?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Acoustic investigations have examined the salts of rare earth metals, such as lanthanum(III) chloride, in sorbitol-water mixed solvent systems of different concentrations at 303.15 K temperature and 1 atmospheric pressure. Various parameters have been calculated from the measured values of ultrasonic velocity, density and viscosity data. These parameters help to characterize the ion-solvent interaction and are also supportive of the structure-promoting tendency in the present solute-mixed solvent system. The limiting solvation number, , of lanthanum(III) chloride is maximum in water, rather than in sorbitol-water mixed solvent systems, which is attributed to an interesting outcome regarding the behaviour of sorbitol and water in comparison to other saccharides.
1 INTRODUCTION
Most of the chemical reactions that are undertaken in industry, biological systems and chemical synthesis involve different types of interactions between solutes and solvents. Therefore, the influence of solvent nature on thermodynamics, kinetics and mechanism is of great importance. One of the fundamental aspects of the solvent effect in electrolyte solutions is the extent of cation-solvent interactions or ionic solvation [Citation1,Citation2]. Wide varieties of physicochemical methods have been employed in the study of ionic solvation and determination of solvation numbers of ions in water and in non-aqueous solvents [Citation3–Citation5].
In an ionic solution, the interaction between the ionic solute and solvent is predominantly ion-dipole. The ion-dipole interaction depends on the size of ions and polarity of solvent. The dissolution of ionic salt in a solvent causes a volume contraction due to interaction between ions and solvent molecules. This might affect various properties involving thermodynamics and acoustics parameters factors.
Extensive research has been conducted to understand the nature of ion-ion and ion-solvent interactions in solutions of electrolytes and electrolytes in mixed solvents [Citation6–Citation11]. However, studies related to trivalent rare earth cations in mixed solvents are scarce. Lanthanum is a trivalent cation and is representative of the lanthanides. It is the second most abundant rare earth metal after cerium. Lanthanum(III) chloride is used widely in various fields such as industry, agriculture, medicine, petrochemicals, etc. In addition, this compound is of great interest due to its solubility both in water as well as in alcohol. Thus, Lanthanum(III) chloride has been widely used in different fields, which often leads to the accumulation of this compound in the human body. Bone formation in mammals is stimulated by the application of lanthanum(III) compounds in low doses, whereas higher doses lead to neurotoxicological disorders [Citation12]. Therefore, understanding the effects of lanthanum(III) chloride on health has become a major concern. On the other hand, sorbitol is commonly known as sugar alcohol and has a wide range of applications such as in food, healthcare, cosmetics, medicines and more. It is also used as a binding agent and syrup base in lanthanum(III) chloride medicines to cure bone disorders. The importance of using this compound is in its ability to form multiple hydrogen bonds with water. Therefore, sorbitol has been chosen as one component of the mixed solvent system.
In continuation of our earlier work on lanthanum(III) chloride in other mixed solvent systems [Citation13–Citation15], this work is a further attempt to investigate systematically the thermo-acoustical behaviours of lanthanum(III) chloride in water and sorbitol-water mixed solvent systems of various compositions at 303.15 K and at 1 atmospheric pressure. These measured values were utilized for the calculation of different thermodynamic and acoustic parameters, which shed light on solvation properties as well as the ion-solvent and solvent-solvent interactions that occur in the solutions.
2 EXPERIMENTAL
Analytical grade sorbitol was procured from Qualigen Chemicals, and hepta hydrated Lanthanum(III) chloride (99.9%) was obtained from Indian Rare Earth Ltd. India. Stock solutions of sorbitol in distilled water were prepared by accurate weighing. Similarly, stock solutions of 2 M lanthanum(III) chloride were prepared in approximate solvents and were estimated by titration against EDTA using xylenol orange as the indicator. Different concentrations of lanthanum(III) chloride (1.0 to 2.0 mol dm−3) were prepared from the stock solution. The solutions were kept for 2 hours in a thermostat at 303.15 K with an accuracy of 0.01 K before the measurements. The densities of all the solutions were measured by a bicapillary pyknometer with deionized double distilled water with 0.9960 × 103 kg m−3 as its density at 303.15 K. The precision of density measurements was within ±0.0003 kg m−3. The ultrasonic velocity was measured at 303.15 K and at atmospheric pressure by a single-crystal variable-path ultrasonic interferometer operating at 5 MHz. The temperature was maintained by circulating water from a thermostatically regulated bath (Toshniwal, India) around the sample holder (within ±0.01 K). The velocity measurements were within ± 0.5 ms−1. The viscosities of the solutions were measured with a calibrated Ostwald viscometer immersed in a constant temperature water bath maintained within ±0.01 K, and the time of flow was determined.
3 RESULTS
Various acoustic parameters, such as isentropic compressibility, βs, acoustic impedance, Z, intermolecular free length, Lf, molar sound velocity, R, and molar compressibility, W, were calculated from the measured values of velocity, U, density, ρ, and viscosity, η, using standard formulae [Citation16–Citation18]. Parameters such as relative association, RA, relaxation time, τ, apparent molar compressibility, ϕk, and apparent molar volume, ϕv, were calculated by the following formulae [Citation10,Citation19]:(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4) where ρ0 and βs0 are the density and isentropic compressibility of the solvent (aqueous-sorbitol), and ρ and βs are those of the solution, respectively, c is the molarity of the solution and M is the molar mass of the solute (lanthanum(III) chloride). The limiting apparent molar volume ϕv0 was determined from Masson’s equation [Citation20] based on Debye- Huckel theory [Citation21]
(5)
(5)
where ϕv0 and Sv are the intercept and slope, respectively, in the plot of ϕv versus c1/2. Similarly, the limiting apparent molar compressibility, ϕk0, is obtained from Gucker’s limiting law [Citation22], which states ϕk is
(6)
(6) where Sk is the slope related to ion-solvent interaction and ϕk0 is the intercept related to ion-ion interaction. The slope and intercept are obtained from the plot of ϕk vs c1/2. The solvation number, Sn, defined by Passynski [Citation23], is calculated using the following relation:
(7)
(7) where n0 and ni are the moles of solvent and solute, respectively. The variation of the solvation number with molar concentration of solute leads to a limiting solvation number S0n which is evaluated by
(8)
(8) where
is the molar volume of the solvent with n1 moles of solvent.
Additionally, Jones and Dole coefficient, B, [Citation24] for concentrated solutions (c ≥ 0.1 M) is calculated from relative viscosity by the following relations [Citation25,Citation26].(9)
(9) where ηr is the relative viscosity and η0 is the viscosity of solvent. In the present investigation, lanthanum(III) chloride in a sorbitol-water mixed solvent system with the concentration range of 1.0 to 2.0 mol dm−3 has been studied and all the parameters have been calculated. The measured values of density, viscosity, ultrasonic velocity, and computed values of other relevant acoustic parameters of lanthanum(III) chloride in water and sorbitol-water mixtures have been summarized in and . Besides, select parameters have been graphically represented (–)
4 DISCUSSION
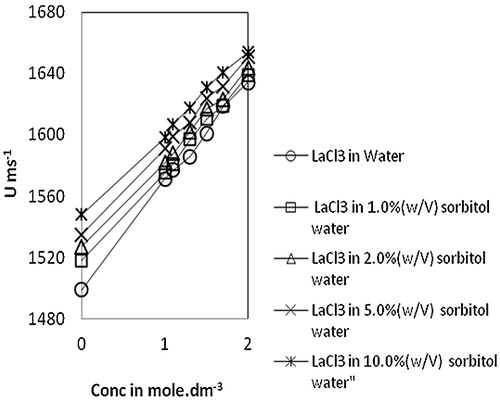
It has been observed from that the ultrasonic velocity, viscosity and density values are increasing with increased concentrations of lanthanum(III) chloride in all systems. This increase in velocity may be due to the cohesive forces by ionic hydration and is indicative of molecular association. The increase in density indicates the existence of solute solvent interactions and may also be interpreted to the structure maker of the solvent due to H-bonding [Citation27]. Similarly, an increase in viscosity may be observed because the solute particles lying across the fluid streamlines tend to rotate and absorb energy, which causes the increase in viscosity of the solutions [Citation28,Citation29]. The results are similar to our observations on dextrose-water systems in the concentration range of 1.0 to 2.0 mol dm−3 of lanthanum(III) chloride [Citation15]. The values of apparent molar volume, ϕv, increase with an increase in concentration of lanthanum(III) chloride concentration and decrease with sorbitol content. The limiting apparent molar volume, ϕv0, values obtained from the plot of ϕv vs c1/2 is a measure of the ion-solvent interaction [Citation20]. Positive values for both ϕv and ϕv0 are indicative of the presence of solute-solvent interactions and both the values are increasing with increase in sorbitol content.
Table 1 Experimentally determined ultrasonic velocity, U, density, ρ, viscosity η, and calculated values of apparent molar volume, ϕv, Relative association, RA, solvation number, Sn , and relaxation time, τ of lanthanum(III) chloride in water and different proportions of sorbitol-water mixture at 303.15 K.
Isentropic compressibility, βs, decreases with an increase in both lanthanum(III) chloride concentration and sorbitol content (). This decrease confirms the presence of ion-solvent interactions through ion-dipole type forces between lanthanum ions and surrounding water molecules. In general, the compressibility of the solvent is higher than that of solution and the compressibility reduces with the increase in concentration of the solution. The values of intermolecular free length, Lf, show a similar trend as that of the compressibility values (). The decreased compressibility brings the molecules closer resulting in a decrease of intermolecular free length. The decrease in free length upon increasing the concentration of solute indicates significant ions-solvent interactions between the molecules and also suggests a structure promoting tendency of the added solute [Citation30].
Fig. 2 Variation of isentropic compresiibility, βs vs Conc. of lanthanum(III) chloride in water and sorbitol-water mixture at temperature 303.15 K.
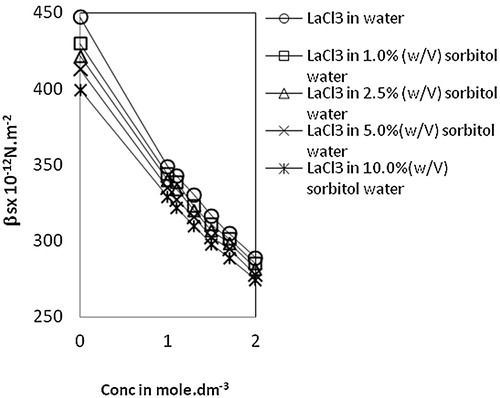
Fig. 3 Variation of intermolecular free length, Lf vs Conc. of lanthanum(III) chloride in water and sorbitol-water mixture at temperature 303.15 K.
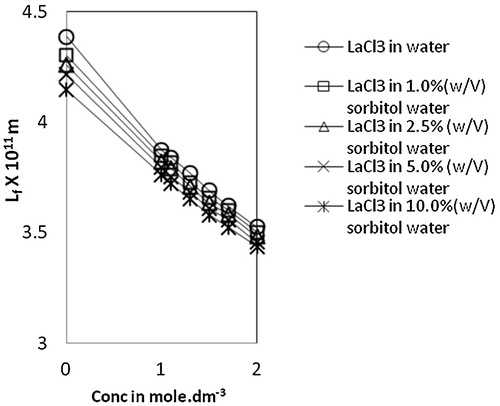
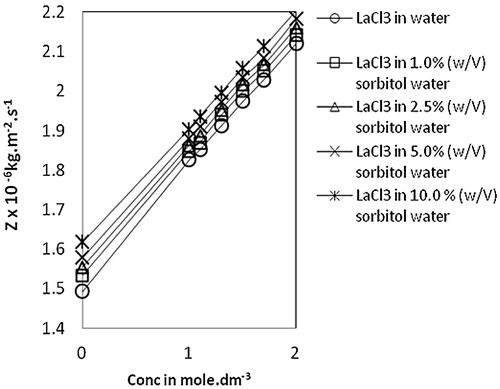
Fig. 4 Variation of acoustic impedance, Z vs Conc. of lanthanum(III) chloride in water and sorbitol-water mixture at temperature 303.15 K.
The apparent molar compressibility, ϕk, is negative for all systems, including pure water and mixed solvent systems. The values decrease with an increase in concentration of lanthanum(III) chloride indicating poor compressibility of the solvent in the vicinity of ions due to electrostrictive stiffening. The same effects are also observed with increasing sorbitol content for a given concentration of lanthanum(III) chloride. The values of and Sk are the intercept and slope of the equation Equation(6)
(6)
(6) and are calculated from the plot of ϕk vs c1/2 for pure water as well as mixed solvent systems. Negative values of
indicate ion-solvent interactions and positive values of Sk indicate ion-ion interactions ().
Table 2 Thermodynamic and acoustic parameters of lanthanum(III) chloride in water and different proportions of sorbitol-water mixture at 303.15 K.
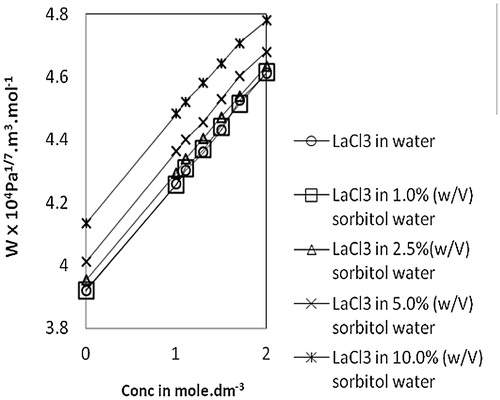
The molar sound velocity, R, also known as Rao’s constant, increases linearly () with an increase in concentration of lanthanum(III) chloride in water, as well as in sorbitol-water mixed solvents. A similar trend is also observed in the case of molar compressibility, W, in due to the presence of solute-solvent interaction [Citation17]. Relaxation time, τ, () increases with an increase in concentration of lanthanum(III) chloride for all the systems and also with an increase in sorbitol content in mixed solvents. This variation of τ suggests the structure making capacity of solutes and is therefore indicative of ion-solvent interactions [Citation26]. The values of relative association, RA, increase with an increase in lanthanum(III) chloride concentration. This reflects ion-solvent interactions leading to structure promotion by lanthanum(III) chloride in all the systems studied. The acoustic impedance, Z, is found to increase linearly with increases in concentration of lanthanum(III) chloride in water as well as sorbitol-water mixed solvents and also increases with the increase in percentage of sorbitol for the same lanthanum(III) chloride concentration (). The increase in Z with increases in lanthanum(III) chloride concentration may be due to the disruption of solvent-solvent interactions, making fewer solvent aggregates available [Citation31]. The solvation number, Sn, also provides insight into solute and solvent interactions and represents the number of solvent molecules attached to the solute molecule. Sn gives the information about the structure breaking or structure making tendency of a compound in solutions. Depending upon the structure of a compound, the solvation number is either positive or negative. In the present study, solvation numbers are positive, which suggests an appreciable solvation of solute [Citation32]. Again, it has been observed that the solvation number decreases with increases in the concentration of lanthanum(III) chloride. According to Kannappan et al., this type of variation may attain the primary solvation in pure crystalline state [Citation5]. The solvent molecules are attached to the ions by strong covalent bonds in the primary sheath of solvation and in the secondary sheath, there are weak forces of attraction between solute and solvent species. Review of the literature [Citation26,Citation33] shows that the values of in the 0–2.5 range are indicative of the presence of unsolvated species and that values above this range suggest the solvation of ions. In the present study,
calculated by the Passynski method are all greater than 2.5 [Eq. Equation(8)
(8)
(8) ], which indicates distinct solvation of the ions. The limiting solvation number for pure water (14) is found to be close to the Bockris data [Citation34].
Fig. 5 Variation of molar volume, R vs Conc. of lanthanum(III) chloride in water and sorbitol-water mixture at temperature 303.15 K.
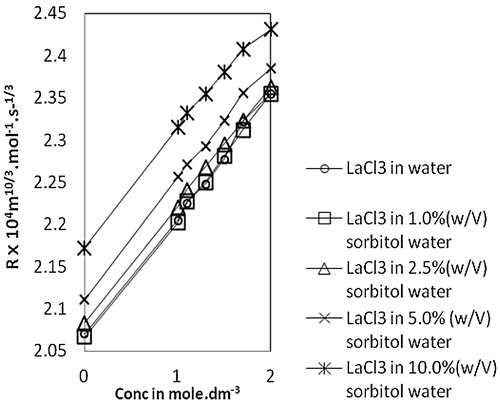
Fig. 6 Variation of molar compressibility, W vs Conc. of lanthanum(III) chloride in water and sorbitol-water mixture at temperature 303.15 K.
The viscosity coefficient, B-value, is a measure of the effective hydrodynamic volume of the solvated ions and is governed by ion-solvent interactions. In the present investigation, the B-values are positive for all the mixed solvent systems, which is similar to our previous observations with dextrose-water systems [Citation15]. It has been observed that the values are positive for all the concentrations of lanthanum(III) chloride and that the B-values increase with the concentration of lanthanum(III) chloride. This observation is due to an increase in ion-solvent interactions. These values also increase with an increase in sorbitol concentration [1.0% w/V − 10.0% w/V] at a given lanthanum(III) chloride concentration. An increase in viscosity is due to the structure making of the ions in the systems that leads to the ordering of the solvent molecules. This, however, predominates over a decrease in solvent viscosity [Citation35]. As a result, there is a net increase in viscosity with increase in lanthanum(III) chloride concentration. The increase of B-coefficient with an increase in sorbitol content for a fixed concentration of lanthanum(III) chloride is due to a strong association between water and sorbitol through multiple hydrogen bonding [Citation31]. Bonding interactions between dextrose-water and sorbitol water systems shows that B-values are higher for sorbitol-water systems for a given lanthanum(III) chloride concentration due to greater ordering of the solvent molecules.
5 CONCLUSIONS
In the present investigation, it is revealed that there is a strong interaction among the solute and solvent. This solute-solvent or ion-solvent interaction promotes the structure-forming tendency, whereas ion-ion or solute-solute interaction enhances the structure-breaking properties in the systems. In addition, the sign and magnitude of various parameters are attributed to the strength and existence of intermolecular association, which are mostly due to hydrogen bonding.
References
- R.M.IzattS.J.BradshawS.A.NielsenJ.D.LanbJ.J.ChristensenChem. Rev.851985271
- O.PopovychR.P.T.TomkinsNon-aqueous solution chemistry1981John Wiley and sonsNewYork
- U.MayerPure Appl. Chem.411975291
- A.PopovPure Appl. Chem.511979101
- V.KannappanS.C.VinayagamInd. J. Pure Appl. Phys.442006670
- A.K.NainR.PalNeetuJ. Chem. Thermodyn.642013172
- C.M.RoneronE.MorenoJ.L.RojasThermochim. Acta328199933
- P.H.Von HippelT.SchleichAccounts Chem. Res.21969257
- E.V.ParfenyukO.I.DavydovaN.S.LebedevaJ. Soln.Chem3320041
- A.K.NainM.LatherNeetuJ. Chem. Thermodyn.63201367
- V.K.DakuaB.SinhaM.N.RoyPhys. Chem. Liquids: An Int. J.452007549
- L.FengH.XiaoX.HeZ.LiF.LiN.LiuY.ZhaoY.HuangZ.ZhangZ.ChaiToxicol. Lett.1652006112
- J.K.DashM.SahuM.ChakraborttyV.ChakravorttyJ. Mol. Liq.842000215
- J.K.DashM.ChakraborttyV.ChakravorttyAcoust. Lett.221999242
- J.K.DashS.KamilaRussian J. Phys. Chem. A8920151578
- S.KamilaS.JenaB.B.SwainJ. Chem. Thermodyn.372005820
- S.KamilaJ.K.DashJ. Mol. Liq.172201271
- S.KamilaV.ChakravorttyS.JenaJ. Solut. Chem.332004365
- N.RohmanS.MahiuddinJ. Chem. Soc. Faraday Trans.9319972053
- D.O.MassonPhil. Mag.81929218
- P.DebyeC.HuckelZ. Phy.241923305
- T.FrankGuckerJ. Am. Chem. Soc.5519332709
- A.PassynskiActa Physico. Chim.81938357
- G.JonesM.DoleJ. Am. Chem. Soc.5119292950
- P.K.DasB.M.SatpathyR.K.MishraB.BeheraInd. J. Chem.16A1978959
- D.V.JahagirdarB.R.ArbadC.S.PatilA.G.ShankarwarInd. J. Chem.40A2001815
- S.ThirumaranA.N.KannapanGlobal J. Mol. Sci42009160
- M.KaminskyDisc. Faraday Soc.241957171
- H.J.V.TyrvellM.KennerlyJ. Chem. Soc. (A)19682724
- V.KannappanV.S.ChidambaraInd. J. Pure Appl. Phys.452007143
- P.K.MishraB.B.SwainV.ChakravarttyPhys. Chem. Liq.31199697
- M.Sethu RamanG.AmirthaganesanInd. J. Phys.7820041329
- K.NishikawaN.KuramotoT.UchiyamaBull.Chem. Soc. Jpn.6719942870
- J.O’M. BockrisP.P.S.SalujaJ. Phys. Chem.7619722140
- R.H.StokesR.MillsViscosity of electrolytes and related properties1965Pergamon PressNew York