4), 2,6-DMBA (5), 2,4-DMBA (6), 3,4-DMBA (7), 2,4,6-TMBA (8), 3,4,5-TMBA (9) and 2,3,4-TMBA (10) by BIFC at 303 K.
The perchloric acid concentration was varied from 0.16 to 0.48 mol dm−3 while keeping the concentrations of all of the other reactant concentrations constant, and the rates were measured (). The increase in [HClO4] in the oxidation reaction increased the rate of the reaction and exhibits a direct first order dependence on [HClO4]. A plot of log k1 as a function of log [H+] was linear (BA: slope = 1.03; 4-MBA: slope = 0.99; 3-MBA: slope = 1.00; 2-MBA: slope = 0.98; 2,6-DMBA: slope = 1.00; 2,4-DMBA: slope = 1.00; 3,4-DMBA: slope = 0.98; 2,4,6-TMBA: slope = 1.00; 3,4,5-TMBA: slope = 1.01; 2,3,4-TMBA: slope = 0.98) (). The experimental data confirms the first order dependence on [H+].
3.2 Effect of MnSO4 concentration
The addition of Mn(II) in the form of MnSO4 (0.003 M) decreased the rate of the oxidation process, indicating two electron oxidation (). This result indicates the involvement of a Cr(IV) intermediate in the oxidation of the aldehydes by the Cr(VI) reagent. The Mn(II) ion reduces Cr(IV) to Cr(III). In the absence of the Mn(II) ion, the formed Cr(IV) reduced Cr(VI) to Cr(V), and the oxidation of the aldehydes by Cr(V) was fast. This result provides evidence that is consistent with the formation of Cr(IV) species, and therefore, BIFC acts as a two-electron transfer oxidant [Citation21,Citation22].
3.3 Effect of solvent polarity on the reaction rate
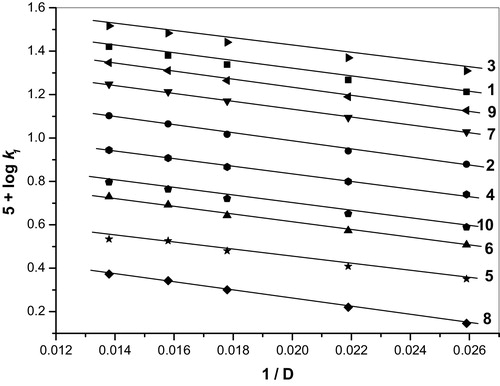
The effect of the solvent composition on the reaction rate was studied by varying the concentration of acetic acid from 30% to 70%. The pseudo-first-order rate constants were estimated for the oxidation of the aldehydes using BIFC in the presence of perchloric acid at a constant ionic strength. The reaction rate decreased substantially as the proportion of acetic acid in the medium increased (). The plot of log k1 as a function of 1/D (dielectric constant) is linear with a positive slope, suggesting the presence of either dipole–dipole or ion–dipole interactions between the oxidant and the substrate [Citation23] (). The plot of log k1 as a function of (D − 1)/(2D + 1) is curved, indicating the absence of dipole–dipole interactions in the rate determining step.
Table 2 Effect of varying solvent polarity on the rate of reaction for the oxidation of BA, 4-MBA, 3-MBA, 2-MBA, 2,6-DMBA, 2,4-DMBA, 3,4-DMBA, 2,4,6-TMBA, 3,4,5-TMBA and 2,3,4-TMBA by BIFC at 303 K.
3.4 Structure reactivity correlation
The effect of the structure on the reactivity was studied, and the reactivity decreased in the following order: 3-MBA > BA > 3,4,5-TMBA > 3,4-DMBA > 4-MBA > 2-MBA > 2,3,4-TMBA > 2,4-DMBA > 2,6-DMBA > 2,4,6-TMBA. The methoxy group at the ortho (σ(OCH3) = −0.39) and para (σ(OCH3) = −0.27) positions to BA decreased the rate. However, a methoxy group at the meta (σ(OCH3) = +0.12) position increased the rate.
Therefore, 3,4-DMBA (σ(OCH3) = −0.15) > 2,4-DMBA (σ(OCH3) = −0.66) > 2,6-DMBA (σ(OCH3) = −0.78) and 3,4,5-TMBA (σ(OCH3) = −0.03) > 2,3,4-TMBA (σ(OCH3) = −0.54) > 2,4,6-TMBA (σ(OCH3) = −1.05).
3.5 Activation parameters
The oxidation kinetics of the BA, 4-MBA, 3-MBA, 2-MBA, 2,6-DMBA, 2,4-DMBA, 3,4-DMBA, 2,4,6-TMBA, 3,4,5-TMBA and 2,3,4-TMBA substrates were investigated using BIFC at four different temperatures (i.e., 298, 303, 308 and 313 K) in a 50% acetic acid–50% water medium in the presence of perchloric acid. The pseudo-first-order rate constants at the four different temperatures are listed in . The second order rate constants were calculated (). The Arrhenius plot of log k2 as a function of 1/T was linear. The enthalpy of activation, entropy of activation and free energy of activation were calculated from k2 at 298, 303, 308 and 313 K using the Eyring relationship using the least squares method, and the results are presented in .
Table 3 Pseudo-first order rate constants for the oxidation of BA, 4-MBA, 3-MBA, 2-MBA, 2,6-DMBA, 2,4-DMBA, 3,4-DMBA, 2,4,6-TMBA, 3,4,5-TMBA and 2,3,4-TMBA by BIFC at various temperatures in aqueous acetic acid medium.
Table 4 Second order rate constants and activation parameters for the oxidation of BA, 4-MBA, 3-MBA, 2-MBA, 2,6-DMBA, 2,4-DMBA, 3,4-DMBA, 2,4,6-TMBA, 3,4,5-TMBA and 2,3,4-TMBA by BIFC in aqueous acetic acid medium.
3.6 Isokinetic temperature
The reaction is neither isoenthalpic nor isoentropic but complies with the compensation law, which is also known as the isokinetic relationship. The isokinetic temperature is the temperature at which all of the compounds in a series react equally fast. In addition, at the isokinetic temperature, variation in the substituent has no influence on the free energy of activation. In an isoentropic reaction, the isokinetic temperature lies at infinity and only the enthalpy of activation determines the reactivity. The isokinetic temperature is zero for an isoenthalpic series, and the entropy of activation determines the reactivity. The operation of the isokinetic relationship is tested by plotting the logarithms of the rate constants at two different temperatures (T2 > T1) against each other according to Eq. Equation(2)(2)
(2) .
(2)
(2)
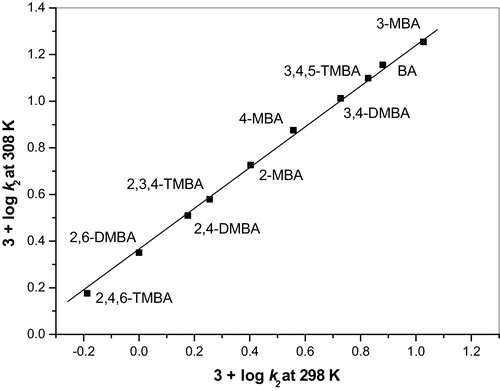
The linear relationship in the Exner plots [Citation24] at 3 + log k2 (308 K) and 3 + log k2 (298 K) in the current study confirm the validity of the isokinetic relationship. The obtained isokinetic temperature was 443 K (). The linear isokinetic correlation implies that all of the methoxy benzaldehydes were oxidized via the same mechanism and that the changes in the rate are governed by the changes in both the enthalpy and entropy of activation [Citation25].
3.7 Mechanism and rate law
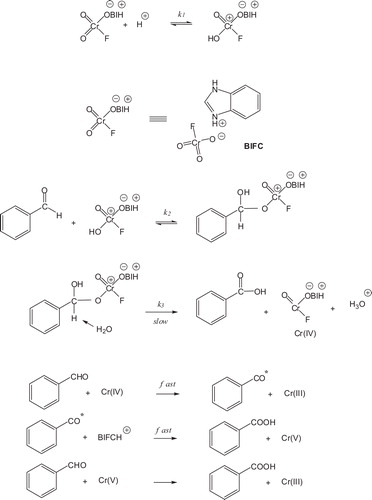
Based on the current experimental observations and the results from previous studies [Citation26], an initial two-electron transfer with the formation of Cr(IV) is proposed. Chromium (IV) reacts with an aldehyde molecule to form an aryl radical and Cr(III). The radical formation was tested with the acrylonitrile monomer when a polymer was precipitated in methanol under a nitrogen atmosphere. The aryl radical reduces BIFC to Cr(V), which further reacts with another aldehyde molecule by a two-electron transfer to form Cr(III). The involvement of the intermediate valance states of chromium in the reaction was investigated with the induced oxidation by Mn(II). The reaction proceeds with the formation of an intermediate (i.e., monochromate ester) (). Because the rate of the reaction is the decomposition of the monochromate ester, the rate law can be expressed as
4 Conclusions
In this study, the detailed kinetics of the oxidation of BA, 4-MBA, 3-MBA, 2-MBA, 2,6-DMBA, 2,4-DMBA, 3,4-DMBA, 2,4,6-TMBA, 3,4,5-TMBA and 2,3,4-TMBA in an acetic acid–water medium was spectrophotometrically studied at 303 K using BIFC as an oxidant. The stoichiometry indicated that 3 mol of aldehydes consumes 2 mol of BIFC. The negative values of ΔS# confirm the formation of a rigid activated complex.
Acknowledgment
Mansoor expresses his gratitude to the Management of C. Abdul Hakeem College (Autonomous), Melvisharam, India, for the facilities and support.
Notes
Peer review under responsibility of Taibah University.
References
- S.PatelB.K.MishraChromium (VI) oxidants having quaternary ammonium ions: studies on synthetic applications and oxidation kineticsTetrahedron63200743674406
- B.KoohestaniZ.JavanshirS.GhammamyK.MehraniH.AfrandL.SaghatforoushSynthesis and characterization of a new oxidation reagent: tetrahexylammonium chlorochromate, (C6H13)4N[CrO3Cl]J. Mex. Chem. Soc.522008116119
- N.MalaniM.BaghmarP.K.SharmaKinetics and mechanism of the oxidation of some organic sulfides by morpholinium chlorochromateInt. J. Chem. Kinet.4120096572
- D.S.BhuvaneshwariK.P.ElangoCorrelation analysis of reactivity in the oxidation of anilines by nicotinium dichromate in non-aqueous mediaInt. J. Chem. Kinet.382006657665
- S.GhammamyS.DastpeymanOxidation of organic substrates using two triethylammonium halochromates (VI), (C2H5)3NH[CrO3X], (X = F, Cl) supported on silica gelJ. Chin. Chem. Soc.552008229232
- P.SwamiD.YajurvediP.MishraP.K.SharmaOxidation of some α-hydroxy acids by tetraethylammonium chlorochromate: a kinetic and mechanistic studyInt. J. Chem. Kinet.4220105055
- S.GhammamyK.MehraniH.AfrandM.HajighahrammaniTetrapropylammonium bromochromate and tetrabutylammonium bromochromate [NR4]CrO3Br, (R = Pr, Bu): two new and efficient reagents for oxidation of alcoholsAfr. J. Pure Appl. Chem.112007008010
- S.GhammamyK.MehraniH.AfrandZ.JavanshirG.RezaeibehbahaniA.MoghimiZ.S.AghbolaghThree new tetraalkylammonium bromochromates, NR4 [CrO3Br], (R = Et, Hex, Hep): mild and efficient reagent for oxidation of primary and secondary alcoholsJ. Chil. Chem. Soc.542009491494
- F.ShiriniI.Mohammadpoor-BaltorkZ.HejaziP.HeraviCaffeinilium chlorochromate: as a mild and efficient reagent for oxidation of alcohols and chemoselective oxidative cleavage of oximesBull. Korean Chem. Soc.242003517518
- V.SivamuruganG.A.RajkumarB.ArabindooV.MurugesanSelective and clean oxidation of alcohols with benzimidazolium fluorochromate (BIFC) under solvent free conditionsIndian J. Chem.44B2005144146
- H.A.A.MedienKinetics of oxidation of benzaldehydes by quinolinium dichromateZ. Naturforsch.58b200312011250
- S.S.MansoorS.S.ShafiStudies on the kinetics of benzyltrimethylammonium fluorochromate oxidation of substituted benzaldehydes in aqueous acetic acid mediumInt. J. ChemTech Res.1200912061212
- K.KrishnasamyD.DevanathanJ.DharmarajaKinetics and mechanism of oxidation of substituted benzaldehydes by 4-(dimethylamino)pyridinium chlorochromateTrans. Met. Chem.322007922926
- B.L.HiranJ.KhuntwalR.K.MalkaniD.SinghOxidation of 3,4,5-trimethoxy benzaldehyde by pyridinium fluorochromate in N,N-dimethyl formamide medium: a kinetic and mechanistic studyArab. J. Chem.201110.1016/j.arabjc.2011.05.017
- B.H.AsgharV.S.MalikS.S.MansoorStudies on kinetics and thermodynamics of oxidation of 3,4,5-trimethoxy benzaldehyde, benzaldehyde and N,N-dimethylamino benzaldehyde by tetraethylammonium bromochromate in dimethyl formamide and acetic acid mixtureArab. J. Chem.201410.1016/j.arabjc.2014.10.047
- S.S.MansoorS.S.ShafiStudies on the kinetics of tetraethylammonium bromochromate oxidation of some meta- and para- substituted benzylalcohols in non-aqueous mediaZ. Phys. Chem.2252011249265
- S.S.MansoorS.S.ShafiOxidation of benzhydrol by tributylammonium chlorochromate: a kinetic and mechanistic studyReact. Kinet. Mech. Catal.10020102131
- S.S.MansoorS.S.ShafiCorrelation analysis of reactivity in the oxidation of some organic diols by tripropylammonium fluorochromate in non-aqueous mediaArab. J. Chem.201010.1016/j.arabjc.2010.11.004
- S.S.MansoorS.S.ShafiOxidation of methionine by tetraethylammonium chlorochromate in non-aqueous media – a kinetic and mechanistic studyArab. J. Chem.201110.1016/j.arabjc.2011.01.031
- S.S.MansoorS.S.ShafiOxidation of aniline and some para-substituted anilines by benzimidazolium fluorochromate in aqueous acetic acid medium – a kinetic and mechanistic studyArab. J. Chem.72014171176
- C.KarunakaranS.SueshIdentical kinetic behavior of dichromates and halochromates of heterocyclic bases: oxidations of pentan-1-olJ. Phys. Org. Chem.1720048893
- D.S.BhuvaneshwariK.P.ElangoCorrelation analysis of reactivity in the oxidation of anilines by nicotinium dichromate in nonaqueous mediaInt. J. Chem. Kinet.382006657665
- E.S.AmisSolvent Effects on Reaction Rates and Mechanisms1967Academic PressNew York42
- O.ExnerJ.R.StreitwiserR.W.TaltProgress in Physical Organic Chemistry1973John WileyNew York41
- J.F.LefflerE.GrunwaldRates and Equilibrium of Organic Reactions1963WileyNew York
- M.K.PillayKinetics of oxidation of para- and meta-substituted benzaldehydes by pyridinium chlorochromateIndian J. Chem.31A19924648