Highlights
| • | Intraspecific diversity of crops harbors different assemblages of flower visiting insects improving agricultural landscape heterogeneity. | ||||
| • | Different structure of floral visitor assemblages could be attributed to volatile signals variability to which some insects are sensitive. | ||||
| • | All insect assemblages presented more non herbivorous than herbivorous insects. | ||||
| • | Combination of different coriander genotypes could enhance insect species diversity, favoring pollination and pest regulation in crops. | ||||
Abstract
Intraspecific diversity of crops producing volatile organic compounds could harbor different assemblages of flower visiting insects, improving agricultural landscape heterogeneity and thus, natural regulation of crop pests. In this context, the objectives of this work were i) to evaluate the composition, abundance and richness of floral visitor assemblages in different coriander crop genotypes and sowing dates and ii) to determine the relationship between insect assemblages and volatile signals emitted by the different coriander genotypes. Two field experiments (Exp. 1 and 2) were conducted in a completely randomized design with four replications, at the Faculty of Agronomy in the University of Buenos Aires, Argentina. Exp. 1 included early and late sowing dates while Exp. 2 included only late sowing date. Treatments were three coriander genotypes from different origins: Leisure 2008 (L) a variety from USA, GSN 2008 (G) a variety from France and a population from Argentina (A). At full flowering, floral visitor insects were sampled using an entomological net. The sampling units were the coriandeŕs umbels contained in squares of 40 × 40 cm. Two squares were randomly placed in each plot and several samplings were made in each of them, along 10 min-periods. Floral visiting insects were classified into pollinator, predator, parasite, herbivore and decomposer functional groups according to their habits and food preferences. Composition and abundance of floral visitor assemblages differed among genotypes, mainly for the early sowing date. Differences could be attributed to the intraspecific variability of volatile signals to which some insects were sensitive. Although richness was similar among assemblages related to each genotype, different species composition suggests that the combination of different coriander genotypes in cropping systems could enhance insect species diversity of the agricultural system and natural pest regulation.
1 Introduction
Plants emit a great variety of volatile signals that can actively participate in plant growth and protection against biotic and abiotic stresses (CitationPichersky and Lewinsohn, 2011). The use of plants to provide signals for enhancing natural enemy activity in agroecosystems is an interesting practice, but candidate crops species and varieties are not always screened for their attractiveness to insects in the system being studied. Signals related to VOCs concentration and composition can vary within and among crop species (CitationGil et al., 2000; CitationBálint et al., 2016) and environments (Citationde la Fuente et al., 2003; CitationLoreto et al., 2014), thus generating different volatile signals that could harbor different insect communities. Up to date, only few studies reported the influence of crop intraspecific variation of volatile emissions on insect attraction, and they were mainly focused on honeybees (CitationKlatt et al., 2013). However, there is growing evidence indicating that intraspecific differences can be important to explain the trophic structure and functioning (CitationJohnson and Agrawal, 2005; CitationCrutsinger et al., 2006; CitationBarbour et al., 2015). For arthropod herbivores in particular, there is strong support demonstrating that genetic variation in host plants is a key factor shaping their diversity and composition but, in many cases, the mechanism determining such variation remains poorly explored (CitationBarbour et al., 2015). For example, a significant effect of genetic variation of Tanacetum vulgare on arthropod abundances (CitationBálint et al., 2016) and of Solidago altissima on floral visitor richness and abundance was detected (CitationGenung et al., 2010; CitationBurkle et al., 2013).
Coriander plants emit signals from vegetative and reproductive structures. Floral volatiles serve as attractants, especially for pollinators and casual visitors, whereas volatiles emitted from vegetative parts, appear to protect plants by deterring herbivores or by attracting natural enemies (CitationPotter and Fagerson, 1990; CitationLenardis et al., 2007; CitationBendifallah et al., 2013). Many of these compounds, mainly terpenes, accumulated in coriander tissues and emitted to the environment may have ecological impact (CitationHarborne, 1997) as chemical signals attracting beneficial insects or repelling herbivores, therefore, protecting the crop (CitationLenardis et al., 2007). Volatile signals may change among coriander genotypes and crop environments (CitationDiederichsen, 2017; Citationde la Fuente et al., 2003). The production of VOCs is strongly regulated by genetics, making VOC emissions site-specific (CitationOlle and Bender, 2010), species-specific (CitationSplivallo et al., 2012) and/or even genotype-specific, as observed in apple accessions and tomato varieties (CitationFarneti et al., 2012; CitationFarneti et al., 2014). For instance, variations in the sowing dates of the crop change the climatic and weather conditions, affecting crop growth, development and terpens synthesis, and thus possibly, also the volatile cues emitted by the crop (CitationOlle and Bender, 2010).
In the agricultural landscape design the inclusion of different coriander genotypes in monocrops or intercrops, could favor functional and species diversity of homogeneous and impoverished agroecosystems such as the Argentinean Pampas. In this region, the lack of rotations and the few crop species included, affected the ecosystem services like natural pest control and pollination (CitationAizen et al., 2009). Intra and interspecific diversity, intended to attract natural enemies of crop pests and pollinators (CitationPatt et al., 1997), could have enormous potential to improve crop pest management and reduce dependence on pesticides (CitationHassanali et al., 2008). Genotype mixtures could be an excellent practice for exploring not only stability (CitationTilman et al., 2001) but also synergies between genotypes (CitationSchöb et al., 2015) and more complex plant–plant interactions (CitationBrooker et al., 2016).
In this context, the hypothesis of this work was that structure and richness of floral visitor assemblages will be related to volatile signals emitted by the crop depending on genotypes and sowing dates. Thus, the objectives of this work were i) to evaluate the composition, abundance and richness of floral visitor assemblages in different coriander crop genotypes and sowing dates and ii) to determine the relationship between insect assemblages and volatile signals emitted by the different coriander genotypes.
2 Materials and methods
2.1 Study site and field experiments
During two consecutive years, field experiments were conducted at the Faculty of Agronomy in the University of Buenos Aires, Argentina (34∘35 S, 58∘25 W) on a silty clay loam soil classified as Vertic Argiudoll according to the USDA taxonomy (1999). The experiments involved three treatments arranged in a completely randomized design (DCA) with four replications. Treatments were three coriander genotypes from different origin: Leisure 2008 (L), a variety from USA, GSN 2008 (G), a variety from France and a population from Argentina (A). Twelve plots of 12 m2 were sown on June 4th (early sowing date) and on August 4th (late sowing date) in the first year (Exp. 1), whereas twelve plots of 8 m2 were sown on August 21st (late sowing date) in the second year (Exp. 2). The soil was ploughed and refined to produce a smooth seed bed. The sowing was made very carefully in order to achieve a uniform coriander seed germination and seedling emergence. Coriander seeds (with over 98% germination) at a rate of 150–200 plants m−2 were placed along the rows, covered with soil, lightly compacted and irrigated (CitationGil et al., 1999). The total crop cycle and sowing-flowering phase durations (days) were different among genotypes and sowing date (for details see supplementary data).
Spontaneous weeds were manually removed throughout the crop cycle. During the experiments, plots were irrigated to supplement natural rainfall with the objective of maintaining the soil near field capacity.
2.2 Measurements
When coriander was at full flowering (from October 15th to November 15th), floral visitor insects were sampled using an entomological net, then killed in situ and preserved to be later identified (CitationTorretta and Poggio, 2013). The sampling units were the coriandeŕs umbels contained in squares of 40 × 40 cm. Two squares were randomly placed in each plot and several samplings were made in each one, along 10 min-periods. The samplings were carried out under similar climatic conditions (sunny and without wind), between 12:00 and 13:00 h in all the experiments.
Floral visitors were taxonomically determined at species level, when possible, or at morphospecies level. The analysis at morphospecies level allows studying insect assemblages since the differences between the number of morphospecies and taxonomic species are, in many cases, very small (CitationDerraik et al., 2002). Floral visiting insects were classified into pollinator, predator, parasite, herbivore and decomposer functional groups according to their habits and food preferences at both immature and adult stages. The main ecological role was assigned based on the information available in the literature (CitationColomo de Correa and Roig-Alsina, 2009; CitationGramajo and Mulieri, 2011). Insect samples are available in the entomological collection in the Faculty of Agronomy, University of Buenos Aires.
The structure of assemblages was analyzed in terms of abundance and richness of floral visitor insects. Floral visitor abundance was the total number of individuals per species or morphospecies captured per plot, and richness was the total number of species or morphospecies captured per plot (CitationMagurran, 1988).
Coriander volatile emissions were evaluated using artificial “nose” technology (CitationSzpeiner et al., 2009). An electronic nose consists of an array of non-specific gas sensors which generates an aromatic pattern of each plot based on the signal received by the nose sensors. Sensors are able to recognize simple and complex odors as a whole blend but not as isolated chemical compounds. Three samples were taken in each plot (at the bottom, the middle and the top of coriandeŕs canopy) to characterize the blend of chemical signals from each genotype. In order to relate insect assemblages and signals, both samplings were made at the same phenological state on all sowing dates.
2.3 Statistical analysis
The abundance of insects from each genotype and sowing date was classified using a cluster analyses PCORD 5 (CitationMcCune and Grace, 2002), to quantify the degree of similarities among treatments. Classification provides useful summary of large data matrices. A Sorensen coefficient version modified by Bray and Curtis (CitationMagurran, 1988) was used as distance measure. Farthest neighbor (complete linkage) was used as similarity measure (CitationVan Torengen, 1987). Results from the classification were presented in tables for each sowing date, where insect groups are shown in rows and insect assemblages related to genotypes are shown in columns.
Abundance and richness of pollinators, natural enemies (predators and parasitoids) and total insects visiting different genotypes on different sowing dates were analyzed with, using Infostat 2016 (CitationDi Rienzo et al., 2016). Abundance was transformed from discrete to continue variable using square root transformations. Means were compared by Tukey’s significant difference test at the 0.05 probability level. Both homogeneity of variance and normal distribution have been tested.
The hypothesis stating differences between species compositions of floral visitor assemblages in different genotypes was tested by using the Multi-Response Permutation Procedure (MRPP) (CitationMielke, 1984). In this analysis, data about presence-absence of morphospecies were considered and genotypes as categorical variables were used (CitationJohnson and Agrawal, 2005).
The relationship between abundance of floral visitors on coriander genotypes and chemical signals was analyzed with principal component analyses (PCA) (CitationterBraak, 1987); using PC-ORD Multivariate Analysis of Ecological Data Version 5.0 (CitationMcCune and Grace, 2002). The chemical signals were used as explanatory variables. To determine association between data and explanatory variables, a biplot from PCA was obtained by overlaying a vector diagram based on coefficients from the canonical functions describing each canonical axis.
3 Results
3.1 Composition, abundance and richness of floral visitor assemblages of coriander
On the early sowing date for Exp. 1, a total of 297 floral visitor insects belonging to 31 morphospecies were surveyed. Functional composition was 16.1% pollinators, 38.7% herbivorous, 22.6% predators, 6.5% parasites, 12.9% decomposers and 3.2% non-identified. On the late sowing date for Exp. 1, 155 insects belonging to 19 morphospecies were surveyed. Functional composition was 21.1% pollinators, 10.5% herbivorous, 36.8% predators, 5.3% parasites 21% decomposers and 5.3% non-identified. In Exp. 2, 180 insects represented by 22 morphospecies were captured. Functional composition was 32% pollinators, 14% herbivorous, 27% predators, 9% parasites and 18% decomposers (–).
Table 1 Order, family, species/morphospecies, function, abundance and richness of floral visitor insects grouped by assemblies (columns) and sociological groups (lines) on early sowing date from Exp. 1. Codes of genotypes: A, Argentinean; L, Leisure; G, GSN; Order: COL, Coleoptera; DIP, Diptera; HYM, Hymenoptera; LEP, Lepidoptera; Species: First three letters of the name identifies the genus and the following three letters identifies the specie. Function: DES, Decomposer; HER, Herbivorous; PAR, Parasite; POL, Pollinator; PRE, Predator; IND, Undetermined.
Table 3 Order, family, species/morphospecies, function, abundance and richness of floral visitor insects grouped by assemblies (columns) and sociological groups (lines) on late sowing date from Exp. 2. Codes of genotypes: A, Argentinean; L, Leisure; G, GSN; Order: COL, Coleoptera; DIP, Diptera; HYM, Hymenoptera; LEP, Lepidoptera; Species: First three letters of the name identifies the genus and the following three letters identifies the specie; Function: DES, Decomposer; HER, Herbivorous; PAR, Parasite; POL, Pollinator; PRE, Predator; IND, Undetermined.
The classification of 31(early sowing date) and 19 (late sowing date) morphospecies from Exp. 1 and 22 morphospecies from Exp. 2 resulted in three assemblages (columns) of floral visitor insects related to genotypes and four insect groups (rows) (–).
Group l of floral visitor insects was common to all the three assemblages. Some species/morphospecies, such as Scaptotrigona jujuyensis, Plebeia droryana, Hylaeus punctatus, Toxomerus sp.1, and Apis mellifera, were present in this group in both experiments. The insect assemblage related to genotype A was characterized by groups I and II; while groups I and III characterized genotype L and groups I and IV characterized genotype G. Thus, group II was present only in A, group III in L and group IV was present only in G. In Exp. 1 the presence of Phaenicia sericata and the absence Tricharaea occidua was distinctive of genotype A (–).
According to MRPP, composition of the assemblages differed among genotypes (p = 0.004) on the early sowing date for Exp. 1. Pair-wise comparison showed that composition of insect assemblages in genotype A significantly differed from genotypes L (p = 0.03) and G (p = 0.02), whereas the insect assemblage composition was not significantly different (p = 0.08) between L and G. Abundance of pollinators and total abundance was significantly higher in genotype A than in the rest of genotypes (p = 0.007, F = 9.19 and p = 0.002, F = 13.61 respectively). While the abundance of natural enemies was not different among genotypes (p = 0.57, F = 0.59). Richness of pollinators (p = 0.41, F = 0.97), natural enemies (p = 0.28, F = 1.48) and total richness (p = 0.76, F = 0.28) of floral visitors was not different among genotypes ().
On the late sowing date for Exp. 1, composition of the assemblages (p = 0.23), abundance (pollinators p = 0.083, F = 3.32, natural enemies p = 0.75, F = 0.30, total p = 0.14, F = 2.45) and richness (pollinators p = 0.12, F = 2.7, natural enemies p = 0.69, F = 0.38 and total p = 0.27, F = 1.53) were not different among genotypes ().
Table 2 Order, family, species/morphospecies, function, abundance and richness of floral visitor insects grouped by assemblies (columns) and sociological groups (lines) on late sowing date from Exp. 1. Codes of genotypes: A, Argentinean population; L, Leisure; G, GSN; Order: COL, Coleoptera; DIP, Diptera; HYM, Hymenoptera; LEP, Lepidoptera; DES, Decomposer; HER, Herbivorous; Species: First three letters of the name identifies the genus and the following three letters identifies the specie. Function: PAR, Parasite; POL, Pollinator; PRE, Predator; IND, Undetermined.
In Exp. 2, composition of the assemblages (p = 0.56), abundance (pollinators p = 0.43, F = 0.90, natural enemies p = 0.89, F = 0.11, total insect abundance p = 0.44, F = 0.90) and richness (pollinators p = 0.70, F = 0.36, natural enemies p = 0.93, F = 0.07 and total insect richness p = 0.51, F = 0.74) were not different among genotypes. However, it is remarkable that in genotype L, no herbivorous nor decomposers were surveyed ().
3.2 Relationship between insect assemblages and volatile signals
On early sowing date for Exp. 1, the PCA main two axes explained 68% of the total variance. Eigenvalues were high for axis 1 and 2 (0.54 and 0.14, respectively). Axis 1 presented a contrast between genotype A related to high abundance of Scaptotrigona jujuyensis, Astylus quadrilineatus and Plebeia droryana (left part of the diagram) and genotypes L and G related to high abundance of Apis mellifera and Dilophusc f.similis (right part of the diagram). The main environmental explanatory variables of insect assemblages were sensors 3, 7 and 2 for axis 1 (r = −0.52, r = −0.48 and r = −0.41, for sensors 3, 7 and 2, respectively) and sensors 8 and 6 for axis 2 (r = 0.48 and r = 0.42, for sensors 8 and 6, respectively) ().
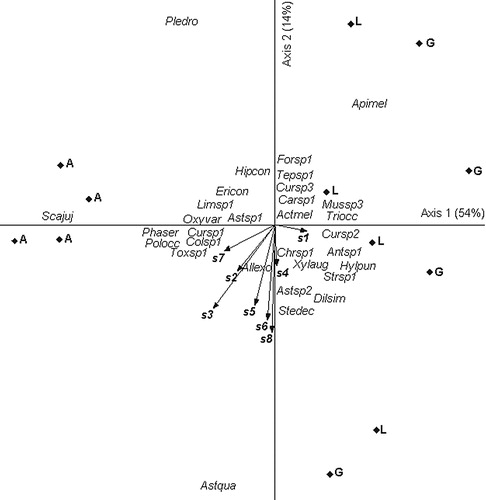
On the late sowing date for Exp. 1, the two main axes explained 81% of the total variance. Eigenvalues were high for axis 1 and 2 (0.62 and 0.19, respectively). Axis 1 presented a contrast between genotype A related to high abundance of Scaptotrigona jujuyensis and Apis mellifera (left part of the diagram) and genotypes L and G related to the presence of Plebeia droryana (right part of the diagram). The main environmental explanatory variables of insect assemblies were sensors 7, 3 and 4 for axis 1 (r = 0.62, r = 0.56 and r = 0.54 for sensors 7, 3 and 4, respectively) ().
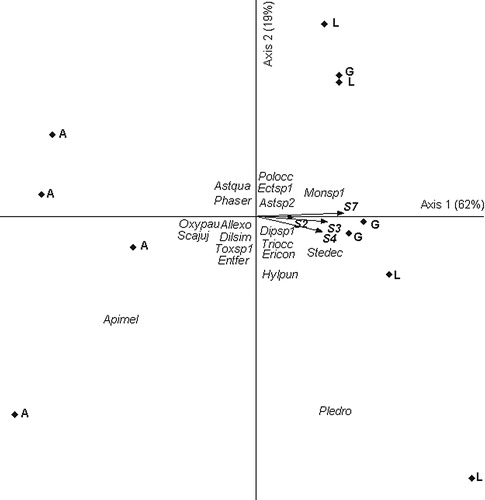
In Exp. 2, the main two axes explained 67% of the total variance. Eigenvalues were high for axis 1 and 2 (0.37 and 0.30, respectively). Axis 1 presented a contrast between genotype L, related to high abundance of Plebeia droryana (right part of the diagram), and genotypes A and G, related to the presence of Scaptotrigona jujuyensis and Hylaeus punctatus (left part of the diagram). The main environmental explanatory variables of insect assemblies were sensors 1 and 8 for axis 1 (r = −0.50 and r = −0.44, respectively) and sensors 7, 3 and 1 for axis 2 (r = −0.84, r = −0.78 and −0.71, respectively) ().
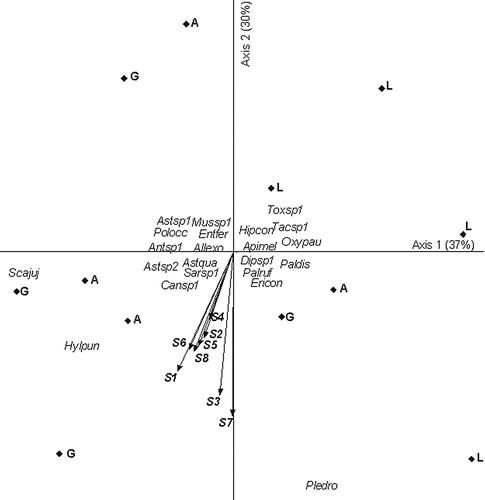
Relationship between insect assemblages and volatile signals emitted by genotypes are mainly explained by changes in the abundance of some insects such as Plebeia droryana. These insects seem to be sensitive to the different blends of signals captured mainly by sensors 3 and 7.
4 Discussion
Composition of the floral visitor assemblages differed among genotypes, according to MRPP analysis, which considered presence-absence data, for the early sowing date (Exp. 1) but not for the late sowing date (Exp. 1 and 2). Changes in the sowing date, affecting the crop and insect phenology, growth and development could partly explain these results. On the one hand, growth cycle differed among genotypes and sowing dates, being the duration of sowing-flowering phase shorter in genotype A than in the rest of the genotypes, and on late sowing dates than on early sowing dates (supplementary data). Although there was a certain overlap of the flowering phases among genotypes, the peak of open flowers, which is highly attractive to some floral visitors (CitationBurkle et al., 2013; CitationBendifallah et al., 2013), occurred at different moments in both experiments, and this could explain in part the differences among insect assemblages. On the other hand, insect phenology, growth and development could also be affected by changes in the sowing date related to environmental temperature variations (CitationLactin et al., 1995; CitationTilman and Pacala, 1993).
The floral visitor assemblages related to each genotype were composed by different groups of insects that were present in some assemblages and absent in the others. This association between genotypes of the same crop and insects groups was previously reported for Oenothera biennis (CitationJohnson and Agrawal, 2005), Solidago altissima (CitationCrutsinger et al., 2006; CitationBurkle et al., 2013) and Tanacetum vulgare (CitationBálint et al., 2016). These results suggest that intraspecific diversity enhances floral visiting insects’ diversity, probably via heterogeneity in plant growth, chemical composition of tissues and odor signals, which causes variability in the behavior and movement of insects (CitationTilman and Pacala, 1993).
On the early sowing date (Exp. 1), both pollinators and total insect abundance were higher for genotype A than for the rest of genotypes but not on the late sowing dates (Exp. 1 and 2). These results are coincident with previous works showing that the abundance of floral visitor insects is influenced by crop genetic (CitationGenung et al., 2010) and environmental variation (CitationLactin et al., 1995). Same considerations could apply for species composition. Differences in the phenology, allowing genotype A to be the first genotype visited, could account for the large quantity of insects captured foraging in their flowers, determining different assemblies, while L and G presented more similar assemblies ( and ). In Exp. 2, flowering in genotype A and G occurred earlier than in L, which could explain in part the assemblies observed (). Moreover, local insects could prefer the local population (genotype A) and, thus, their phenology could be more related to this genotype explaining differences in assemblies’ composition and insect abundance of genotype A with respect to the rest of the genotypes.
Total insect richness was similar among genotypes ranging from 18 to 19 species on early sowing dates and from 10 to 14 species on late sowing dates. Likewise, the richness of functional groups as pollinators and natural enemies were not different among genotypes. These results are not coincident with previous works showing that the richness of floral visitor insects is influenced by genetic variation (CitationGenung et al., 2010). However, considering that species number was similar but species composition was different on early sowing dates and more non herbivores than herbivores were observed, combining different genotypes of coriander could increase regional floral visiting insect’s diversity, as observed combining different crops in intercropping (Citationde la Fuente et al., 2014). For this reason, sowing several coriander genotypes in intercropping or other polycultural systems could be a very interesting strategy to reduce of crop pests by enhancing their natural enemies (CitationTschumi et al., 2015).
In this work, the electronic nose technology detecting volatile signals discriminated among genotypes of coriander for both experiments. Environmental variables may have contributed to the variation in blends of signals detected in the different genotypes and sowing dates (CitationKesselmeier and Staudt, 1999). Mainly sensors 3 and 7 explain part of the observed variability, similarly to previous works showing that the electronic nosé sensors can discriminate between different types of damage inflicted on rice plants (CitationZhou and Wang, 2011). The stingless bee Plebeia doryana was particularly sensitive to the variation of VOCs. In addition to volatile blends, several features such as abundant nectar and pollen, zygomorphic flowers and compact umbels (CitationDiederichsen, 2017), which make coriander attractive to a wide variety of insect species (CitationBendifallah et al., 2013) can explain the rest of the variability. For instance, the number of perfect and staminate flowers is not different among genotypes, while pistillate flowers shows differences in petal longitude; short petals on G; and long petals on A (personal observation). On the other hand, the high abundance of eusocial bees Scaptotrigona jujuyensis and Apis mellifera observed in local genotype A, mainly in Exp. 1, could be explained in part by the preference of insects frequently found in this experimental area, which can be influenced by earlier experience (CitationAnderson and Anton, 2014).
In this study, the composition, abundance and richness of the assemblages of flower visiting insects in different coriander genotypes and sowing dates and the relationship between insect assemblages and volatile signals were evaluated. Composition and abundance differed among genotypes, mainly for the early sowing date. Differences could be in part attributed to the intra specific variability of volatile signals to which some insects were sensitive. Although local richness was similar among genotypes, different species composition suggests that the combination of different genotypes of coriander could enhance regional species diversity. These results, together with the fact that insect assemblages presented more non herbivorous than herbivorous insects, suggest that the combination in space or time of different genotypes of coriander could be an interesting option to maintain population of pests at economic levels, favor pollination of crops and sustain biodiversity.
tjls_a_12128500_sm0001.docx
Download MS Word (12.4 KB)Acknowledgment
This research was financially supported by UBACyT (2010–2012): 20020090200069BA and UBACyT (2013–2016): 20020120200041BA.
References
- M.A.AizenL.A.GaribaldiM.DondoExpansión de la soja y diversidad de la agricultura argentinaEcología Austral1920094554(Asociación Argentina de Ecología)
- P.AndersonS.AntonExperience-based modulation of behavioural responses to plant volatiles and other sensory cues in insect herbivoresPlant Cell Environ.20141826183510.1111/pce:12342
- J.BálintS.E.ZytynskaR.V.SalamonM.MehrparvarW.W.WeisserO.J.SchmitzK.BenedekA.BalogIntraspecific differences in plant chemotype determine the structure of arthropod food websOecologia180201679780710.1007/s00442-015-3508-y
- M.A.BarbourM.A.Rodriquez-CabalE.T.WuR.Julkunen-TiittoC.E.RitlandA.E.MiscampbellE.S.JulesG.M.CrutsingerMultiple plant traits shape the genetic basis of herbivore community assemblyFunct. Ecol.2982015995100610.1111/1365-2435.12409
- L.BendifallahK.LouadiS.DoumandjiBee fauna potential visitors of coriander flowers Coriandrum sativum L. (Apiaceae) in the Mitidja area (Algeria)J. Apicult. Sci.57220135970
- R.W.BrookerA.J.KarleyA.C.NewtonR.J.PakemanCh.SchöbFacilitation and sustainable agriculture: a mechanistic approach to reconciling crop production and conservationFunct. Ecol.301201698107
- L.A.BurkleL.SouzaM.A.GenungG.M.CrutsingerPlant genotype, nutrients, and GxE interactions structure floral visitor communitiesEcosphere49201312010.1890/ES13-00039
- M.V.Colomo de CorreaA.Roig-AlsinaPompilidaeL.E.ClapsG.DebandiS.Roig-JuñetBiodiversidad de artrópodos argentinos2009435460
- G.M.CrutsingerM.D.CollinsJ.A.FordyceZ.GompertC.C.NiceN.J.SandersPlant genotypic diversity predicts community structure and governs an ecosystem processScience3132006966968
- E.de la FuenteA.GilA.LenardisM.López PereiraS.A.SuárezC.M.GhersaM.Yaber GrassResponse of winter crops differing in grain yield and essential oil production to some agronomic practices and environmental gradient in the Rolling Pampa ArgentinaAgric. Ecosys. Environ.991–32003159169
- E.B.de la FuenteS.A.SuarezA.E.LenardisS.PoggioIntercropping sunflower and soybean in intensive farming systems: evaluating yield advantage and effect on weed and insect assemblagesNJAS − Wageningen J. Life Sci.70–7120144752
- J.G.B.DerraikG.P.ClossK.J.M.DickinsonP.SirvidB.I.P.BarrattB.H.PatrickArthropod morphospecies versus taxonomic species: a case study with Araneae, Coleoptera, and LepidopteraConserv. Biol.164200210151023
- J.A.Di RienzoM.BalzariniL.GonzalezF.CasanovesM.TabladaC.W.RobledoINFOSTAT Versión2016Grupo InfoStat, FCA, Universidad Nacional de Córdoba Argentina
- A.DiederichsenCoriander (Coriandrum Sativum L.). Promoting the Conservation and Use of Underutilized and Neglected Crops. 32017Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute Rome199689
- B.FarnetiS.M.CristescuG.CostaF.J.M.HarrenE.J.WolteringRapid tomato volatile profiling by using proton-transfer reaction mass spectrometry (PTR-MS)J. Food Sci.772012551559
- B.FarnetiI.KhomenkoL.CappellinV.TingA.RomanoF.BiasioliG.CostaF.CostaComprehensive VOC profiling of an apple germplasm collection by PTR-ToF-MSMetabolomics1142014838850
- M.A.GenungJ.P.LessardC.B.BrownW.A.BunnM.A.CreggerW.N.ReynoldsE.Felker-QuinnM.L.StevensonA.S.HartleyG.CrutsingerJ.A.SchweitzerJ.K.BaileyNon-additive effects of genotypic diversity increase floral abundance and abundance of floral visitorsPLoS One512010e871110.1371/journal.pone.0008711(Published online 2010 Jan 14)
- A.GilE.de la FuenteA.LenardisS.LorenzoJ.MarengoCoriander (Coriandrum sativum L.) yield response to plant population. Journal of HerbsSpices Med. Plants6319996373
- A.GilS.LeicachC.M.GhersaEssential oil yield and composition of Tagetes minuta accessions from ArgentinaBiochem. Syst. Ecol.283200026127410.1016/S0305-1978(99)00062-9
- M.C.GramajoP.R.MulieriRedescripción de Archytascirphis (Diptera: Tachinidae) y primer registro del hospedero para la región NeotropicalRevista de la Sociedad Entomológica Argentina701-22011(2011) Mendoza, ISSN 0373-5680
- J.HarborneBiochemical plant ecologyP.M.DeyJ.B.HarbornePlant Biochemistry1997Academic Press San DiegoUSA503516
- A.HassanaliH.HerrenZ.KhanJ.PickettC.WoodcockIntegrated pest management: the push-pull approach for controlling insect pests and weeds of cereals, and its potential for other agricultural systems including animal husbandryPhilos. Trans. R. Soc. B3632008611621
- M.T.JohnsonA.A.AgrawalPlant genotype and environment interact to shape a diverse arthropod community on evening primrose (Oenothera biennis)Ecology862005874885
- J.KesselmeierM.StaudtBiogenic volatile organic compounds (VOC): an overview on emission: physiology and ecologyJ. Atmos. Chem.3319992388
- B.K.KlattC.BurmeisterC.WestphalT.TscharntkeM.von FragsteinFlower volatiles, crop varieties and bee responsesPLoS One882013e7272410.1371/journal.pone
- D.J.LactinN.J.HollidayD.L.JohnsonR.CraigenImproved rate model of temperature-dependent development by arthropodsEnvironment Entomology24119956875
- A.LenardisC.Van BarenP.Di Leo LiraC.M.GhersaPlant-soil interactions in wheat and coriander crops driving arthropod assemblies through volatile compoundsEur. J. Agron.26200741041710.1016/j.eja.2006.12.007
- F.LoretoM.DickeJ.P.SchnitzlerT.C.J.TurlingPlant volatiles and the environmentPlant Cell Environ.37201419051908
- A.E.MagurranA variety of diversitiesA.E.MagurranEcological Diversity and Its Measurement1988Princeton Univ. Press PrincetonNJ8199
- A.McCuneJ.B.GraceAnalysis of Ecological Communities2002Mjm Software DesignGleneden Beach, OR, US(with Urban, D.L.)
- P.W.MielkeMeteorological applications of permutation techniques based on distance functionsP.R.KrishnaiahP.K.SenHandbook of Statistics Vol. 4 (1984) North-Holland. Amsterdam. 813–830.
- M.OlleI.BenderThe content of oils in umbelliferous crops and its formationAgron. Res.8Special Issue III2010687696
- J.M.PattG.C.HamiltonJ.H.LashombForaging success of parasitoid wasps on flowers: interplay of insect morphology: floral architecture and searching behaviorEntomol. Exp. Appl.8319972130
- E.PicherskyE.LewinsohnConvergent evolution in plant specialized metabolismAnnu. Rev. Plant Biol.622011549566
- T.L.PotterI.S.FagersonComposition of coriander leaf volatilesJ. Agric. Food Chem.38199020542056
- Ch.SchöbS.KerleA.J.KarleyL.MorcilloR.J.PakermanA.C.NewtonR.W.BrookerIntraspecific genetic diversity and composition modify species-level diversity-productivity relationshipsNew Phytolog.205201572073010.1111/nph.13043
- R.SplivalloN.ValdezN.KirchhoffM.C.OnaJ.P.SchmidtI.FeussnerP.KarlovskyIntraspecific genotypic variability determines concentrations of key truffle volatilesNew Phytolog.1942012823835
- A.SzpeinerM.A.Martínez-GhersaC.M.GhersaWheat volatile emissions modified by top-soil chemical characteristics and herbivory alter the performance of neighbouring wheat plantsAgric. Ecosyst. Environ.13420099910710.1016/j.agee.2009.06.005
- C.J.F.terBraakOrdinationR.H.G.JongmanC.J.F.terBraakO.F.R.van TongerenData Analysis in Community and Landscape Ecology1st. ed.1987Pudoc WageningenThe Netherlands91173
- D.TilmanS.PacalaThe maintenance of species richness in plant communitiesR.E.RickleftsD.SchluterSpecies Diversity in Ecological Communities1993University of Chicago Press Chicago557569
- D.TilmanP.B.ReichJ.KnopsD.WedinT.MielkeC.LehmanDiversity and productivity in a long-term grassland experimentScience294200184384510.1126/science.1060391
- J.P.TorrettaS.L.PoggioSpecies diversity of entomophilous plants and flower-visiting insects is sustained in the field margins of sunflower cropsJ. Nat. Hist.473–4201313916510.1080/00222933.2012.742162
- M.TschumiM.AlbrechtM.H.EntlingK.JacotHigh effectiveness of tailored flower strips in reducing pests and crop plant damageProc. Royal Soc.2015B28210.1098/rspb.2015.1369
- O.F.R.Van TorengenCluster analysisR.H.G.JongmanC.J.F.terBraakJ.R.van TongerenData Analysis in Community and Landscape Ecology vol 6 (1987) Pudoc Wageningen. The Netherlands. 174–206.
- B.ZhouJ.WangDiscrimination of different types damage of rice plants by electronic noseBiosyst. Eng.1092011250257
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi:10.1016/j.njas.2017.09.004.
