?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The Instrumental Neutron Activation Analysis (INAA) technique was used to analyse 10 cigarette brands sold in the Egyptian market. The concentrations of trace and major constituents were measured to better understand the hazardous effects of smoking Egyptian cigarette tobacco. The samples were irradiated at the core of the Second Research Egyptian Reactor ET-RR-2, and the induced activities were counted by <gamma>-ray spectrometry using an efficiency-calibrated High Purity Germanium (HPGe) detector. The NAA k0-standardization method was used to determine the concentrations of 14 elements (Ba, Br, Ca, Cd, Eu, K, Hf, Mg, Na, Rb, Sb, Sc, Th and Yb). The high concentrations found for some of these elements pose a health hazard to individuals exposed to these cigarettes
1 Introduction
Cigarette smoking is an important source of particles in indoor air and potentially causes human respiratory diseases due to the presence of hazardous substances in tobacco smoke, trace metals, carbon monoxide and nicotine, which can lead to cancer, heart and lung diseases [Citation1–Citation7]. Tobacco use currently kills approximately 5.2 million people worldwide each year, a number that is expected to grow to more than 8 million a year by 2030. Half of those deaths occur between the ages of 30 and 69 years, ensuing in a loss of 20-25 years of life for smokers versus non-tobacco users. Depending on WHO, at least one person dies every 10 seconds as a result of cigarette smoking. It is well established that tobacco (Nicotiana tabacum) can easily accumulate certain heavy metals, in particular cadmium, in leaves [Citation8–Citation12]. Cadmium is a non-essential, potentially toxic and largely pollutant ion that is absorbed by tobacco plants and transferred in tobacco smoke to humans. Unless current trends change, the vast bulk of these deaths occur in developing countries. Tobacco use is widespread throughout the world owing to low prices, aggressive and widespread tobacco company marketing, lack of awareness about its dangers and inconsistent public policies against its use. Smoked forms of tobacco include numerous varieties of cigarettes (manufactured, hand-rolled, filtered, unfiltered, and flavoured), cigars and pipes. Although manufactured cigarettes are the most usual case of smoked tobacco, other smoked tobacco products such as beds, critics and Shisha are gaining in popularity, often in the mistaken belief that they are less hazardous to health. Even then, all strains of tobacco are lethal. Smoked tobacco in all forms causes up to 90% of all lung cancers and is a hazard factor for six of the eight leading causes of death worldwide. Smoking in Egypt is prevalent, and 19 billion cigarettes are smoked annually in the country, making it the largest market in the Arab world [Citation13]. Inside cafes, hookah (shisha) smoking is widespread. As of 2012, smoking in Egypt had reached an all-time high, with an estimated twenty percent, or ten million people, regularly using tobacco products [Citation14]. A tobacco control law prohibits smoking in the following specified public places: inside cafes, health and educational facilities, governmental venues, sporting and social societies, and youth centres. Smoking is also forbidden on public transports. The World Health Organization indicates that, according to 2013 estimates, over 20% of the Egyptian population, or approximately 18 million people, smoke tobacco daily. The World Lung Foundation reported that 50,000 Egyptians die from tobacco-related diseases every year, making smoking the fourth largest cause of death in the country. In addition to causing multiple diseases, cigarette smoking has many additional adverse effects on the body such as causing inflammation and impairing immune functions. Tobacco plants are known for easily absorbing nickel, cadmium and other metals from the soil and concentrating them in the leaves. These metals are present in tobacco rolls and are then ingested during the smoking procedure. The smoke may contain arsenic, particularly when the tobacco has been dressed with lead arsenate insecticide. The content of arsenic in tobacco grown on soils not treated with arsenic compounds is usually below 3 mg/kg. Out of the entire amount of heavy and trace metals originally present in tobacco, 10-15% were found to be from mainstream smoke, and the remainder were mainly distributed in the ash and cigarette. Some subjects have confirmed increased respiratory cancers in published studies of other inhaled nickel compounds. Considerable data also confirm the statement that tobacco smoking acts sympathetically with a number of respiratory carcinogens. For most relevant metals, their percentage transfers from a glowing cigarette into smoke have been calculated. Studies have found that cigarette smoking is a substantial factor in spontaneous abortions among pregnant women who smoke cigarettes and that it leads to a number of other threats to the wellness of foetuses. Access trace metals and radioactive elements such as radium, 210 Pb and 210 Po in counterfeit cigarettes have been attributed to heavy applications of less expensive and contaminated phosphate fertilizers produced from apatite rock. Dangers from modern cigarettes include contamination from pesticides used during tobacco growth. Insecticides are used during storage to protect curing leaves from insect attack, and other chemicals are added to enhance taste and make cigarettes burn better. Technological processes can significantly increase the total trace metal concentration in cigarettes. Not all traces of metals in cigarettes are the result of human actions. Some arise through the concentration of naturally occurring soil components. Smoking appears to stimulate oxidative stress, in that smokers have diminished levels of anti-oxidants such as vitamin C, vitamin E and carotenoids. Metals such as cadmium, tin, lead and mercury affect the transmission of synaptic messages, both in the brain and peripheral nervous system. They can disrupt calcium metabolism, which can affect cellular function. The chemical behaviour of barium, for example, is the same as calcium. When taken up into the blood stream, it is deposited in delicate organs (liver, spleen, brain, and nerves), in hair and in fingernails in a similar manner to Ca. In another survey, the displacement of calcium from cell membranes by barium increased the permeability and stimulation of muscles, which eventually resulted in paralysis of the central nervous system. Antimony has found in tobacco smoke and can interact similarly to arsenic in producing toxicity. Several methods are in place to determine trace metals in cigarette tobacco, but the demand for multiple component analyses is receiving greater attention because toxicity might be greatly influenced by the synergistic effects of various metals. The k0-NAA technique’s inherent quality control [Citation15–Citation17] has been utilized for the detection of trace metals and major elements in tobacco. The levels of trace and major elements in several sorts of tobacco have up to now been poorly studied, and knowledge of these ground substances is important both from the point of view of health studies connected with smoking and, more generally, aspects of the uptake of trace elements by plants. Because of its outstanding sensitivity, neutron-activation analysis is ideal for the determination of trace heavy metals.
Table 3 Trace element levels in (ppm) in cigarette tobacco, as determined by INAA.
2 Materials and methods
In the present study, instrumental neutron activation analysis (INAA) was used to study the essential elemental contents in a variety of cigarettes randomly collected in the Egyptian market. The samples were irradiated with thermal neutrons in a nuclear reactor, and the induced activities were counted by <gamma>-ray spectrometry using an efficiency-calibrated high resolution High Purity Germanium (HPGe) detector. The samples were irradiated in the Second Research Egyptian Reactor, ET-RR-2, at a neutron flounce rate of approximately 1.3 × 1013n/(cm2 s). CRMs, RMs and gold monitors were also irradiated. Certified reference materials such as IAEA-153, IAEA-155, and CTA-VTL-2 [Citation18] were used to determine the elemental concentrations by the relative technique. At the same time, the analytical results obtained for the CRMs were used for internal quality control. All samples, monitors and standards were placed in clean cylindrical aluminium vials for the long irradiations. Data acquisition was performed using Genie-2000 software from Canberra.
2.1 Sample preparation
Twenty cigarettes in each box were used for the experiments. During sample preparation, the cigarette wrappers were removed, and 50–100 mg of tobacco were powdered by mortar and pestle and sieved through a 30 mesh, dried in an oven at 90 °C and allowed to cool in desiccators. The samples and the standard were prepared under the same geometry. The analytical samples for both short-lived and long-lived irradiations were weighed and wrapped in well prepared, contaminant free polyethylene film. Approximately 50–100 mg of tobacco were used to estimate the trace and major constituents.
2.2 Sample irradiation procedure
The samples were placed in a homogenous flux of thermal neutrons for periods of time sufficient to produce measurable amounts of radionuclides of the elements to be determined. In this work, short-lived and long-lived irradiations were adopted. In the first scheme, nuclides with shorter half-lives were measured to determine Na, K, Ca, Br and Mg. The listed short-lived nuclides () were measured utilizing the nuclear data. For those elements leading to short lived activation products, no further packaging was necessary. The samples were sent for irradiation in an outer irradiation channel designated C3, which had a soft neutron spectrum. The outer irradiation channel was selected to eliminate corrections due to nuclear interferences caused by threshold reactions, notably Mg in the presence of Al and Al in the presence of Si. The number of standards applied with the short irradiations was one per seven samples. In the second scheme, nuclides with relatively longer half-lives were examined to determine Rb, Br, Sb, Sc, Ba, Hf, Cd, Eu, Yb and Th. The prepared samples were irradiated for 6 hours in two of the small inner irradiation channels, designated C1 and C2, to take advantage of the higher thermal neutron flux in the internal positions. The samples and a standard were packaged in a polyethylene capsule and irradiated together to avoid flux variations over time. A power rating of 20 MW was used to provide a thermal neutron flux of 2.5 × 1013 (n/cm2 s), which was suitable for long-lived activation, and a flux of 1.3 × 1013 n/(cm2 s) for short-lived activation.
Table 1 Half-life and energy results for some of the radionuclides produced.
2.3 Counting procedure
After successfully irradiating the samples in the reactor, a pneumatic transfer system was used to place the irradiated samples for counting. For the short irradiation schemes, after a 5 minute irradiation with a 1.3 × 1013 n/(cm2 s) thermal neutron flux and 5 minute waiting time, the samples were counted for 10 minute counting periods for the first round of counting (). The second cycle of counting was also performed for 10 minutes after a waiting time of 3–4 hours. For elements with long-lived radionuclides, the conditions necessary for effective counting after irradiation with a 2.5 × 1013 n/(cm2 s) thermal neutron flux for 6 hours included 4–5 days cooling time to remove short-lived gamma-rays and 30 minutes counting time on a Plexiglas holder designated H1 corresponding to a source detector geometry of 5 cm. The second cycle of counting after the long irradiation was performed for 30 minutes after a waiting period of 10 days on a Plexiglas holder designated H1. The choices of cooling time and sample detector geometry were such that the detectors had dead times of less than 10%. Using a multichannel analyser (MCA) card, the spectral intensities of the samples were collected. Each spectrum was examined using the Genie 2000 gamma-ray spectrum analysis software program. The software computes an average background by integrating the areas designated by the analyst on each side of the desired peak region. This was subsequently subtracted from the peak. Where peaks overlapped, a Gaussian fitting routine was used. The routine was able to resolve up to 5 overlapping peaks by varying the height, full width at half-maximum and centroid of each peak until the best fit to the data was obtained. The result of the above were corrected for dead time, tobacco weights and half-life and then compared to the mean value of all the criteria for the same peak. The computer then processed these data using Genie 2000 and estimated the concentrations in parts per million (ppm) for each sample.
2.4 The k0-standardization method
The neutron flux at the irradiated position was measured by detecting the 411.8 keV gamma line from the reaction 197 Au (n, g) 198 Au, using a thin gold foil with a 1-cm2 circular shape. The obtained results were found to be f = 16.5 ± 0.55, and α = 0.03 ± 0.013. A FORTRAN computer program was designed and used to calculate the values of for the analysed elements.
The k0-factors for the majority of the elements that can be determined via NAA were experimentally measured with high accuracies and tabulated.
Once the k0 values were determined, the concentration of the analyte ρa were obtained by the co-irradiation of a sample and a suitable flux monitor (comparator):(1)
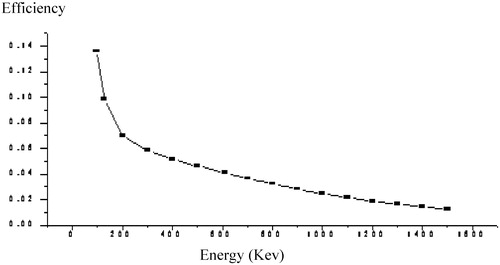
(1) where the subscript “Au” refers to the co-irradiated gold monitor, and Np is the net number of counts under the full-energy gamma-ray peak measured at t life time, W is the weight of the sample, w is the weight of the gold monitor, S = 1 − exp(−λti), λ is the decay constant, ti is the irradiation time, D = exp(−λtd), td is the decay time, C = {{1 − exp(−λtm)}/λtm}, tm is the measurement real time, and f is the thermal to epithermal flux ratio. α is the deviation in the real epithermal neutron spectrum (1/E1+a) from the ideal 1/E law, ε is the absolute full energy gamma-ray peak efficiency, and Q0 is the ratio between the resonance neutron integral and the thermal neutron cross-section at 2200 m/s. shows the efficiency curve for the detector used in this work.
3 Results and discussion
To evaluate the accuracy and precision of our analytical procedure, standard reference materials IAEA-153 and IAEA-155 and CTA-VTL-2 were analysed in a manner similar to our samples. shows the radioisotopes produced, half-lives and gamma-ray energies. The measured elemental concentrations found in the selected brands of cigarettes analysed in this survey are shown in and . In the present study, the concentrations of 14 trace elements in cigarette tobacco of ten different brands of Egyptian cigarettes were determined using the k0-NAA method.
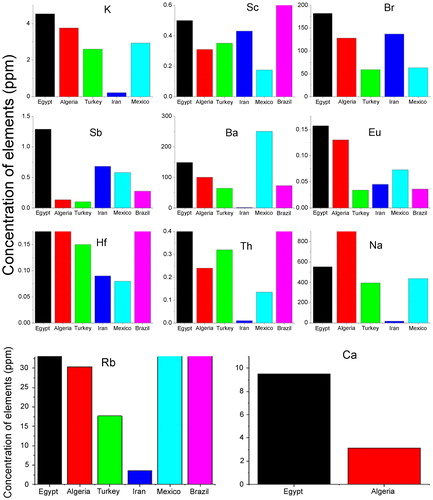
For the elements measured in the three reference materials, the values found using the k0 technique were usually within 10% of the certified values. The results were reported with respect to those obtained for samples from Iranian, Turkish, Brazilian and Mexican cigarette tobacco. For 11 elements, Ca, K, Sc, Br, Sb, Ba, Eu, Hf, Th, Na, and Rb, the mean concentrations and the standard-deviations of the 10 results for the 10 brands of Egyptian cigarettes were calculated and compared in with those obtained for samples from Iranian, Turkish, Brazilian and Mexican cigarette tobacco. As can show in , for many of the elements, the concentrations found in Egyptian cigarettes were higher than those found in cigarettes from other countries. The average levels of essential and non-essential elements analysed in each brand of cigarette in this study are shown in and . The nutritionally essential elements, which were detected in all cigarette brands investigated in this study, are low molecular weight cations that do not possess the physical properties of metals. Notwithstanding, those cations are essential to human health due to their essential metabolic roles. The levels of Sb detected in the cigarette brands analysed in the present study in conjunction with arsenic could contribute to environmental toxicity and have health effects on both dynamic and passive smokers. The comportment of those ingredients in cigarettes available in the Egyptian market should be examined due to the short-term and long-term devastating effects of those constituents on body vital organs. The finding of toxic and trace constituents in cigarette tobacco is important both from the perspective of health studies connected with smoking and, more generally, aspects of the uptake of trace elements by plants. The results for the Egyptian tobacco () were compared with analyses of Turkish [Citation19], Iranian [Citation20], Mexican [Citation21] and Brazilian tobacco [Citation22]. compares the results of studies of trace element contents of cigarettes in various countries.
Table 2 Trace element levels (in ppm or %) in cigarette tobacco, as determined by INAA.
Table 4 Comparability of the results of studies of trace element contents in cigarettes from various countries [Citation18–Citation21].
The concentrations of elements detected in the selected brands of cigarettes analysed in terms of risk assessment are important due to potential interactions with the principal metals. Barium’s (Ba) chemistry is similar to that calcium and is deposited in delicate organs such as nerves, the liver and brain and even in hair and bones in the same manner as calcium. The shift in calcium from cell membranes causes increased muscle permeability and stimulation, a probable cause of central nervous system paralysis. The levels of Sb detected in the cigarette brands analysed in the present study, when in conjunction with arsenic, could contribute to environmental toxicity and health effects on both dynamic and passive smokers. The comportment of these ingredients in cigarettes available in the Egyptian market should be examined due to their short-term and long-term devastating effects on the vital organs of the physical structure.
shows a comparison of the results of studies of the highest trace element contents (ppm) of cigarettes from several countries. shows the relationships among the concentration levels of K, Sc, Br, Sb, Ba, Eu, Hf, Th, Na, Rb and Ca in Egypt and various other countries. The results are reported with those from samples of Iranian, Turkish, Brazilian and Mexican cigarette tobaccos. The cigarette tobacco toxic and trace constituent findings are essential both from the perspective of health studies connected with smoking and more general aspects of the uptake of trace elements by plants. Due to its great sensitivity, the k0-NAA method is highly suited for the determination of heavy metals.
4 Conclusions
NAA using the k0 method is considered to be a useful method for the determination of heavy metal concentrations in a variety of matrices, including toxic heavy metals in cigarettes, and is more accurate and precise.
Using k0-NAA with an Au monitor, the concentrations of (14) elemental concentrations (Ba, Br, Ca, Cd, Eu, K, Hf, Mg, Na, Rb, Sb, Sc, Th and Yb) were determined. The observed average metals concentrations for sodium in all cigarette brands was 554.66 and ranged from 805.1 to 144.6%, for potassium, 4.48, and ranging from 8.11 to 2.81%, calcium, 9.5, and ranging from 21.75 to 2.65%, rubidium, 45.45, and ranging from 84.35 to 27.35 ppm, magnesium, 1.87, and ranging from 2.83 to 0.53 ppm, cadmium, 4.42, and ranging from 6.4 to 1.6 ppm, bromine, 183.69, and ranging from 226.3 to 135.7 ppm, antimony, 1.29, and ranging (2.6 to 0.03) ppm, scandium, 0.499, and ranging from 2.1 to 0.10 ppm, barium, 149.18, and ranging from 214.00 to 122.00 ppm, hafnium, 0.994, and ranging from 1.92 to 0.5 ppm, europium, 0.157, and ranging from 0.4 to 0.11 ppm, ytterbium, 0.612, and ranging from 1.22 to 0.16 ppm, and thorium, 0.58, and ranging from 0.9 to 0.2 ppm. The results were compared with those obtained for samples from Algerian, Turkish, Iranian, Mexican and Brazilian cigarette tobaccos, and the concentrations in Egyptian cigarette tobacco were found to be higher. The results for some of the elements were sufficiently high that they pose health hazards to individuals exposed to those cigarette constituents. Higher concentrations of Cd, Sb, K and Yb were observed in local cigarette brands relative to those in import cigarette brands. This poses a heavy health risk to individuals exposed to cigarettes in Egypt. Cigarette manufacturers should conduct research on constructing filters capable of trapping metals found in cigarette smoke. In consequence, the government should outlaw advertisements and the public purpose of cigarettes in Egypt through adequate legislation. This work could provide new data, which would be a useful tool for health organizations. Measured levels of toxic metals in cigarette tobacco brands provide extra public health information.
Acknowledgements
We would like to thank the staff of the Second Research Egyptian reactor (ET-RR-2) for irradiating the samples used in this work and the editor and referees for their valuable comments.
Notes
Peer review under responsibility of Taibah University.
References
- AshrafHamidaM.Abd ElSamadaN.F.SolimanbH.A.HanaficDetection of minor and trace elements in powdered milkJ. Taibah Univ. Sci.201710.1016/j.jtusci.2016.01.005
- R.S.PappasG.M.PlozinC.H.WatsonD.L.AshleyCadmium, lead and thallium in smokeparticulate from counterfeit cigarette compared toauthentic us brandsFood Chem. Toxicol.452007202209
- R.S.PappasS.B.StanfillC.H.WatsonD.L.AshleyAnalysis of toxic metals in commercial moistsnuff and Alaskan IqmikJ. Anal. Toxicol.3242008281291
- D.WuS.LandsbergerS.M.LarsonDetermination of the elemental distribution in cigarettecomponents and smoke by instrumental neutron activation analysisJ. Radioanal. Nucl. Chem.21719977782
- E.L.CarminesEvaluation of the potential effects of ingredients added to cigarettes Part 1: cigarette design, testing approach, and review of resultsFood Chem. Toxicol.4020027791
- A.RodgmanT.A.PerfettiThe Chemical Components of Tobacco and Tobacco Smoke2008CRC PressBoca Raton, FL, USA
- D.DavisM.T.NielsenTobacco: Production, Chemistry and Technology2017Wiley-Blackwell
- G.G.YebpellaG.A.ShallangwaC.HammuelA.MagomyaM.O.A.OladipoA.N.NokJ.J.BonirePac. J. Sci. Technol.1212011356362
- A.MassadehF.AlaliQ.JaradatActa Chim. Slov.502003375381
- A. Massadeh, F. Alali, Q. Jaradat, Determination of Cadmium and Lead in Different Brands of Cigarettes in Jordan, (2004).
- G.SchneiderV.KrivnaMulti-element analysis of tobacco and smoke condensate by instrumental neutron activation analysis and atomic absorption spectrometryInt. J. Environ. Anal. Chem.532199387100
- R.NessJ.GrissoN.HirschengaerN.MarkovicL.ShawN.DayJ.KlineN. Engl. J. Med.3402002333339
- Yolande Knell (9 June 2010). Egypt Introduces Alexandria Smoking Ban. BBC News. Retrieved 3 May 2012.
- Egypt Global Adult Tobacco Survey. Tobacco Use in Egypt (PDF). Retrieved 1 May 2012.
- H. Ozaki, M. Ebihara, Res. Cent. Nucl. Sci. Tech., Univ. of Tokyo, Tokai, Ibaraki, Japan, 2007, 1195, 319.
- R.AcharyaA.G.C.NairK.SudarshanA.V.R.ReddyA.GoswamiAppl. Radiat. Isot.200765
- L.AlghemM.RamdhaneS.KhaledT.AkhalNucl. Instrum. Methods Phys. Res.202006556
- Rajimincl Dybczyriski, Halina Polkowska-Motrenko, Zbigniew Samczynski, Zygmunt Szopa, Krzysztof Kulisa. Marek Wasek (Department of Analytical Chemistry, Institute of Nuclear Chemistry and Technology, Warsaw. Poland) Certification of A New Biological Reference Material – Virginia Tobacco Leaves (Cta-Vtl-2) and Homogeneity Study by NAA On This And Other Candidate Reference Materials.
- M.C.GülovaliG.GunduzJ. Radioanal. Chem.781983189198
- Z.AbedinzadehM.RazechiB.ParsaJ. Radioanal. Chem.351977373379
- H.R.Vega-CarrilloF.Y.IskanderE.Manzanres-AcunaJ. Radioanal. Nucl. Chem. Lett.2001995137145
- C.S.MunitaB.P.MazzillilJ. Radioanal. Nucl. Chem. Lett.1081986217227