?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
An experimental study was conducted to evaluate the effect of 0.129 NaCN mg/l for 28 days on the behavior of tilapia and the activities of adenosine triphosphatases enzyme (Na+/K+, Ca2+ and Mg2+-ATPase) within gill filaments, liver and muscles. Also, to evaluate the stability of cyanide in stored, frozen fish tissues. The present results showed that the exposed fish had shown different modes of behaviors. During the first 14 days, the fish lost its equilibrium with excessive mucous secretion on its gill filaments and skin. On the other hand, tilapia showed a normal swimming behavior with no excessive mucus secretion at 21st and 28th days.
In addition, the present result showed that the NaCN decreased the activities of ATPase within the investigated tissues during the exposure periods except for the 1st day. Furthermore, the present result showed that the mean concentration of cyanide gradually decreased within the frozen tissues and completely disappeared after 48 h within frozen liver and muscle. But, it disappeared after 72 h within frozen blood and gills.
1 Introduction
Catching fish by using toxic chemicals such as cyanide is considered illegal. However, it is usually employed to stun and capture fishes alive from their natural habitat. After catching the fishes, they are transferred and stocked in fresh water to recover and reduce the amount of cyanide since nearly 80% of all cyanide is converted to thiocyanate anion (SCN−). Thiocyanate anion (SCN−) is produced by the main metabolic pathway for cyanide anion (CN−) detoxification and then execrated in the urine “self depuration” (CitationRubec et al., 2002).
Many studies showed that freshwater fishes are the most cyanide-sensitive group of aquatic organisms. Free cyanide at concentration of >5 μg/l can cause negative impact on the swimming and reproduction of fresh water fish. While, at concentration of >20 μg/l, the cyanide induces high fish mortality (CitationEisler and Wiemeyer, 2004; CitationHossein and Reza, 2011). Cyanide exists in water in the form of free state (CN− and HCN), simple cyanide (e.g., NaCN), complex cyanide and total cyanide. Almost all cyanide persists in the undissociated form (HCN) in pH below 7. But at a pH value of 11, all of the cyanide appears as the free (CN) ion (CitationBroderius et al., 1977). Toxicity of cyanide is primarily determined by the concentration of undissociated HCN in the water column (even in case of simple cyanides and metal cyanide complexes). Hydrocyanic acid (HCN) is also more toxic than the free cyanide and the degree of its toxicity depends on the solubility and the dissociation rate to form free cyanide (CitationSorokin et al., 2008).
Cyanide has low persistence in the aquatic environment and does not accumulate in or store within the tissue of fish because of its rapid detoxification at sub-lethal doses and death at lethal doses. The toxicity of cyanide is attributed to the presence of HCN derived from dissociation of the complexes that penetrate cell walls (CitationPablo et al., 1996) and cause fish mortality (CitationPrashanth et al., 2011). When the fish is subjected to direct contact with sodium cyanide, it shows some behavioral changes during its movement (CitationRichmonds and Dutta, 1992; CitationShwetha and Hosetti, 2009). Such behaviors can be used as biological indicators which provide a unique perspective linking the physiology and ecology of an organism and its environment (CitationDube and Hosetti, 2010). Few studies have been done to evaluate the effect of cyanide on behavior of fish. One of those studies, showed that sodium cyanide has tremendous effect on the behavior of Clarias gariepinus resulting from depression of the central nervous system which may be attributed to the combination of lactate acidosis with cytotoxic hypoxia (CitationKhalid and Shahid, 2012).
Other studies have been done to evaluate the effect of the cyanide on the activity of adenosine triphosphate enzyme. This complex enzyme plays a central role in the physiological process as energy transducers by coupling chemical reaction (CitationTakao, 1985; CitationCarfagna et al., 1996). ATPases require Na+/K+, Mg2+ and Ca2+ ions for their activity and cleavage of ATP to ADP/AMP and inorganic phosphate with liberation of energy (CitationBegum, 2011; CitationPraveen et al., 2012). The studies revealed that the reduction of ATPase may cause disturbances in cellular metabolism, leading to histotoxic hypoxia in fish. Concerning the effect of cyanide, free cyanide ions can pass through the gill membranes but it can act as a metabolic inhibitor that prevents re-synthesis of adenosine triphosphate (ATP) in the axon. So, cyanide is expected to reduce the efflux of ions to a very low value (CitationUnnisa and Devaraj, 2007). Therefore, the enzymatic activities can be used as physiological indicators to detect damages within different organs related to water contamination (CitationAdamu and Iloba, 2008).
The primary aim of this study is to evaluate the effect of sodium cyanide (NaCN) on the behavior of fresh water fish, Nile tilapia, Oreochromis niloticus and on the activities of adenosine triphosphatases enzyme (Na+/K+, Ca2+ and Mg2+-ATPase) within gills, liver and muscles. In addition the aim is also to evaluate the stability of cyanide in stored frozen fish tissues.
2 Related work
The concentration and rate of transformation of cyanide in tissues is dependent on the initial concentration in the sample, storage time and the preservation method (e.g., addition of sodium fluoride to sample), storage condition (e.g., temperature) of the sample, types of cyanide compound and pH. There are no reports of cyanide biomagnifications or cycling in living organisms, probably owing to its rapid detoxification (CitationHagelstein, 1997). So that the opinions were divided between those that believe that cyanide is quickly metabolized and excreted in a matter of hours (CitationBruckner and Roberts, 2008) and those that believe that cyanide is retained for longer time periods (CitationRubec et al., 2001). Upon the differentiation between the previous opinions, the present laboratory work aimed to evaluate the stability of cyanide concentration in stored frozen tilapia tissues with time.
3 Methodology
To evaluate the effect of sodium cyanide (NaCN)–free cyanide on the fish, O. niloticus, 1000 healthy fish weighing 30 ± 0.6 g each with an average length of 16 ± 0.3 cm were obtained from El-Qanater, Qalubiya Governorate, Egypt. The fish were transported to the laboratory in a plastic ice-box containing oxygenated, de-chlorinated water. In the laboratory, the fish were acclimated by keeping them in a tank with aerated water for about one month. The bioassay test used dechlorinated tap water with physico-chemical characteristics as following: pH: 7.9 ± 0.05, temperature: 25 ± 0.03 °C, hardness 94.03 ± 1.83 mg/l as CaCO3, Na: 25.72 mg/l, K: 6.49 mg/l, Mg: 13.5 mg/l, F: 0.458 mg/l, Cl: 36.88 mg/l, NO3: 2.41 mg/l, SO4: 38.00 mg/l, dissolved oxygen: 6.9 ± 0.052 mg/l, and alkalinity 2.41 ± 0.18 meq/l. These measurements were analyzed according to American Public Health Association (CitationAPHA, 2005). The fish were fed once/day with a commercial diet (30% protein, fish meal, soya bean, bran, corn yellow and oil) at a rate of 2% of the fish body weight (CitationSprague, 1969). The tank was cleaned daily and provided with clean water weekly to keep the fish in healthy condition. After acclimation the following steps were carried out.
3.1 Preparation of sodium cyanide stock solution
NaCN is a hygroscopic crystalline powder with a faint acid-sodium generally soluble in water and slightly soluble in alcohol. The aqueous solution of this chemical is strongly alkaline and rapidly decomposes. NaCN produces hydrogen cyanide on contact with acids or acids salts.
The stock solution was prepared by dissolving NaCN (97% purity) in double distilled water in standard volumetric flask. The required quantity of sodium cyanide was drawn directly from this stock solution using a micropipette. The concentrations of test compounds used in short term definitive tests were between the highest concentrations at which mortality was 100%, and the lowest concentration at which mortality was 0% in the range finding tests. The speciation of CN− in water samples using Visual Minteq program (CitationGustafsson, 2012) indicated that 4.9% of cyanide was CN− and 95% in the form of HCN. The activity of cyanide value was 2.3 × 10−7.
3.2 Determination of LC50 of cyanide for Nile tilapia
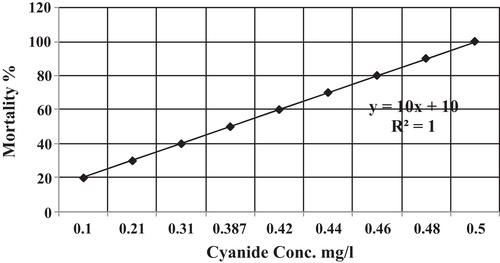
Nine concentrations of cyanide (0.5, 0.48, 0.46, 0.44, 0.42, 0.387, 0.31, 0.21, and 0.1 mg/l) were prepared in 9 aquaria (80 cm × 40 cm × 60 cm) containing 100 l of aerated dechlorinated tap water. In addition, one control aquarium was prepared with only aerated dechlorinated tap water. Then, ten healthy fish were stocked in each aquarium. To keep the concentration of cyanide constant in each aquarium, water that provided with its fixed dose was changed daily within the period of the experiment (96 h). After 96 h from the exposure time, the percentage of fish mortality was calculated in each aquarium according to probit analysis method (CitationFinney, 1971).
This experiment was repeated twice and the average lethal concentration (LC50) value of cyanide was found to be 0.387 mg/l. shows the percentage of fish mortality that are exposed to special concentration of cyanide in aquatic environment following the linear equation (10x + 10) with R2 = 1.
3.3 Determination of adenosine triphosphatase (ATPase) activities
To study the effect of cyanide on the activity of adenosine triphosphatases within the liver, muscle and gills of O. niloticus, 50 healthy fish were stocked in aquaria (10 fish/aquarium) and subjected to 1/3 LC50 of cyanide (0.129 mg/l) for a period of 1, 7, 14, 21 and 28 days. Similarly, 50 fish were stocked in 5 glass aquarium (10 fish/aquarium) containing only 100 l of aerated dechlorinated tap water and remained for the same period of time to act as a control. During the experiment, fish were fed on commercial diet (30% protein) at rate 2% of fish weight. In addition, fish were monitored to record their behavioral changes.
After each exposure period of time, the fish was stunned on its head to prevent sensation. Blood was obtained directly from puncture of the heart of each fish. Each fish was then dissected to collect pieces of muscle, liver and gill tissues. The activity of total ATPase was measured as the rate of release of inorganic phosphate (CitationSamson and Quin, 1967). Gill, liver and muscle tissues homogenized in pre cold 0.32 m sucrose buffer pH 7.5 containing 1.0 mm EDTA and 10.0 mm imidazole using Potter-Elvehjem glass homogenizer with a Teflon piston, and then centrifuged. Each assay was formed from 25 μl of the tissue sample homogenate and the supernatant was used to determine the enzyme activities in treated cyanide fish tissues by determination of inorganic phosphate in the supernatant which liberated during the hydrolysis of the substrate adenosine triphosphate at 37 °C. The assay of inorganic phosphate was done using CitationLowry and Lopez (1946) method.
The detailed ATPases activities were recorded and subjected to Kruskal–Wallis one-way analysis to reveal the significant differences within the gill filaments, the liver and muscles when they are compared with the control. The Kruskal–Wallis one-way analysis of variance by ranks is a non-parametric method for testing whether samples originate from the same distribution. It is used for comparing two or more samples that are independent.
3.4 Determination of cyanide concentration in stored treated tilapia tissues
To evaluate the possibility of cyanide to accumulate in or stored within the tissue of fish, 70 fish (10 fish/aquarium) were exposed to 0.129 mg/l NaCN for a period of 24 h. Then, they were sacrificed to collected blood sample. Then, each fish is dissected to collect pieces of gills, liver and muscles.
The samples were stored in refrigerator at −4 °C for a period of time 20 min, 1, 12, 24, 48, 72 and 96 h in order to determine the concentration of cyanide in each time.
The procedure of cyanide determination is outlined according to the standard operating procedures for cyanide testing used by Philippines (CitationCDT, 2001). The method was applied as following.
Approximately 10 g of fish sample was weighted and intended for distillation. The tissue was blended at high speed for 3–5 min with 2 ml of normal dilute NaOH and brought it up to 500 ml using distilled water. Then 10 ml of 1 M NaOH solution was added to the absorber tube. Diluted with distilled water about 50 g of lead carbonate powder was added into the absorber tube to precipitate sulfide which can interfere with ISE-CN− reading. The absorber tube was attached to the vacuum and connected it to the condenser. The vacuum was turned on and the air flow adjusted to approximately 1–2 bubble/s entering the boiling flask through the air inlet tube. 20 ml of magnesium chloride solution was added through the inlet tube (acted as catalyst) and about a minute later, 15 ml of sulfamic acid was added to reduce nitrite or nitrate which can act as interfering substances in the flask. The air inlet tube was rinsed with a few milliliters of water and the air flow was allowed to mix the content of the flask for three minutes. Carefully 50 ml of H2SO4 was added then turned on the flow of cooling water to the reflux condenser. The solution was heated in the flask to boiling. The sample was refluxed for one hour, then, turned off the heat, while maintaining the air flow for at least an additional 15 min. The absorption solution was transferred into the volumetric flask and diluted the later to 250 ml by adding distilled water and mixed thoroughly. The concentration of cyanide was determined in the sodium hydroxide solution by using cyanide ISE (the Ion Selective Electrode – Meter) connected to pH/ISE meter from EquationEq. (1)(1)
(1) :
(1)
(1)
4 Results and discussions
The experimental results showed that sodium cyanide was highly toxic to O. niloticus. The lethal concentration (96 h LC50) of sodium cyanide for fish was 0.387 mg/l. While at sub-lethal concentrations, the fish survived without any sign of fish mortality during the exposure periods.
Within the aquaria, the experimental results showed that when fish was subjected to 1/3 LC50 of cyanide (0.129 mg/l) for a period of 28 days, the fish revealed different mode of behaviors. During the first 14 days, fish exhibited disrupted schooling behavior, appeared sluggish in their movement and swim independently in erratic mode near the bottom at the corners of the aquarium. Also the present study showed that the cyanide induced excessive mucus secretion on the gill filaments and the skin of fish. As the result of the excessive mucus secretion on the gill filaments, the movement of the operculum increased to enlarge and lead to increase the breathing rate. It seems that the excessive mucus secretion that covering the whole gill filaments and skin of the fish may act as defensive mechanism and barrier that prevent the diffusion of the cyanide from water to fish body. Similarly, CitationPrashanth et al. (2011), CitationPrashanth and Patil (2006) and CitationThorat (2001) mentioned that the secretion of mucus over the gills may be an adaptive response to provide additional protection against corrosive nature of toxicant and its absorption by the general body surface; however it may inhibit the diffusion of oxygen during the process of gaseous exchange. Also, the increase in opercula movement and corresponding increase in frequency of surfacing of fish clearly indicates that fish adaptively shifts towards aerial respiration to escape the toxic aquatic medium and ovoid cyanide contaminated media. Such unusual behavior was detected among Labeo rohita fish due to obstructed functions of neurotransmitters.
On the other hand, the present results showed that when the fish was subjected to the same concentration for a longer period of time (21–28 days), fish recovered and swim normally such as the control fish. Such behavior is strongly related to the acceleration of compensatory mechanism that may occur in the activity of some enzymatic pathways to counteract the deleterious action of cyanide as possible by increasing the enzymatic detoxification rate of cyanide. Similarly, CitationBrian et al. (2010) mentioned that the cyanide detoxification processes of fish seem to be very similar to those existing in mammals. The detoxification is probably achieved through the action of the enzyme rhodanese, which, in the presence of the thiosulfate, transforms cyanide into nontoxic thiocyanate.
For the activities of ATPase (μmol Pi liberated/mg protein/h), the present results showed that when Nile tilapia was subjected to the sub-lethal concentration of sodium cyanide, the mean concentrations of Na+/K+-ATPase within liver, muscles and gills were significantly changed. Within the gill filaments of fish (Chi square = 27.003, df = 5, p = 0.05), the mean concentration of Na+/K+-ATPase (μmol Pi liberated/mg protein/h) increased from 13.51 ± 0.61 to 15.01 ± 0.37 during 24 h and then gradually decreased to 10.76 ± 0.62, 7.45 ± 0.41, 6.49 ± 0.74 and 6.12 ± 0.35 after 7, 14, 21 and 28 days, respectively as shown in and . The same phenomenon occurred among liver (Chi square = 27.183, df = 5, p = 0.05) and muscles (Chi square = 27.452, df = 5, p = 0.05). After 24 h, the mean concentrations of Na+/K+-ATPase (μmol Pi liberated/mg protein/h) increased from 11.15 ± 0.53 to 13.45 ± 0.54 μmol Pi liberated/mg protein/h in the liver and from 8.37 ± 0.57 to 10.02 ± 0.41 μmol Pi liberated/mg protein/h in the muscles. However, they decreased to 9.72 ± 0.46, 7.86 ± 0.69, 6.95 ± 0.75 and 5.44 ± 0.75 μmol Pi liberated/mg protein/h in the liver, and 7.47 ± 0.67, 5.68 ± 0.49, 4.53 ± 0.46 and 4.00 ± 0.29 μmol Pi liberated/mg protein/h in the muscles after 7, 14, 21 and 28 days of exposure, respectively (). Based on these results, it is clear that fish is subjected to the stress of toxicant during the exposure periods of time except for fish that exposed to the sub-lethal dose for only 24 h. This may relate to the activity of muscles after exposure to cyanide stress (CitationBesty, 2011). On the other hand, the inhibition of the Na+/K+-ATPase activities after the first day of exposure periods might be due to osmoregulation (CitationParvez et al., 2006). So, it is expected that the reduction of Na+/K+-ATPase may have a metabolic or ionic regulation when the fish is subjected to toxic exposure. Coinciding with this, CitationBegum (2011) revealed that the inhibition of this enzyme by cyanide exposure thus build up high ion concentrations in the extracellular spaces resulting in the obstruction of the movement of internal destructive extra ions towards the external medium via the leakage junctions.
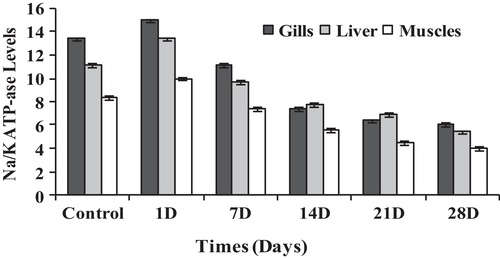
Table 2 The mean activities of Na+/K+, Mg2+ and Ca2+-ATPase (μmol Pi liberated/mg protein/h) in stored frozen different tissues of tilapia in different times.
It was shown that when fish was subjected to the sub-lethal concentration of sodium cyanide, the mean concentrations of Mg2+-ATPase (μmol Pi liberated/mg protein/h) within the liver, muscles and gill filaments were significantly changed as shown in . Within the gill filaments of fish (Chi square = 27.988, df = 5, p = 0.05), the mean concentration of Mg2+-ATPase (μmol Pi liberated/mg protein/h) increased from 8.91 ± 0.05 to 9.65 ± 0.08 during the first day of exposure, however it decreased gradually to 6.73 ± 0.13, 5.42 ± 0.06, 5.03 ± 0.21 and 4.32 ± 0.41 μmol Pi liberated/mg protein/h after 7, 14, 21 and 28 days of exposure, respectively. Similarly, such behavior occurred among liver tissues (Chi square = 28.232, df = 5, p = 0.05) and the muscles (Chi square = 27.885, df = 5, p = 0.05) when the fish was subjected to the same dose. Within 24 h after the exposure time, the mean concentrations of Mg2+-ATPase (μmol Pi liberated/mg protein/h) increased from 3.63 ± 0.22 to 5.83 ± 0.19 in the liver and from 2.14 ± 0.09 to 4.49 ± 0.10 in the muscles. However, they decreased to 2.52 ± 0.06, 1.92 ± 0.04, 1.64 ± 0.06 and 1.00 ± 0.04 μmol Pi liberated/mg protein/h in the liver and 1.24 ± 0.08, 0.91 ± 0.02, 0.88 ± 0.02 and 0.53 ± 0.02 μmol Pi liberated/mg protein/h in the muscles after 7, 14, 21 and 28 days of exposure, respectively ().
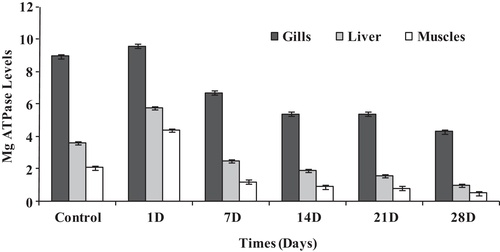
It is clear that Mg2+-ATPase has a unique role in energy synthesis through oxidative phosphorylation in mitochondria within all types of cells (CitationSeraj et al., 2010). As a result, Mg2+-ATPase can be taken as an index of general ATPase activity (CitationShwetha and Hosetti, 2012). Also, it is clear that the decrease in the mean values of Mg2+-ATPase within the gill filaments, liver and muscles of fish are highly related to the metabolic activity of fish when it is subjected to sub-lethal concentration of sodium cyanide for a period of time.
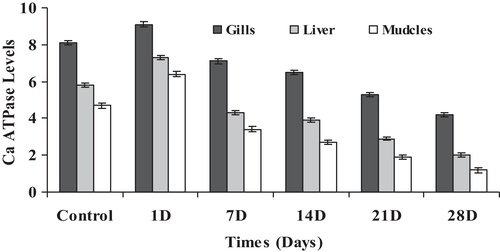
As it is illustrated in , Ca2+-ATPase had the same behavior as the previous ATPases activities when the fish was subjected to the same concentration of cyanide. The mean concentrations of Ca2+-ATPase revealed a significant differences within the gill filaments (Chi square = 28.226, df = 5, p = 0.05), liver (Chi square = 27.454, df = 5, p = 0.05) and muscles (Chi square = 28.232, df = 5, p = 0.05). In the gill filaments of fish, the mean concentrations of Ca2+-ATPase increased from 8.13 ± 0.07 to 9.13 ± 0.25 within 24 h after the exposure time and then gradually decreased to 7.18 ± 0.13, 6.51 ± 0.08, 5.39 ± 0.09 and 4.21 ± 0.13 after 7, 14, 21 and 28 days of exposure, respectively. The same observation was detected in both the liver and the muscles of fish since the level of Ca2+-ATPase increased after 24 h from 5.82 ± 0.57 to 7.35 ± 0.44 in the liver and from 4.77 ± 0.16 to 6.41 ± 0.25 in muscles. Then, it gradually decreased to 4.32 ± 0.43, 3.97 ± 0.31, 2.98 ± 0.24 and 2.02 ± 0.21 in liver and 3.47 ± 0.10, 2.75 ± 0.12, 1.96 ± 0.12 and 1.24 ± 0.08 in the muscles after 7, 14, 21 and 28 days of exposure, respectively. Such a decrease is highly related to the inhibition phosphorylation process. Similarly, CitationTiwari et al. (2002) and CitationUnnisa and Devaraj (2007) showed that the inhibition of Ca2+-ATPase activity may be due to the inhibition of phosphorylation and degradation of products of lipid peroxidation of the enzyme molecule. Thus, ATPases are very sensitive to chemical interaction and can be used as a reliable biomarker for the mechanistic toxicity studies of toxicant (CitationShwetha and Hosetti, 2012).
Concerning the stability of cyanide in stored frozen blood, gills, liver and muscles of O. niloticus, the present results listed in showed that the mean concentrations of sodium cyanide and their percentage within the tissue of fish decreased with the time of storing. Sodium cyanide remained within the tissues of fish but it gradually decreased and completely disappeared from the liver and the muscles after 48 h and completely disappeared from both the gill filaments and the blood after 72 h. Absorbed hydrogen cyanide and cyanides are distributed within the body by blood. Up to 80% of absorbed cyanides are metabolized to thiocyanate that execrated later in urine. Majority of cyanide metabolism occurs within the tissues. The major route of metabolism for hydrogen cyanide and cyanide is detoxification in the liver by the mitochondrial enzyme rhodanese, which catalyses the transfer of the sulfane sulfur of thiosulfate to the cyanide ion to form thiocyanate. Although rhodanese is present in the mitochondria of all tissues, the species and tissue distributions of rhodanese are highly variable, the highest concentrations of rhodanese are found in the liver, kidney, brain and muscles. The rate of spontaneous detoxification of cyanide in humans is about 1 μg/kg body weight per minute, which is considerably slower than animals (CitationAminlari et al., 1994; CitationBrian et al., 2010). There was no reason to believe that fish would be different from mammals since the cyanide is quickly metabolized in humans (CitationLogue et al., 2010). There is no evidence that cyanide is bioaccumulated in humans, animals, or aquatic organisms (CitationSmith and Mudder, 1994). There is no report of cyanide biomagnifications or cycling in living organisms, probably owing to its rapid detoxification (CitationHagelstein, 1997). The concentration of cyanide present in the living fish over time is dependent on several factors e.g., concentration of the cyanide solution used during exposure, the length of time of the exposure, time for holding and duration of transport and the conversion rate of hydrogen cyanide (HCN) to SCN− in blood (CitationLeduc, 1984). The metabolism and excretion of SCN− from the fish may be related more to osmoregulation than to temperature mediated enzyme kinetics. Freshwater fish have a higher blood ion concentration (hyper-osmotic) in relation to the surrounding water, while marine fish have a lower blood ion concentration (hypo-osmotic) in relation to seawater. Hence, freshwater fish have a high rate of urinary excretion and marine fish have a low rate of urinary excretion, which helps the fish to maintain osmotic equilibrium with surrounding aquatic environments (CitationSmith, 1982). Research presented by CitationMak et al. (2005) suggested that CN− in solution associated with blended tissue samples declined to zero in about one hour; this may be due to the rapid taken up of cyanide in the blood by the fish's organs including the liver, kidney, heart, spleen, and brain. Hence, the CN− may only be detectable in the blood of fish for a few hours with law storage ability (CitationBrian et al., 2010).
Table 1 Mean concentration of cyanide (mg/kg) and its percentage (%) in stored frozen different tissues of tilapia in different times.
5 Conclusion and future work
The experimental study showed that cyanide is highly toxic and cause high mortality to the fish O. niloticus. When the fish is subjected to sub-lethal concentration, cyanide induces alterations on the behavior and the metabolic activity of fish. The metabolic activities of ATPase can be used as biomarkers for the mechanistic toxicity to detect sodium cyanide toxicity in water. Also, the present study showed that cyanide anion in the tissues of fish can readily metabolize to thiocyanate (SCN−) with a half-life of approximately one hour. So, it is important to determine the concentration of cyanide in freshwater fish in the laboratory once the fish is immediately caught and transferred from the contaminated area. More studies should be focused on the cyanide metabolism compounds and their half-time in fish tissues. Developing an environmental law to criminalize of using cyanide for fishing is of utmost importance. Increasing the awareness of fishermen about the dangers of using cyanide for fishing that have a serious negative impact on the fishery and fish production as well as the overall human health.
Notes
Peer review under responsibility of National Water Research Center.
References
- K.M.AdamuK.I.IlobaEffect of sublethal concentrations of Portland cement powder in solution on the aminotransferases of the African catfish (Clarias gariepinus (Burchell, 1822))Acta Zool. Lit.18120085054
- M.AminlariT.VaseghiM.A.KargarThe cyanide metabolizing enzyme rhodanese in different parts of the respiratory systems in sheep and dogToxicol. Appl. Pharmacol.12419946471
- APHAStandard Methods for the Examination of Water and Wastewater (APHA)21st ed.2005American Public Health AssociationWashington, DC, USA
- G.BegumOrgan-specific ATPase and phosphorylase enzyme activities in a food fish exposed to a carbamate insecticide and recovery responseFish Physiol. Biochem.37120116169
- E.B.BestyLactate dehydrogenase and Na+/K+-ATPase activity in Leiostomus xanthurus (Spot) in response to hypoxiaExplorations620112229
- A.L.BrianM.H.DianeI.B.StevenA.R.GaryThe analysis of cyanide and its breakdown products in biological samplesCrit. Rev. Anal. Chem.402010122147
- S.J.Broderius.L.L.SmithD.T.LindRelative toxicity of free cyanide and dissolved sulphide forms to the fathead minnow (Pimephales promelas)J. Fish. Res. Board Canada34197723232332
- A.W.BrucknerG.RobertsProceedings of the International Cyanide Detection Testing WorkshopNOAA Technical Memorandum NMFS-OPR-40, Silver Spring, MD 1642008
- M.A.CarfagnaG.D.PonslerB.B.MuhoberacInhibition of ATPase activity in rat synaptic plasma membranes by simultaneous exposure to metalsChem. Biol. Interact.10019965365
- Cyanide Detection Test (CDT)Standard operating procedures for cyanide testing used by Philippines2001128
- P.N.DubeB.B.HosettiBehaviour surveillance and oxygen consumption in the freshwater fish Labeo rohita (Hamilton) exposed to sodium cyanideBiotechnol. Anim. Husb.261–2201091103
- R.EislerS.N.WiemeyerCyanide hazards to plants and animals from gold mining and related water issuesRev. Environ. Contam. Toxicol.18320042154
- D.J.FinneyProbit Analysis3rd ed.1971Cambridge University PressLondon, UK
- J.P.GustafssonVisual Mintiq Program, Ver.32012Department of Land and Water Resources EngineeringStockholm, Sweden
- S.Hagelstein‘The ecotoxicological properties of cyanide’, in short course on management of cyanide in mining1997ACMRRPerth
- T.N.HosseinR.RezaSome biochemical properties of rhodanese from liver of rainbow troutInternational Conference Medical, Biological, Pharmaceutical SciencesPattaya2011
- A.A.KhalidM.ShahidEffect of sodium cyanide on the activities of some oxidative enzymes and metabolites in Clarias gariepinusAfr. J. Biotechnol.1141201298499854
- G.LeducCyanides in water: toxicological significanceL.J.WeberAquatic Toxicologyvol. 21984Raven PressNew York, NY, USA153224
- B.A.LogueD.M.HinkensS.I.BaskinG.A.RockwoodThe analysis of cyanide and its metabolites in breakdown products in biological samplesCrit. Rev. Anal. Chem.402010122147
- O.H.LowryJ.A.LopezThe determination of inorganic phosphorus in the presence of labile phosphate esterJ. Biol. Chem.1621946421428
- K.K.W.MakH.YanaseR.RennebergNovel optical biotest for determination of cyanide traces in marine fish using microbial cyanide hydratase and formate dehydrogenaseMicrochim. Acta1492005131135
- F.PabloR.T.BuckneyR.P.LimToxicity of cyanide and iron–cyanide complexes to Australian bass Macquaria novemaculeata and black bream Acanthapagrus butcheriAust. J. Ecotoxicol.219967584
- S.ParvezI.SayeedS.RaisuddinDecreased gill ATPase activities in the freshwater fish Channa punctata exposed to a diluted paper mill effluentEcotoxicol. Environ. Saf.6520066266
- M.S.PrashanthB.Y.PatilBehavioral surveillance of Indian major carp, Catla catla (Hamilton) exposed to free cyanideJ. Cur. Sci.912006313318
- M.S.PrashanthH.A.SayeswaraA.G.MaheshEffect of sodium cyanide on behavior and respiratory surveillance in fresh water fish, Labeo rohita (Hamilton)Rec. Res. Sci. Technol.3220112430
- N.D.PraveenA.ShwethaB.H.BasalingIn vivo changes in the activity of (gill, liver and muscle) ATPases from Catla catla as a response of copper cyanide intoxicationEur. J. Exp. Biol.24201213201325
- S.C.RichmondsH.M.DuttaEffect of malathion on the optomotor behavior of bluegill sunfish, Lepomis macrochirusComp. Biochem. Physiol.1021992523
- P.J.RubecF.CruzV.PrattR.OellersB.McCulloughF.LalloCyanide-free net-caught fish for the marine aquarium tradeAquar. Sci. Conserv.320013751
- P.J.RubecV.R.PrattSuplidoB.McCulloughB.ManipulaJ.AlbanT.EseberoTrends determined by cyanide testing on marine aquarium fish in the PhilippinesJ.C.CatoC.L.BrownMarine Ornamental Species: Collection, Culture and Conservation2002Iowa State Press327340
- E.F.SamsonJ.D.QuinNa+/K+ activated ATPase in rat developmentJ. Nerochem.141967421427
- A.D.ShwethaB.B.HosettiAcute effects of zinc cyanide on the behavior and oxygen consumption of the Indian major carp, Cirrhinus mrigalaWorld J. Zool.432009238246
- A.D.ShwethaB.B.HosettiEffect of exposure to sublethal concentrations of zinc cyanide on tissue ATPase activity in the fresh water fish, Cirrhinus mrigala (HAM)Arch. Biol. Sci. Belgrade6412012257263
- M.S.SerajR.S.RajeswaraK.L.AnandaAcephate induced alterations in Mg2+ ATPase and Na+/K+-ATPases of different brain regions of Albino ratsThe Bioscan512010153156
- L.S.SmithOsmoregulationIntroduction to Fish Physiology1982TFH Publications, Inc.Neptune, New Jersey1986 (Chapter 2)
- A.SmithT.I.MudderAn environmental perspective on cyanideMining World News691994
- N.SorokinC.AtkinsonE.AldousK.RuleD.MaycockS.ComberProposed EQS for Water Framework Directive Annex VIII substances: cyanide (free) (For consultation) by Water Framework Directive – United Kingdom Technical Advisory Group (WFD-UKTAG), Scotland2008
- J.B.SpragueMeasurement of pollutant toxicity to fish. Bioassay methods for acute toxicityWater Res.31969793882
- K.TakaoThermodynamic analysis of muscle ATPase mechanismsPhysiol. Rev.651985467
- S.R.ThoratChronic effect of endosulfan on freshwater fish, Catla catlaJ. Ecotoxicol. Environ. Monit.1142001221223
- B.S.TiwariB.BelenghiA.LevineOxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell deathPlant Physiol.128200212711281
- Z.A.UnnisaN.S.DevarajEffect of methacrylo-nitrile on membrane bound enzymes of rat brainInd. J. Physiol. Pharmacol.5142007405409