?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This work reports the biogenic synthesis of silver nanoparticles (AgNPs) using the pod extract of Cola nitida, the evaluation of their antibacterial and antioxidant activities, and their application as an antimicrobial additive in paint. The AgNPs were characterized with UV–Vis spectroscopy, Fourier-transform infrared (FTIR) spectroscopy, and transmission electron microscopy (TEM). The AgNP solution was dark brown with a maximum absorbance occurring at 431.5 nm. The FTIR spectrum showed strong peaks at 3336.85, 2073.48, and 1639.49 cm−1, indicating that proteins acted as the capping and stabilization agents in the synthesis of the AgNPs. The AgNPs were spherical, with sizes ranging from 12 to 80 nm. Energy dispersive X-ray (EDX) analysis showed that silver was the prominent metal present, while the selected area electron diffraction pattern conformed to the face-centred cubic phase and crystalline nature of AgNPs. At various concentrations between 50 and 150 μg/ml, the AgNPs showed strong inhibition of the growth of multidrug resistant strains of Klebsiella granulomatis, Pseudomonas aeruginosa, and Escherichia coli. In addition, at 5 μg/ml, the AgNPs completely inhibited the growth of Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Aspergillus niger, A. flavus and A. fumigatus in a paint-AgNP admixture. The AgNPs exhibited a potent antioxidant activity with an IC50 of 43.98 μg/ml against 2,2-diphenyl-1-picrylhydrazyl and a ferric ion reduction of 13.62–49.96% at concentrations of 20–100 μg/ml. This study has demonstrated the biogenic synthesis of AgNPs that have potent antimicrobial and antioxidant activities and potential biomedical and industrial applications. To the best of our knowledge, this work is the first to use the pod extract of C. nitida for the green synthesis of nanoparticles.
1 Introduction
The numerous potential applications of nanoparticles have played a prominent role in the search for eco-friendly processes for generating nanoparticles using different biological materials because conventional nanoparticles syntheses involve the use of toxic solvents, high pressure, and high energy, all of which may be harmful to the environment. Such syntheses are capable of yielding nanoparticles that have unique attributes and properties that may influence their utility. Because of the rich biodiversity of microbes and plants, the potential for using biological materials in the synthesis of nanoparticles has yet to be fully explored. In this regard, several biological resources of the tropics, particularly those of Nigeria, have not been adequately evaluated for their potential use in synthesizing nanoparticles.
The green synthesis of nanoparticles has continued to receive unprecedented attention due to the simplicity of the processes, the minimal chemical handling needed, and the eco-friendliness [Citation1]. In addition, the availability of several biological macromolecules/substances that can serve as capping and stabilization molecules for the green synthesis of nanoparticles has also contributed to the steady rise of this synthesis route. Various authors have reported using bacteria, fungi, algae, spider cobwebs and plant extracts for the green synthesis of different types of nanoparticles [Citation1–Citation13].
Kola is a caffeine-containing nut, and the two most common species are Cola nitida and Cola acuminata [Citation14]. The nut is chewed in many West African countries, as it is known to ease hunger pangs. It also has ethnomedicinal values, with reports of its use to treat whooping cough and asthma, and it contains not only caffeine but also essential oils, phenolic compounds and alkaloids [Citation15–Citation17]. It is also a flavouring ingredient that is used in the production of flavoured beverages and chocolates, as well as in the production of dyes [Citation18]. Cola sp., an evergreen plant originally endemic to West Africa, mainly Cote d’Ivoire, Ghana, Liberia, Nigeria, and Sierra Leone, has been introduced to many tropical countries [Citation19]. The exotic species can now be found in countries such as Jamaica, India, and the United States. The world production of kola nuts from C. nitida and allied species has been estimated to be approximately 180,000 t, of which approximately 120,000 t is produced in Nigeria and used either internally or in neighbouring countries [Citation20]. Despite the large potential that kola offers, it has not been fully exploited for applications in the food and pharmaceutical industries. This work therefore seeks to extend the frontiers of the potential applications of the pod extract of kola nut with a particular interest in nanobiotechnology. The work presented here pioneers the effort of evaluating the kola nut pod for use in the green synthesis of nanoparticles. Most recently, we established the possibility of using seed shells and seed extracts of C. nitida to form AgNPs that have potent antibacterial activities against multi-drug resistant bacteria [Citation21].
Metal nanoparticles and their alloys made of some combination of silver, iron, cadmium, zinc, platinum, gold, graphene, among others have diverse applications in different aspects of human endeavours, with catalytic [Citation8,Citation22,Citation23], optical [Citation24], electronic [Citation25], magnetic, antimicrobial [Citation3,Citation6,Citation9,Citation12,Citation26] and biomedical utility [Citation27,Citation28]. Silver nanoparticles, for instance, are of interest because of their unique properties (size- and shape-dependent optical, electrical, and magnetic properties), which can be incorporated into coating materials, antimicrobial applications, biosensor materials, composite fibres, and electronic components [Citation29]. In this work, we report the green synthesis of AgNPs using the pod extract of Cola nitida and the evaluation of its antibacterial activities towards several drug-resistant strains of bacteria. The study also assessed the application of the nanoparticles as an antimicrobial additive in emulsion paint and included an evaluation of their antioxidant activity. To the best of our knowledge, this is the first report of the green synthesis of nanoparticles using the fruit pod of C. nitida.
2 Materials and methods
2.1 Sample collection
C. nitida plants with voucher number LH0431 were obtained from Aroje village, Ogbomoso and deposited in the herbarium of the Department of Pure and Applied Biology at Ladoke Akintola University of Technology in Ogbomoso. The fruit was split open to remove the seeds, while the pod was cut into pieces, air-dried for 5 days under ambient conditions and then milled into powder form using an electric blender.
2.2 Preparation of pod extract
Approximately 0.1 g of the milled pod (CPE) was suspended in 10 ml of distilled water and heated in a water bath at 60 °C for 1 h to obtain the extract. The extracts were then filtered using Whatman No. 1 filter paper and centrifuged at 4000 rpm for 20 min. The supernatant was collected and used without further purification.
2.3 Biogenic synthesis of AgNPs
The pod extract was used to synthesize AgNPs as previously described by Lateef et al. [Citation3,Citation4,Citation21]. Approximately 1 ml of the extract was added to a reaction vessel containing 40 ml of a 1 mM silver nitrate (AgNO3) solution to reduce the amount of silver ions. The reaction was carried out under static conditions at room temperature (30 ± 2 °C) for 2 h. The formation of AgNPs was observed as a change in the solution colour and was monitored both visually and through absorbance measurements of the reaction mixture performed using a UV–Visible spectrophotometer (Cecil, USA).
Fourier transform infrared (FTIR) spectroscopic analysis was carried out on the powdered AgNPs sample using an IRAffinity-1S Spectrometer (Shimadzu, UK) according to the method of Bhat et al. [Citation30]. The AgNPs solution was centrifuged at 10,000 rpm for 20 min. The solid residue obtained was then dried at room temperature, and the powder obtained was used for FTIR measurements using KBr pellets.
The TEM micrograph was obtained as follows. A drop of nanoparticles in suspension was placed on a 200-mesh hexagonal copper grid (3.05 mm) (Agar Scientific, Essex, UK) coated with 0.3% formvar dissolved in chloroform. The particles were allowed to settle for 3–5 min on the grid, the excess liquid was flicked off with a wick of filter paper, and the grids were air dried before being placed in the TEM. The micrograph was obtained using a JEM-1400 microscope (JEOL, USA) operating at 200 kV.
2.4 Antimicrobial activities of synthesized AgNPs
The antibacterial properties of the synthesized AgNPs were investigated using the agar diffusion method. Clinical isolates of Escherichia coli, Klebsiella granulomatis, and Pseudomonas aeruginosa obtained from LAUTECH Teaching Hospital in Ogbomoso were used as test organisms. Each bacterium was grown overnight in peptone water, and an 18-h culture was used to seed plates of Mueller-Hinton Agar (Lab M Ltd., UK). The plates were then bored using a cork borer (7 mm) to create wells. The wells were irrigated with 100 μl of graded concentrations of AgNPs prepared by dispersion in sterile distilled water. The plates were thereafter incubated at 37 °C for 24 h. At the end of incubation, the plates were examined for zones of inhibition, which were measured.
2.5 Antibacterial susceptibility test
The test bacterial isolates were screened for susceptibility using a panel of antibiotics on Mueller Hinton Agar plates by disc diffusion as previously described [Citation31,Citation32]. The bacterial isolates were tested on discs (Abtek Biologicals Ltd. UK) impregnated with antibiotics containing (in μg): ceftazidime (Caz), 30; cefuroxime (Crx), 30; gentamicin (Gen), 10; cefixime (Cxm), 5; ofloxacin (Ofl), 5; augmentin (Aug), 30; nitrofurantoin (Nit), 300; and ciprofloxacin (Cpr), 5. The plates were incubated at 37 °C for 48 h, and afterwards, the zones of inhibition were examined and interpreted according to Chortyk et al. [Citation33] considering the appropriate breakpoints [Citation34].
2.6 Evaluation of the antimicrobial properties of the synthesized AgNPs as additives in paint
The potential protective attributes of the synthesized AgNPs were studied by incorporating AgNPs in emulsion paint that was inoculated with bacteria and fungi isolated from soil samples as previously described by Lateef et al. [Citation4]. Commercially available white emulsion paint was procured and prepared according to manufacturer's instructions. The paint was dispensed into McCartney bottles in 19-ml volumes and was autoclaved at 121 °C for 15 min. Afterwards, the paints were inoculated with 1 ml (∼106 cfu/ml) of 18 h broth cultures of E. coli and P. aeruginosa. For fungal strains of Aspergillus flavus, A. fumigatus and A. niger, 1 ml (∼106 cfu/ml) of a 48-h culture was used as the inoculum. While the control experiment consisted of the paints and test organisms only, the test experiments consisted of the paint, test organism and 1 ml of 100 μg/ml of biosynthesized AgNPs to produce a final AgNP concentration of 5 μg/ml. The bottles were incubated at 37 °C and 30 ± 2 °C for 48 h for the bacteria and fungi, respectively. At the end of incubation, 1 ml of the contents of each bottle was used to inoculate fresh plates of nutrient agar for the bacteria and potato dextrose agar for the fungi using the pour plate method. The plates were then incubated at appropriate temperatures for up to 48 h. The plates were observed for growth at the end of incubation.
2.7 Antioxidant activities of biosynthesized AgNPs
2.7.1 DPPH radical-scavenging activity
The modified method of William et al. [Citation35] was used to study the free radical-scavenging activity of the AgNPs using 2,2-diphenyl-1-picrylhydrazyl or DPPH (Sigma–Aldrich, Germany). One millilitre of graded concentrations of AgNPs dispersed in methanol was added to 4.0 ml of a methanolic solution of 0.1 mM DPPH. The mixture was shaken and left in a dark box to stand for 30 min at room temperature (30 ± 2 °C). The blank was prepared with 1.0 ml of absolute methanol mixed with 4.0 ml of 0.1 mM methanolic DPPH. The absorbance of the resulting solution was measured at 517 nm on a model 6405 UV-Vis spectrophotometer (Jenway Ltd. Essex, UK). The inhibitory percentage of DPPH was calculated accordingly [Citation36].(1)
(1) The IC50, the concentration of AgNPs that decreases the initial concentration of the DPPH radical by 50%, was obtained by interpolation from linear regression analysis [Citation37]. The same procedure was used for standard antioxidant compounds, namely quercetin and β-carotene.
2.7.2 Ferric reducing activity
This was investigated using the Oyaizu [Citation38] method as follows: 1 ml of different concentrations of AgNPs was prepared using distilled water, to which 250 μl of phosphate buffer (pH 6.6) and 2.5 ml of potassium ferricyanide were added, and the solution was incubated at 50 °C for 20 min. After cooling, 250 μl of trichloroacetic acid was added and centrifuged for 10 min at 500 rpm. Then, 250 μl of the supernatant was mixed thoroughly with 250 μl of deionized distilled water and 500 μl of ferric (II) chloride, and the absorbance was read at 700 nm. A blank was prepared with all of the reagents without AgNPs. The reducing activity was calculated as follows:(2)
(2)
3 Results and discussion
3.1 Biogenic formation of AgNPs
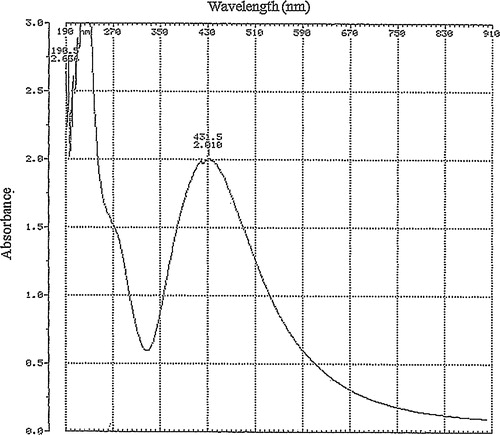
The production of AgNPs was catalysed by the pod extract over a 5 min period, with the development of a dark brown colour stabilizing within 20 min (). Colloidal AgNP solutions of varying colours ranging from light yellow, yellow brown to dark brown have been reported by several authors [Citation3,Citation4,Citation21,Citation39–Citation41] due to the composition of different macromolecules responsible for the catalytic formation and stabilization of the particles. The biosynthesized AgNPs showed a maximum absorbance wavelength of 431.5 nm (), which falls within the range previously reported for AgNPs [Citation3,Citation4,Citation39,Citation42,Citation43].
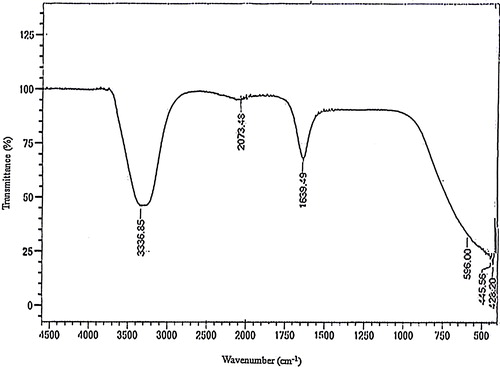
The FTIR results showed strong peaks at 3336.85, 2073.48, and 1639.49 cm−1 (), which showed that the biogenically synthesized AgNPs were capped and stabilized by proteins. The band at 3336 cm−1 is associated with the N–H bond of amines, while the 1639 cm−1 band is indicative of the C=C stretch of alkenes and the C=O stretch of the carbonyl in amides [Citation39,Citation44], both of which are important for the bio-reduction of AgNO3 to AgNPs and subsequent capping and stabilization. The kola nut pod has been previously reported to contain ∼9.5% of crude protein and also to be rich in alkaloids, caffeine (2.8%), theobroine (0.05%), nicotine and kolatine [Citation20,Citation45].
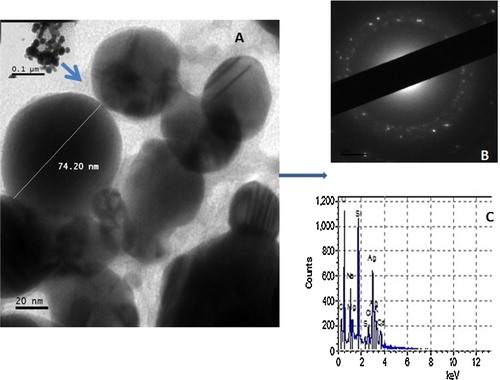
The AgNPs were spherical in shape and poly-disperse in nature, with sizes ranging from 12 to 80 nm (A). This shape and these sizes are in agreement with those reported earlier [Citation3,Citation4,Citation21,Citation41,Citation42,Citation46]. The particles were well dispersed, an indication of good stability against aggregation. The EDX pattern (B) showed that silver was the predominant element present in the AgNPs solution [Citation1,Citation21,Citation47] and had the characteristic ring-like SAED pattern (C) typical of face-centred cubic crystalline silver [Citation21,Citation44]. The presence of other elements, such as magnesium, sulphur, calcium, sodium and carbon, was from the pod extracts and are impurities on the surface of the biosynthesized AgNPs.
Fig. 4 Transmission electron micrograph (A), selected area electron diffraction pattern (B) and energy dispersive X-ray signal (C) of the biosynthesized AgNPs using pod extract of C. nitida.
The major by-product of kola nut processing is the kola nut pod, and its disposal creates a serious challenge to the environment, as more than approximately 30,000 t of kola nut pod husk are wasted annually in Nigeria [Citation48]. However, several authors have demonstrated a viable utilization of this important agro waste in the formulation of poultry and snail feeds [Citation16,Citation45,Citation49], nematicide [Citation50], and fertilizer [Citation51], as well as in the production of caustic potash [Citation52] and liquid detergent [Citation16]. It has also been evaluated for the production of activated carbon [Citation53] for use in the adsorption of dye [Citation54]. In our previous study, we reported the first reference to the utilization of kola nut pod by a local strain of Aspergillus niger for the production of fructosyltransferase [Citation55], an important enzyme in the biocatalytic transformation of sucrose to fructooligosaccharides [Citation56–Citation59]. This work has therefore expanded the frontier of the utilization of the kola nut pod for the biogenic and eco-friendly synthesis of AgNPs, which is the first report of its kind.
3.2 Antibacterial activities of biosynthesized AgNPs
The biosynthesized AgNPs displayed considerable inhibitory activity against several clinical isolates of bacteria (). Even though the bacterial isolates were multi-drug resistant strains, showing resistance to 2–8 antibiotics (), the AgNPs at concentrations of 50–150 μg/ml effectively inhibited strains of E. coli, P. aeruginosa and Klebsiella granulomatis to 12–30 mm (). We have previously documented the high burden of antibiotic resistance among bacterial isolates obtained from diverse sources in Nigeria [Citation31,Citation32,Citation60], which may constitute a threat to public health. The antibacterial activities of the AgNPs obtained in this study are similar to those reported in previous studies, [Citation1,Citation3,Citation4,Citation21,Citation44,Citation61,Citation62], demonstrating the efficacy of the particles to combat multi-drug resistant microbes in the environment in diverse applications.
Fig. 5 The antibacterial activities of synthesized AgNPs using pod extract of C. nitida against some clinical bacterial isolates (A, E. coli Wound; B, P. aeruginosa Ear; C, P. aeruginosa Wound; and D, K. granulomatis Ear).
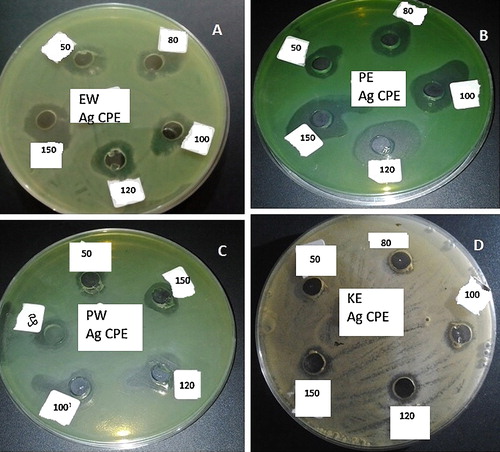
Table 1 Resistance pattern of the test isolates.
Table 2 Zone of inhibition (mm) of AgNPs on some bacterial isolates.
3.3 Antimicrobial activities of AgNPs in a AgNP-paint admixture
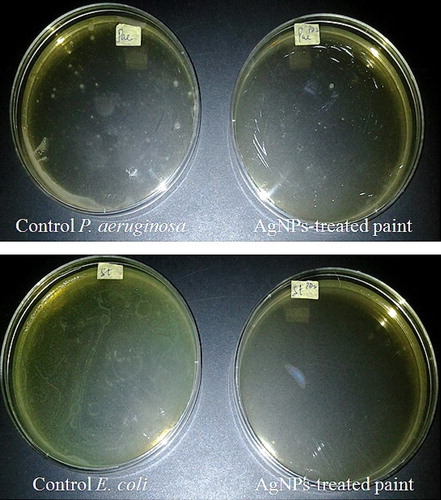
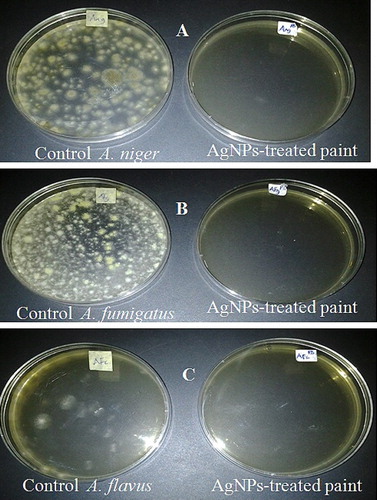
The results of the antimicrobial analysis showed the total obliteration of E. coli and P. aeruginosa (), as well as of A. niger, A. fumigatus and A. flavus (), in AgNPs-treated paint compared to the abundant growth observed in the control. The results obtained are similar to those obtained in a previous study using cobweb-mediated AgNPs as an additive in paint [Citation4]. The potential application of nanoparticles as additives in paints has been previously reported [Citation63,Citation64], indicating that the paint industry may benefit from the use of novel nanomaterials, including nanoparticulate silver, to improve the quality of the paint against microbial attack, biodegradation and chemical deterioration [Citation65].
3.4 Antioxidant activities
The DPPH-scavenging activities of the nanoparticles are presented in . The biosynthesized AgNPs showed inhibitions of 32.81–100% that were dose-dependent at the tested concentrations of 20–100 μg/ml, while the quercetin and β-carotene standards produced inhibitions of 43.87–74.62% and 11.11–66.67%, respectively, at concentrations of 200–1000 μg/ml. The IC50 values of AgNPs, quercetin and β-carotene were obtained as 43.98, 430 and 710 μg/ml, respectively, showing the dominant potency of the AgNPs. The results of the ferric ion reduction tests showed activities of 13.62–49.96% for the AgNPs present at concentrations of 20–100 μg/ml, while at concentrations of 200–1000 μg/ml, β-carotene and quercetin displayed reduction activity of 11.53–65.38% and 12.05–100%, respectively (). The scavenging activities of the AgNPs used in this work are similar to those that were previously reported [Citation66–Citation68]. The free radical scavenging activity of the AgNPs have been attributed to the functional groups of the bioreductant molecules adhered to the surface of the particles [Citation67].
Table 3 The DPPH-radical scavenging activity of the biosynthesized AgNPs.
Table 4 The ferric ion reducing activity of the biosynthesized AgNPs.
4 Conclusions
This study has shown that the extract obtained from the pod of C. nitida, which is an important agro waste, can be used for the biogenic, green and eco-friendly synthesis of AgNPs. The synthesis incorporating this extract led to the formation of poly-disperse spherical-shaped AgNPs, with sizes ranging from 12 to 80 nm. The particles showed strong activities against multi-drug resistant clinical bacterial strains, thereby establishing the relevance of the biosynthesized AgNPs for biomedical applications. Similarly, the AgNPs completely suppressed the growth of bacteria and fungi when used as an additive in emulsion paint and yielded an IC50 of 43.98 μg/ml against DPPH and ferric ion reducing activity of 49.96% at 100 μg/ml. This work has therefore established the relevance of the kola nut pod in nanobiotechnological applications, particularly in the cost-effective green synthesis of AgNPs. We believe that this is the first report on the effective use of the C. nitida pod in the green synthesis of AgNPs and the first demonstration of its antimicrobial and antioxidant activities.
Acknowledgement
AL thanks the authority of LAUTECH, Ogbomoso, Nigeria, for access to some of the facilities used in this study.
Notes
Peer review under responsibility of Taibah University.
References
- W.M.SalemM.HaridyW.F.SayedN.H.HassanAntibacterial activity of silver nanoparticles synthesized from latex and leaf extract of Ficus sycomorusInd. Crops Prod.622014228234
- A.LateefI.A.AdelereE.B.Gueguim-KanaThe biology and potential biotechnological applications of Bacillus safensisBiologia702015411419
- A.LateefI.A.AdelereE.B.Gueguim-KanaT.B.AsafaL.S.BeukesGreen synthesis of silver nanoparticles using keratinase obtained from a strain of Bacillus safensis LAU 13Int. Nano Lett.520152935
- A.LateefS.A.OjoM.A.AzeezT.B.AsafaT.A.YekeenA.AkinboroI.C.OladipoE.B.Gueguim-KanaL.S.BeukesCobweb as novel biomaterial for the green and eco-friendly synthesis of silver nanoparticlesAppl. Nanosci.201510.1007/s13204-015-0492-9
- S.ShivajiS.MadhuS.SinghExtracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteriaProcess Biochem.46201118001807
- R.AugustineN.KalarikkalS.ThomasA facile and rapid method for the black pepper leaf mediated green synthesis of silver nanoparticles and the antimicrobial studyAppl. Nanosci.42014809818
- B.KumarK.SmitaL.CumbalA.DebutSacha inchi (Plukenetia volubilis L.) oil for one pot synthesis of silver nanocatalyst: an ecofriendly approachInd. Crops Prod.582014238243
- A.MishraM.KumariS.PandeyV.ChaudhryK.C.GuptaC.S.NautiyalBiocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp.Bioresour. Technol.1662014235242
- G.M.NazeruddinN.R.PrasadS.R.PrasadY.I.ShaikhS.R.WaghmareP.AdhyapakCoriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activityInd. Crops Prod.602014212216
- N.ShanmugamP.RajkamalS.CholanN.KannadasanK.SathishkumarG.ViruthagiriA.SundaramanickamBiosynthesis of silver nanoparticles from the marine seaweed Sargassum wightii and their antibacterial activity against some human pathogensAppl. Nanosci.42014881888
- A.I.El-BatalN.M.ElKenawyaA.S.YassinM.A.AminLaccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticlesBiotechnol. Rep.520153139
- S.YallappaJ.ManjannaB.L.DhananjayaPhytosynthesis of stable Au, Ag and Au–Ag alloy nanoparticles using J. sambac leaves extract, and their enhanced antimicrobial activity in presence of organic antimicrobialsSpectrochim. Acta A: Mol. Biomol. Spectrosc.1372015236243
- M.AgharkarS.KochrekarS.HidouriM.A.AzeezTrends in green reduction of graphene oxides, issues and challenges: a reviewMater. Res. Bull.592014323328
- R.AkinosoA.K.AremuI.S.BalogunSome physical properties of kola nuts – a response surface approachInt. Agrophys.282014251255
- A.C.OdebodePhenolic compounds in the kola nut (Cola nitida and Cola acuminata) (Sterculiaceae) in AfricaRev. Biol. Trop.441996513515
- E.U.AsogwaJ.C.AnikweF.C.IhokwunyeKola production and utilization for economic developmentAfr. Sci.7200645
- E.A.DewoleD.F.A.DewumiJ.Y.T.AlabiA.AdegokeProximate and phytochemical of Cola nitida and Cola acuminataPak. J. Biol. Sci.16201315931596
- G.JarvisThe rise and fall of cocaine cola2002 https://www.lewrockwell.com/2002/05/gail-jarvis/the-rise-and-fall-of-cocaine-cola/ (accessed 25.04.15)
- H.I.C.LoweC.T.WatsonS.BadalP.PeartN.J.ToyangJ.BryantPromising efficacy of the Cola acuminata plant: a mini reviewAdv. Biol. Chem.42014240245
- C.OrwaA.MutuaR.KindtR.JamnadassS.AnthonyAgroforestry tree database: a tree reference and selection guide version 4.02009 http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp (accessed 19.06.15)
- A.LateefM.A.AzeezT.B.AsafaT.A.YekeenA.AkinboroI.C.OladipoF.E.AjetomobiE.B.Gueguim-KanaL.S.BeukesCola nitida-mediated biogenic synthesis of silver nanoparticles using seed and seed shell extracts and evaluation of antibacterial activitiesBioNanoScience52015196205
- T.J.I.EdisonM.G.SethuramanInstant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blueProcess Biochem.47201213511357
- N.MuniyappanN.S.NagarajanGreen synthesis of silver nanoparticles with Dalbergia spinosa leaves and their applications in biological and catalytic activitiesProcess Biochem.49201410541061
- S.S.KumarP.VenkateswarluV.R.RaoG.N.RaoSynthesis, characterization and optical properties of zinc oxide nanoparticlesInt. Nano Lett.320133036
- S.RajeshkumarM.PonnanikajamideenC.MalarkodiM.MaliniG.AnnaduraiMicrobe-mediated synthesis of antimicrobial semiconductor nanoparticles by marine bacteriaJ. Nanostruct. Chem.4201496102
- K.GopinathK.S.VenkateshR.IlangovanK.SankaranarayananA.ArumugamGreen synthesis of gold nanoparticles from leaf extract of Terminalia arjuna, for the enhanced mitotic cell division and pollen germination activityInd. Crops Prod.502013737742
- A.M.FayazK.BalajiM.GirilalR.YadavP.T.KalaichelvamR.VenketesanBiogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteriaNanomed. Nanotechnol. Biol. Med.62010103109
- K.L.NiraimathiV.SudhaR.LavanyaP.BrindhaBiosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activitiesColloids Surf. B: Biointerfaces1022013288291
- A.S.KhannaNanotechnology in high performance paint coatingsAsian J. Exp. Sci.2120082532
- R.BhatR.DeshpandeS.V.GanachariD.O.HuhA.VenkataramanPhoto-irradiated bio-synthesis of silver nanoparticles using edible mushroom Pleurotus florida and their antibacterial activity studiesBioinorg. Chem. Appl.2011 Article ID: 650979
- A.LateefT.E.DaviesA.AdelekanI.A.AdelereA.A.AdedejiA.H.FadahunsiAkara Ogbomoso: microbiological examination and identification of hazards and critical control pointsFood Sci. Technol. Int.162010389400
- A.LateefM.O.OjoPublic health issues in the processing of cassava (Manihot esculenta) for the production of ‘lafun’ and the application of hazard analysis control measuresQual. Assur. Saf. Crops Foods82016165177
- T.O.ChortykR.F.SeversonH.C.CutlerV.A.SiessonAntibiotic activities of sugar esters isolated from selected Nicotiana speciesBiosci. Biotechnol. Biochem.57199313551356
- J.M.AndrewsBSAC standardized disc susceptibility testing method (version 4)J. Antimicrob. Chemother.5620056076
- B.W.WilliamsM.E.CuverlierC.BersetUse of free radical method to evaluate antioxidant activityFood Sci. Technol. – LWT2819952530
- A.A.OlajireL.AzeezTotal antioxidant activity, phenolic, flavonoid and ascorbic acid contents of Nigerian vegetablesAfr. J. Food Sci. Technol.22011022029
- A.LateefJ.K.OlokeE.B.Gueguim-KanaS.O.OyeniyiO.R.OnifadeA.O.OyeleyeO.C.OladosuA.O.OyelamiImproving the quality of agro-wastes by solid state fermentation: enhanced antioxidant activities and nutritional qualitiesWorld J. Microbiol. Biotechnol.24200823692374
- M.OyaizuStudies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamineJpn. J. Nutr.441986307315
- E.E.EmekaO.C.OjiefohC.AleruchiL.A.HassanO.M.ChristianaM.RebeccaE.O.DareA.E.TemitopeEvaluation of antibacterial activities of silver nanoparticles green-synthesized using pineapple leaf (Ananas comosus)Micron57201415
- P.AramwitN.BangJ.RatanavarapornS.EkgasitGreen synthesis of silk sericin-capped silver nanoparticles and their potent anti-bacterial activityNanoscale Res. Lett.9201417
- V.R.NetalaV.S.KotakadiL.DomdiS.A.GaddamP.BobbuS.K.VenkataS.B.GhoshV.TartteBiogenic silver nanoparticles: efficient and effective antifungal agentsAppl. Nanosci.201510.1007/s13204-015-0463-1
- K.AnandalakshmiJ.VenugobalV.RamasamyCharacterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activityAppl. Nanosci.201510.1007/s13204-015-0449-z
- T.KathiravenA.SundaramanickamN.ShanmugamT.BalasubramanianGreen synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogensAppl. Nanosci.52015499504
- S.ShankarL.JaiswalR.S.L.AparnaR.G.S.V.PrasadSynthesis, characterization, in vitro biocompatibility, and antimicrobial activity of gold, silver and gold silver alloy nanoparticles prepared from Lansium domesticum fruit peel extractMater. Lett.13720147578
- B.B.BabatundeR.A.HamzatEffects of feeding graded levels of kolanut husk meal on the performance of cockerelsNig. J. Anim. Prod.3220056166
- R.R.R.KannanR.ArumugamD.RamyaK.ManivannanP.AnantharamanGreen synthesis of silver nanoparticles using marine macroalga Chaetomorpha linumAppl. Nanosci.32013229233
- K.ShameliM.B.AhmadM.ZargarW.M.Z.Wan YunusN.A.IbrahimP.Sha-banzadehM.Ghaffari-MoghadamSynthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activityInt. J. Nanomed.62011271284
- J.O.EdeworChemical production from local fossil fuels and the Nigeria balance of tradeJ. Nig. Soc. Chem. Eng.3198459
- J.AbioyeA.O.FanimoA.M.BamgboseM.A.DipeoluO.OlubamiwaNutrient utilization, growth and carcass performance of broiler chickens fed graded levels of kolanut huskJ. Poult. Sci.432006365370
- E.E.A.OyedunmadeJ.O.BabatolaT.I.OlabiyiThe effects of two crop residues on nematodes in cowpea cultivationNematol. Mediterr.2319956164
- E.A.MakindeR.R.IpinmorotiG.O.IremirenL.S.AyeniUtilization of kola pod husk for Telfaria occidentalis, Corchorus olitorius and Amaranthus cruentus production in Ikorodu, Lagos StateRes. Rev: J. Bot. Sci.22013913
- A.A.TaiwoI.OluwadareA.O.ShoboS.A.AmolegbeExtraction and potential application of caustic potash from kolanut husk, ugwu pod husk and plantain peelsSci. Res. Essay32008515517
- O.AdeyiProximate composition of some agricultural wastes in Nigeria and their potential use in activated carbon productionJ. Appl. Sci. Environ. Manage.1420105558
- O.S.AyandaO.AdeyiB.DurojaiyeO.OlafisoyeAdsorption kinetics and intraparticulate diffusivities of congo red onto kola nut pod carbonPol. J. Environ. Stud.21201211471152
- A.LateefJ.K.OlokeE.B.Gueguim-KanaO.R.RaimiProduction of fructosyltransferase by a local isolate of Aspergillus niger in both submerged and solid substrate mediaActa Aliment.412012100117
- A.LateefJ.K.OlokeS.G.PrapullaPurification and partial characterization of intracellular fructosyltransferase from a novel strain of Aureobasidium pullulansTurk. J. Biol.312007147154
- A.LateefJ.K.OlokeS.G.PrapullaThe effect of ultrasonication on the release of fructosyltransferase from Aureobasidium pullulans CFR 77Enzyme Microb. Technol.40200710671070
- A.LateefJ.K.OlokeE.B.Gueguim-KanaS.O.OyeniyiO.R.OnifadeA.O.OyeleyeO.C.OladosuRhizopus stolonifer LAU 07: a novel source of fructosyltransferaseChem. Pap.622008635638
- M.A.GanaieA.LateefU.S.GuptaEnzymatic trends of fructooligosaccharides production by microorganismsAppl. Biochem. Biotechnol.172201421432159
- A.LateefThe microbiology of a pharmaceutical effluent and its public health implicationsWorld J. Microbiol. Biotechnol.202004167171
- S.PriyadarshiniV.GopinathN.M.PriyadharsshiniD.M.AliP.VelusamySynthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its applicationColloids Surf. B: Biointerfaces1022013232237
- P.KanmaniS.T.LimSynthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogensProcess Biochem.48201310991106
- N.C.MuellerB.NowackExposure modeling of engineered nanoparticles in the environmentEnviron. Sci. Technol.42200844474453
- M.RajarathinamD.DhanapalG.MorukattuS.JosephK.P.ThangaveluImparting potential antibacterial and antifungal activities to water based interior paint using nanoparticles of silver as an additive – an ecofriendly approachAdv. Sci. Eng. Med.62014676682
- J.P.KaiserS.ZuinP.WickIs nanotechnology revolutionizing the paint and lacquer industry? A critical opinionSci. Total Environ.4422013282289
- C.ShanmugamG.SivasubramanianB.ParthasarathiK.BaskaranR.BalachanderV.R.ParameswaranAntimicrobial, free radical scavenging activities and catalytic oxidation of benzyl alcohol by nano-silver synthesized from the leaf extract of Aristolochia indica L.Appl. Nanosci.201510.1007/s13204-015-0477-8
- S.BhakyaS.MuthukrishnanM.SukumaranM.MuthukumarBiogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activityAppl. Nanosci.201510.1007/s13204-015-0473-z
- J.R.NakkalaE.BhagatK.SuchiangS.R.SadrasComparative study of antioxidant and catalytic activity of silver and gold nanoparticles synthesized from Costus pictus leaf extractJ. Mater. Sci. Technol.201510.1016/j.jmst.2015.07.002