Abstract
Antibiotic resistance has led to the search for more effective antimicrobial agents among plant materials that can serve as source and template for the synthesis of new antimicrobial drugs. The current study aims to analyses the oil, extracted from the leaves of Crotalaria pallida for its physicochemical and antimicrobial properties. Leaf oil was extracted by petroleum ether (40–60 °C) and its fatty acid constituents were isolated as a mixture after saponification. Mixture of fatty acid was purified by TLC and characterized by IR, GC and GC–MS analysis by converting them into their FAME. MIC of the oil against the Gram positive and Gram negative bacteria was determined by agar diffusion method. Nineteen fatty acids were identified by gas-liquid chromatography followed by GC–MS. It was found that unsaturated fatty acids were present in greater amounts than saturated fatty acids. Most predominating unsaturated and saturated fatty acids were linolenic acid (34.06 ± 0.23%) and palmitic acid (24.47 ± 0.22%) respectively. The acid value (19.63 ± 0.22) and saponification value (109.08 ± 2.87) were also estimated to evaluate the quality of the oil. The oil showed good antimicrobial activities against Gram-positive bacteria, Bacillus subtilis as well as Gram-negative bacteria, Escherichia coli and Acinetobacter junii.
1 Introduction
The indiscriminate use of antimicrobials exaggerates the problem of antibiotic resistance, resulting in the limited life span of antimicrobials. Antibiotic resistance makes the treatment of infectious disease difficult, costly or even impossible. The non-availability and high cost of new-generation antibiotics with a limited effective span have resulted in an increase in morbidity and mortality [Citation1]. The magnitude of the problem is worldwide. The impacts of antibiotic resistance on human health, the costs for the healthcare sector and the wider societal impact are still largely unknown [Citation2]. The potential risks of using synthetic drugs have been reported [Citation3]. It necessitates the continuous search for alternatives [Citation4]. This has led to the search for more effective antimicrobial agents of plant origin with potential useful active ingredients capable of serving as a source and template for the synthesis of new antimicrobial drugs [Citation5,Citation6]. Consequently, the utilization of traditional plant extracts as well as other alternative forms of medicinal treatments has been gaining momentum since the 1990s [Citation7]. It has been estimated that 60–90% of the population of developing countries use traditional and botanical medicines almost exclusively, considering them a normal part of primary healthcare [Citation8].
Oil constituents are typically produced by plants for their own uses. They are liquid at room temperature, though a few of them are solid or resinous. Their colour ranges from pale yellow to emerald green and from blue to dark brownish red [Citation9]. All plant organs synthesize oil, which is stored in secretary cells, cavities, canals, epidermis cells or glandular trichomes [Citation10]. In addition to the usefulness of oil in the food and beverage industry, their broad spectrum of biological activities has led to an increased interest among researchers [Citation11,Citation12]. The mechanism of action of oil is still unclear. Some studies suggest that after penetrating the cell, these compounds disturb the cellular membrane, interfere with cellular metabolism and/or react with the active sites of enzymes [Citation13].
The plant Crotalaria pallida Aiton family Fabaceae is an annual erect herb approximately 1.50 m in height that grows widely in tropical and subtropical regions of India [Citation14]. The stems yield fibre similar to sunn hemp, and the seeds are used as a substitute for coffee [Citation14–Citation16]. The plant is used as a good cover crop in tea, coconut and rubber plantations to check soil erosion and also serves as a green manure [Citation16]. Various parts of this plant are used in folk medicine to treat urinary infections and the swelling of joints [Citation16]. Chemical investigations on this plant are mainly confined to alkaloids and flavonoids [Citation17–Citation21]. Chemical analysis of leaf oil and its antibacterial activities are yet to be reported. The present communication deals for the first time with the physicochemical characterization and antimicrobial activities of oil extracted from the leaf of C. pallida.
2 Materials and methods
2.1 Plant materials and chemicals
Fresh and matured fully expanded and undamaged leaves of Crotalaria pallida were collected from the campus area of the University of Burdwan, Burdwan, W. Bengal, India, in the morning in the middle of December 2013 and authenticated by Prof. A. Mukherjee, Department of Botany, The University of Burdwan, Burdwan, W. Bengal. A voucher specimen (Sushobhan 203) has been deposited at the herbarium of the Department of Botany at the University of Burdwan, Burdwan, bearing the acronym BURD.
Standard fatty acid methyl esters (FAMEs), including a mixture of 37 components, were purchased from Supelco, USA. All chemicals used in this experiment were of analytical grade and were purchased from Sigma Chemical Co. (USA) except petroleum ether (40–60 °C) and chloroform, which were procured from Merck (India).
2.2 Isolation of oil from the leaves of C. pallida
Fresh leaves (150 g) were air dried and finely powdered and extracted with petroleum ether (40–60 °C) in a soxhlet for 72 h. After complete removal of the solvent under vacuum, an oily substance was obtained. The total oil was weighed and stored at 4 °C for further analysis. The colour and state of the oil were noted visually. Chemical analysis of the oil of the leaves (including acid value and saponification value) was performed according to the methods of the Association of Official Analytical Chemists [Citation22]. The density and specific gravity of the oil were also determined.
2.3 Infrared spectral analysis
Infrared spectral analysis of the mixture of fatty acids was obtained after saponification of the oil was carried out in a Perkin-Elmer FT-IR spectrometer (Model No. Spectrum RX 1, Holland) using solid KBr, and the bands were characterized in the usual way.
2.4 Preparation of FAME
The extraction of fatty acids from the leaf oil was carried out according to the method described by Wilkfors et al. [Citation23]. Fatty acid methyl esters (FAMEs) were prepared by methylation with 12.5% boron trifluoride (BF3) in methanol [Citation23]. The methyl ester of the fatty acid mixture was purified by preparative TLC using hexane:ethyl acetate (1:1) as a chromatographic solvent. The fatty acid methyl ester band was eluted with chloroform (Merck, India) and stored in a refrigerator for further analysis.
2.5 GC-analysis of FAME
Analysis of the FAMEs using a capillary gas chromatograph (GC) was carried out on a Shimadzu Gas Chromatograph (Model: GC-2010; Shimadzu, Japan) with a flame ionization detector (FID) on a split injector. A SP-2560 capillary column (100 m long × 0.25 mm i.d.) was used for FAME analysis. The temperature of the injection and detector ports was set to 260 °C. The oven temperature programme was initially set to 140 °C for 5 min, increased at a rate of 4 °C/min to 240 °C and finally held for 20 min at 240 °C. The carrier gas used was nitrogen with a total flow rate of 33.9 ml/min; volume injected 1 μl; split ratio 1:30. Peaks were identified by comparison of their retention times with a Supelco 37 component FAME standard mixture (Catalogue No. 18919 – 1 AMP) from Supelco, USA. The percentage composition of the sample was computed from the GC peak area.
2.6 GC–mass spectrographic analysis
The methyl esters of fatty acids were analyzed by gas chromatography–mass spectrometry on a Shimadzu GCMS-QP 2010 Plus (Shimadzu, Japan) fitted with a SP-2560 capillary column (100 m × 0.25 mm i.d.). The temperature of the injection and detector ports was set to 260 °C. The oven temperature was initially set to 140 °C for 5 min, increased at a rate of 4 °C/min to 240 °C and finally held at 240 °C for 5 min. The carrier gas was nitrogen with a total flow rate of 16.3 ml/min. The MS condition: ionization voltage, ion source temperature and mass range were 70 eV, 270 °C and 30–700 mass units, respectively. The individual peaks were identified by comparing their retention indices and mass spectra with the NIST/Wiley mass spectral database library.
2.7 Antimicrobial assay
Three bacterial strains Escherichia coli ATCC 25922, Bacillus subtilis ATCC 6633 and Acinetobacter junii MTCC 11818 maintained in nutrient agar medium (Hi Media) were used for the assay. The agar diffusion method (Washington, 1985) on nutrient agar medium was followed with a crude extract (250 μg/ml) against test organisms (107 cfu/ml) having an individual cup diameter of 9 mm. The antimicrobial capability was estimated visually by measuring the inhibition zone. The MIC of the fatty acid constituents of the oil was determined according to the method of Washington [Citation24].
3 Results and discussion
3.1 Physicochemical characterization
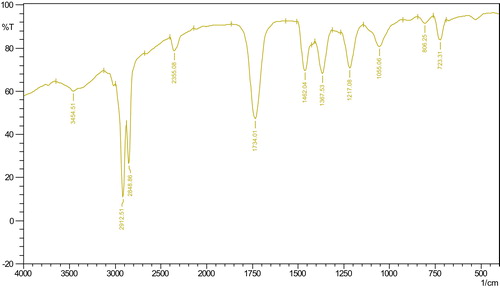
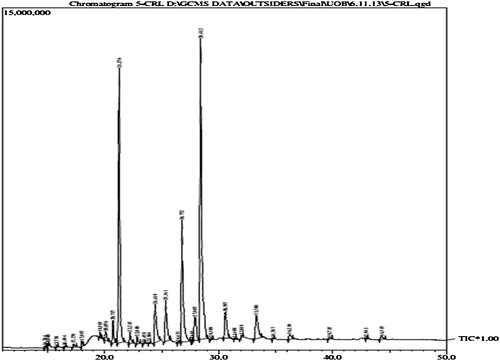
From the present experimental data, it was found that the extracted oil from the leaves of C. pallida was dark green in colour and semi-solid in nature at room temperature (27 °C). The oil contains chlorophyll, and the yield of chlorophyll-containing oil was 8.60 g/100 g leaf. The density and specific gravity of the oil were 1.356 ± 0.003 and 1.240 ± 0.002, respectively. The acid value and saponification value were determined to be 19.63 ± 0.22 and 109.08 ± 2.87, respectively (). The infrared spectra () showed a broad band at 3454.51 cm−1 for the –OH str. of the carboxyl group; 2912.51 cm−1 and 2848.86 cm−1 for –CH2; 1734.61 cm−1 for the C=O str.; 1462.04 cm−1 for C=C un-saturation and 1217.08 cm−1 for the C–O str. of the carboxyl group). Nineteen fatty acids were identified from 28 bands in the GC analysis () followed by GC–MS analysis and quantification (), representing 96.72% of the total fatty acid content. The balance (3.18%) was made up of nine unidentified compounds. The amount of unsaturated fatty acids (53.25%) in the oil was somewhat greater than that of saturated fatty acids (43.57%). Their ratio in the oil was 1.22:1 (). However, the number of identified unsaturated fatty acids was only 5 whereas the number of identified saturated fatty acids was 14. The most abundant unsaturated and saturated fatty acids were linolenic acid (34.06 ± 0.23%) and palmitic acid (24.47 ± 0.22%), respectively. Other unsaturated fatty acids were linoleic acid (13.50 ± 0.12%), oleic acid (4.60 ± 0.11%), 7-hexadecanoic acid (1.00 ± 0.01%) and a very small amount of 7,10-hexadecadienoic acid (0.09 ± 0.01%). Among the saturated fatty acids other than palmitic acid, stearic acid (4.84 ± 0.05%), eicosanoic acid (3.61 ± 0.10%), behenic acid (3.67 ± 0.11%) and tetrasanoic acid (4.08 ± 0.08%) were present in considerable amounts. Nine other saturated fatty acids were present in minute amounts ().
Table 1 Some properties of the leaf oil of C. pallida.
Table 2 Fatty acid composition of the leaf oil of C. pallida by GC–MS.
The low saponification value and pleasant odour of the leaf oil of C. pallida may be an indication of its suitability for nutritional use. Moreover, linoleic acid (omega-6 fatty acid) was present as a third major fatty acid, which is one of the naturally occurring essential fatty acids. Linoleic acid is found to be beneficial for human health due to its regulation of body fat gain, enhanced immunity, reduced inflammation and minimized adverse reactions, which occur with increased immunity of the body system. Both linoleic acid and oleic acid are important from a nutritional point of view as well as for the stability of the oil. Another important fatty acid, linolenic acid, was present in very high amounts. This fatty acid is also an omega-6 fatty acid and is found to exhibit anti-inflammatory activity by some metabolic end products [Citation25–Citation27].
3.2 Antibacterial activity
The oil from the leaf extract of C. pallida showed considerable activities against Gram-positive and Gram-negative bacteria, though more significantly on Gram-negative bacteria (). The MIC of the fatty acid constitutes of the oil against E. coli and A. junii was determined to be 10 ± 0.14 and 10 ± 0.25, respectively, while in the case of B. subtilis, it was 80 ± 0.58 ().
Table 3 MIC of the Fatty acid constituents of the leaf oil of C. pallida against some bacteria.
The Gram-negative test organism E. coli is one of the most important bacteria responsible for human infection, which may be enterohaemorrhagic, enterotoxigenic, uropathogenic and meningitis/sepsis-associated [Citation28]. Conversely, A. junii is a human pathogen, which is particularly associated with outbreaks of septicaemia in neonates and paediatric oncology patients [Citation29]. Bacillus subtilis is a common cause of food spoilage, and its heat-resistant spores often pose a challenge to the thermal efficacy of heat processes resulting in the reduced shelf life of many processed foods [Citation30]. The MIC of the experimental oil against both E. coli and A. junii was 10 μg/ml, while in the case of B. subtilis, it was much higher (80 μg/ml). Soković et al. [Citation31] evaluated the MIC of oil from 10 commonly consumable herbs, namely, Citrus aurantium, C. limon, Lavandula angustifolia, Matricaria chamomilla, Mentha piperita, M. spicata, Ocimum basilicum, Origanum vulgare, Thymus vulgaris and Salvia officinalis. The MIC of oil against B. subtilis varies from 1.5 to 7 μg/ml, while it was between 2.5 and 10 μg/ml against E. coli. Our findings more or less corroborate the antimicrobial effects of essential on Gram-negative bacteria but are much higher for Gram-positive bacteria. Rahman et al. [Citation32] reported the MIC of oil from Ferula asafoetida against B. subtilis and E. coli to be 165 μg/ml and 110 μg/ml, respectively. The exact mechanism of the antibacterial activities of fatty acids remains unclear. The prime target seems to be the bacterial cell membrane and various essential processes involving it. The important processes include cell lysis, induction of autolysis, leakage of cell metabolites, disruption of the electron transport chain, enzyme inhibition, inhibition of nutrient uptake, and interference with oxidative phosphorylation [Citation33].
4 Conclusion
This is the first report about the physicochemical properties, fatty acid profile and antimicrobial activities of oil isolated from C. pallida leaves. Nineteen fatty acids were identified by gas-liquid chromatography followed by GC–MS. A pleasant odour, low saponification value and the presence of linoleic acid, oleic acid and linolenic acid in good amounts indicate its suitability for nutritional use. The broad spectrum of activity may make the oil attractive as an antibacterial agent for various applications in medicine, agriculture, food preservation, cosmetics and nutraceuticals, especially where the use of conventional antibiotics is undesirable or forbidden.
Acknowledgements
The authors are thankful to the University of Burdwan, Burdwan, for financial assistance and to Prof. A. Mukherjee, Department of Botany, for the authentication of the plant.
Notes
Peer review under responsibility of Taibah University.
References
- R.WilliamsAntimicrobial resistance: the factEssential Drug Monitor2000World Health OrganizationGeneva, Switzerland
- WHOAntimicrobial Resistance: Global Report on Surveillance2014WHO PressGeneva, Switzerland
- R.P.BorrisNatural products research: perspectives from a major pharmaceutical companyJ Ethnopharmacol.5119962938
- K.M.HazraR.N.RoyS.K.SenS.LaskarIsolation of antibacterial pentahydroxy flavones from the seeds of Mimusops elengi Linn AfrJ. Biotechnol.6200714461449
- J.C.PretoriusS.MagamaP.C.ZietsmanGrowth inhibition of plant pathogenic bacteria and fungi by extracts from selected South African plant speciesSouth Afr. J. Bot.202003188192
- P.MoreillionY.A.QueM.P.GlauserStaphylococcus aureus (including Staphyloccal Toxic shock)6th ed.G.L.MandellJ.E.BennettR.DolinPrinciples and Practice of Infectious diseasesvol. 22005Churchill LivingstonePennyslyvania23332339
- M.M.CowanPlant products as antimicrobial agentsClin. Microbiol. Rev.121999564582
- WHOTraditional Medicine Growing Needs and Potential – WHO Policy Perspectives on Medicines, No. 002May 2002World Health OrganizationGeneva, Switzerland
- R.BalzThe Healing Power of Essential Oils1st ed.1999Lotus PressTwin Lakes, WI, USA2780
- F.BakkaliS.AverbeckD.AverbeckM.IdaomarBiological effects of essential oils – a reviewFood Chem. Toxicol.462008446475
- D.KalembaA.KunickeAntibacterial and antifungal properties of essential oilsCurr. Med. Chem.102003813829
- S.PrabuseenivasanM.JayakumarS.IgnacimuthuIn vitro antibacterial activity of some plant essential oilsBMC Complement. Altern. Med.6200639
- M.E.GuynotA.J.RamosL.SetóP.PurroyV.SanchisS.MarínAntifungal activity of volatile compounds generated by essential oils against fungi commonly causing deterioration of bakery productsJ. Appl. Microbiol.942003893899
- R.N.ChopraS.L.NayarI.C.ChopraGlossary of Indian Medicinal Plants1956Publications and Information Directorate, Council of Scientific and Industrial ResearchNew Delhi81
- Editorial Board, CSIRThe Useful Plants of India1986Publication and Information Directorate, Council of Scientific and Industrial ResearchNew Delhi147
- N.O.AguliarCrotalaria pallida AitonI.Farida HanumL.J.G.Vander MaesenPlant Resources of South-East Asia No.11; Auxiliary Plants1997Backhuys PublisherLeiden, The Netherlands103105
- G.MahranG.WasselB.El-MenshawiG.El-HossaryA.SaeedPyrrolizidine alkaloids of Crotalaria aegyptiaca and Crotalaria madurensisActa Pharm. Suec.161979333338
- D.S.BhakuniR.ChaturvediChemical constituents of Crotalaria madurensisJ. Nat. Prod.471984585591
- C.C.W.WanjalaR.R.T.MajindaFlavonoid glycosides from Crotalaria podocarpaPhytochemistry511999705707
- H.S.YooJ.S.LeeC.Y.KimJ.KimFlavonoids of Crotalaria sessilifloraArch. Pharm. Res.22004544546
- H.KoJ.WengL.TsaoJ.WangC.LinAntiinflammatory flavonoids and pterocarpanoid from Crotalaria pallida and Crotalaria assamicaBioorg. Med. Chem. Lett.13200410111014
- Association of Official Analytical ChemistsOfficial Methods of Analysis16th ed.1995AOACWashington, DC
- G.H.WilkforsG.W.PattersonP.GhoshR.A.LewinB.C.SmithJ.H.AlixGrowth of post-set oysters, Crassastrea virginica on high lipid strains of algal flagellates Tetraseleis sp.,Aquaculture1431996411419
- J.A.WashingtonManual of Clinical Microbiologyfourth ed.1985American Society for MicrobiologyWashington, DC967
- P.C.CalderPolyunsaturated fatty acids, inflammation and immunityLipids36200110071024
- M.W.ParizaPerspective on the safety and effectiveness of conjugated linoleic acidAm. J. Clin. Nutr.7920041132S1136S
- P.C.Caldern-3 Polyunsaturated fatty acids, inflammation and inflammatory diseasesAm. J. Clin. Nutr.8320061505S1519S
- J.B.KaperJ.P.NataroH.L.MobleyPathogenic Escherichia coliNat. Rev. Microbiol.22004123140
- I.KappsteinH.GrundmannT.HauerC.NiemeyerAerators as a reservoir of Acinetobacter junii: an outbreak of bacteraemia in paediatric oncology patientsJ. Hosp. Infect.4420002730
- A.JagannathaT.TsuchidoaJ.-M.MembreComparison of the thermal inactivation of Bacillus subtilis spores in foods using the modified Weibull and Bigelow equationsFood Microbiol.222005233239
- M.SokovićJ.GlamočlijaD.MarinD.Brkić van GriensvenAntibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro modelMolecules15201075327546
- M.R.RahmanS.GulE.A.OdhanoAntimicrobial activities of Ferula assafoetida oil against Gram-positive and Gram-negative bacteriaAmerican-Eurasian J. Agric. Environ. Sci.42008203206
- A.P.DesboisV.J.SmithAntibacterial free fatty acids: activities, mechanisms of action and biotechnological potentialAppl. Micribiol. Biotechnol.85201016291642