Abstract
Magnetic materials have been extensively used for the extraction of heavy metal ions from contaminated aqueous streams. This inherent characteristic of the magnetic particles has received considerable attention in recent years. The external magnetic field employed in the sorption process overcomes many hindrances established during the application of conventional sorbents for metal ion removal. Recent studies illustrate the severity of arsenic toxicity to be a major environmental health hazard in the contaminated ground water. Available literature has been reviewed to highlight the problem, including its malignancies. Magnetic sorbents with demonstrated high specific surface area and specific affinity for metal ions have been exceedingly beneficial for removing the toxic arsenic ions. In addition to this, these sorbents have demonstrated a promising performance in practical applications also. This review paper aims to summarize the magnetic structures and all recent progress in the research of novel magnetic materials for arsenic removal making it a promising technique in the frame of engineering chemistry is showcased herein and reviewed scrupulously.
1 Introduction
The insufficiency of water has become a considerable threat to the well-being of human beings and animals. The drinking water quality has become a grave alarm with the swift boom in industrialization heading towards a developed society (CitationChen et al., 2009). The effluent generated from industries including textiles, chemicals, mining and metallurgy are accountable for contaminating the water streams (CitationDambies et al., 2000; CitationBourlinos et al., 2001). This contaminated water contains heavy metal ions including arsenic, zinc, copper, nickel, mercury, cadmium, lead and chromium which are a deleterious threat to human beings and harmful to the natural water resources. Of these, the most toxic is arsenic. Arsenic is found in abundance in the earth’s crust. The long term geological changes and the anthropogenic sources contribute to its existence in profusion (CitationSmedley and Kinniburgh, 2002; CitationLi et al., 2012). It occurs in both organic and inorganic forms. In addition to its contribution by natural profusion methods into water streams, their intentional discharge from industries in the form of effluents also contributes to the toxicity existing in the water streams. In aquatic systems, though the organic form of arsenic undergoes bio-transformation, the inorganic arsenic exists in four oxidation states (−3, 0, +3, and +5). Arsenic has been known as deadly fatal since ancient time due to its several side effects (CitationGoldschmit and Peters, 1934; CitationFerguson and Gavis, 1972). The order of lethality of arsenic species is dimethyl arsenic acid (DMA) < monomethyl arsenic acid (MMA) < arsenate < arsenite. Contamination of water streams with As(III) and As(V) pretense a severe and potential threat to the human and animal health (CitationATSDR, 2000). They cause damage to central nervous system, liver, and skin. Furthermore, arsenic causes liver, bladder, skin, and kidney cancers (CitationIPCS, 2001; CitationFeng et al., 2012). Stoppage of drinking the arsenic contaminated water is the mainstay in the management of arsenicosis, as specific chelation therapy has limited value. Therefore, the emergent techniques to remove As from contaminated water is an imperative task for a healthy society.
1.1 Health hazards caused by arsenic
Arsenic is an omnipresent element in the environment. It is produced by the reduction of arsenic trioxide with charcoal during the metal smelting operations. Power plants using high arsenic-containing coal, could be a major source of pollution in the environment through the source of arsenic contaminated wastewater discharged (CitationWang, 1997). Human epidemiological data, classify inorganic arsenics as Group I carcinogens (CitationIARC, 1987). Hyperpigmentation, hypopigmentation, keratosis, hypertension, cardiovascular diseases, diabetes, and cancer, especially of skin, lung and bladder are the clinical demonstration of relentless arsenicosis in humans (CitationATSDR, 2000). Cancers involving other organs have also been implicated (CitationIPCS, 2001). For example, sodium arsenate was found to cause tumors in mice (CitationNg et al., 1999). Long-term exposure to arsenic results in chronic arsenic poisoning known as arsenicosis, a serious condition reported to transpire in people who live in widespread areas with high arsenic concentrations in drinking water or in burning coal (CitationPi et al., 2000; CitationBerg et al., 2001). Occupational exposure to arsenic can result in elevated un-metabolized inorganic arsenic in the urine due to the reduction of methylation capacity of the kidney (CitationNg et al., 1998). Skin lesions, which include melanosis and keratosis of the exposed regions of the hands and feet are comprehensive characteristics of chronic arsenic poisoning. These usually appear only after 5–15 years of arsenic exposure (CitationTseng, 1977). Chronic arsenic poisoning may also lead to the damage of internal organs, including the circulatory, neural, respiratory, renal and digestive systems. Black Foot Disease (BFD) has been the most severe manifestation of the exposure (CitationChen et al., 1999). Long-term exposure to inorganic arsenic in drinking water leads to peripheral neuropathy (CitationHindmarsh et al., 1977). Moreover, people occupationally exposed to arsenic through contaminated water are reported to have an increased risk of diabetes (CitationRahman and Axelson, 1995). Exposure to inorganic forms of arsenic has tremendously increased the risks of liver and kidney cancer among the human population (CitationTseng et al., 2002). It has been estimated that about 60–100 million people in Asia are at risk due to the ingestion and drinking arsenic-contaminated water (CitationAhmad, 2001). WHO recommended guideline value for arsenic in drinking water is 10 mg/L, however, many developing countries still have their standards set at 50 mg/L. Arsenic contamination in the groundwater has reached a very distressing level and requires abrupt consideration. Intrusion options may include, dug wells and deep tube wells in regions of low arsenic concentrations. Rainwater harvesting, pond sand filtration, low cost domestic filtration systems and most importantly, arsenic removal technologies such as iron hydroxide precipitation are the solution to the existing chaos related to arsenic contamination (CitationMeng et al., 2002).
The arsenic removal technologies based on adsorption cum co-precipitation using iron or aluminum salts; adsorption on activated carbon, activated alumina, activated bauxite; reverse osmosis, ion exchange followed by the other alternative water supplies and watershed management strategies are the solution to the situation arisen due to the present circumstances (CitationZaw and Emett, 2002). The ground water level is being contaminated with arsenic beyond the limit recommended by the World Health Organization (WHO). The arsenic standard for drinking water was at 10 parts per billion (10 μg/L) in 2006, as set standard by the US Environmental Protection Agency (EPA) but in most parts of the world the contamination level has gone beyond 10 μg/L (CitationUSEPA, 2011). The globe is on the verge of fighting the arsenic contamination. Many literatures have reported the arsenic pollution in areas of China, Argentina, Bangladesh, Chile, India, Mexico, New Zealand, Taiwan, Thailand, South Africa and USA (CitationBose and Sharma, 2002; CitationMasue et al., 2007; CitationBanerjee et al., 2008). An efficient arsenic amputation expertise is thus highly enviable to provide protected drinking water to the population. Literature has reported that the arsenic removal depends on water chemistry and sorbent characteristics. Arsenic removal has been studied thoroughly by many researchers around the globe using different technologies. These include oxidation (CitationHug and Leupin, 2003; CitationLeupin and Hug, 2005), coagulation using alum/iron (CitationHug and Leupin, 2003; CitationBose and Sharma, 2002; CitationMasue et al., 2007), sorption/ion-exchange using activated alumina, iron coated sand, ion-exchange resin (CitationBanerjee et al., 2008; Spperlich et al., 2005) and membrane filtration (CitationBrandhuber and Amy, 2001; CitationYoon et al., 2009). A comparison of adsorption method was carried out with other water treatment technologies. The order of complexity in relation to the cost is as follows: solvent extraction > oxidation > distillation > precipitation > reverse osmosis > micro- and ultra filtration > electrodialysis > ion exchange > anaerobic > aerobic > evaporation > adsorption. This provides a proof that the membrane separation, coagulation-precipitation and adsorption are the three major categories of the arsenic disposal techniques (CitationJiang, 2001; CitationVaclavikova et al., 2008; CitationChoong et al., 2007). Among these techniques, the adsorption method is considered to be more advantageous over others because of its removal effectiveness, treatment cost, simplicity, eco-friendliness and ease in equipment handling. In addition to this, adsorption also offers good suppleness in design and operation with the advantage of producing quality effluents suitable for safe and healthy usage (CitationMohan and Pittman, 2007; CitationAnuradha Jabasingh and Pavithra, 2010; CitationHu et al., 2012). The adsorption of heavy metals on solid sorbents and biosorbents in dispersed as well as bound forms has been studied to a larger extent (CitationAnuradha Jabasingh and Sheeba Varma, 2010; CitationAnuradha Jabasingh and Valli Nachiyar, 2010; CitationZhang and Fang, 2010; CitationAnuradha Jabasingh and Valli Nachiyar, 2012). Besides, the reversible nature of most sorption processes, demands the regeneration of the sorbents employed using a suitable desorption process. The sorbents so far used to remove arsenic from aqueous solutions and waste water streams include, novel fabricated copper ferrite (CitationYao et al., 2012), magnetic ion exchange resins, magnetic graphene oxide composites, granular ferric hydroxide, hydrous ion oxide particles, sulfur modified iron, activated alumina, iron oxide-coated microsand (CitationSinha et al., 2011), iron oxide/activated carbon magnetic composite (CitationYao et al., 2014), metal oxide heterostructures (CitationChen et al., 2014), nanocrystalline magnetite(CitationMayo et al., 2007), Fe3O4 nanoparticle-coated boron nitride nanotubes (CitationChen et al., 2011), FeO nanoparticles (CitationWu et al., 2009), dendrimer-conjugated magnetic nanoparticles (CitationChou and Lien, 2011), magnetic multi-granule nanoclusters (CitationLee et al., 2014), maghemite nanoparticles (CitationTuutijärvi et al., 2009), bi-metal doped nanosorbent (CitationKumar et al., 2011), poly(acrylo-amidino ethylene amine) nanofiber (CitationDinhthao et al., 2013). Moreover, development of materials with heterogeneous structures, like porous (CitationLi et al., 2007; CitationXin et al., 2008), spheres (CitationLi et al., 2013; CitationJia et al., 2012; CitationWu et al., 2010), hierarchical materials (CitationKoekkoek et al., 2011; CitationXin et al., 2013; CitationZheng et al., 2010; CitationXin et al., 2010), nanotubes (CitationYan et al., 2010), nanocomposites (CitationWang et al., 2013a), ultrafine magnetic nanoparticles (CitationRostamnia et al., 2013; CitationTang et al., 2011), sheets (CitationYan and Xue, 2005; CitationKoekkoek et al., 2013; CitationWang et al., 2013b), nanowires (CitationWang et al., 2007; CitationHan et al., 2011), and binary metal oxides (CitationXin et al., 2004; CitationZhao et al., 2013). These sorbents are anticipated to detect and rectify the problem of water pollution by various researchers.
Precedent few decades have received more attention towards the purification of arsenic contaminated water. Moreover, a new method for arsenic removal from water, which would operate efficiently over a wide range of pH and also in the presence of competitive ions is the present need of the existing scenario. The chitosan bio-polymer is also known for its scavenging abilities and is used for the removal of metal cations from water. Since arsenic is a chalcophile with a strong affinity for sulfur, studies have been conducted by cross linking a thiol (–SH) functionalized compound to chitosan eventually producing a cross-linked chitosan–DMSA bead that can be effectively used as sorbent. After synthesis of the adsorbent beads, the beads were subjected to characterization to determine their size, specific surface area and composition. Preliminary batch sorption tests were carried out to determine the arsenic removal capacity of the produced sorbent. The aim of this review is to present a broad view of magnetic materials as sorbents for removing arsenic from water systems. Studies have been carried out using low cost ferruginous manganese ore (CitationChakravarty et al., 2002), molybdate-impregnated chitosan beads (Dambies et al., 2002) and α-Al2O3 (CitationHalter and Pfeifer 2001). Arsenic sorption onto pillared clays and iron oxides has also received considerable attention (CitationLenoble et al., 2002). Sorption characteristics of arsenic(III) and arsenic(V) on iron(III)-loaded chelating resin having lysine-Nα, Nα-diacetic acid moiety have been carried out (CitationMatsunaga et al., 1996). The combined effects of anions on arsenic removal by iron hydroxides have been investigated (CitationMeng et al., 2002). Laboratory and field studies have been carried out using zero-valent iron (CitationNikolaidis et al., 2002). Research has been carried out with emphasis onto the sorbate/sorbent ratios and co-occurring solutes. Cerium doped iron oxide sorbent (CitationZhang et al., 2003) and hydrous ferric oxide (CitationWilkie and Hering, 1996) were investigated for their effectiveness to remove arsenic(V) ions.
2 Magnetic sorbents
In recent times, the magnetic sorbents are widely used for the purification of industrial waste waters containing metal ions. These magnetic adsorbents find extensive application in chemical industry and their treatment procedures decrease the purification time and residual volume deposits to 5–6 times and 2–3 times, respectively, in comparison with other wastes cleaning treatment methods including adsorption onto conventional adsorbents, photocatalysis and electrodialysis (CitationBarakat, 2011). For different applications, several chemical methods including sol–gel synthesis, flow injection synthesis, electrospray synthesis, sonochemical reactions, co-precipitation, micro-emulsion technology, reverse micelles technology, hydrolysis, thermolysis and hydrothermal reactions can be used to manufacture magnetic nanoparticles (CitationLaurent et al., 2008). The purification system includes pH correction of water, addition of sorbent, mixing of the received suspension and the magnetic separation of the sorbent (CitationBose and Sharma, 2002). The basic advantages of magnetic sorbents are the reduction in the extent of waste cleaning in comparison with reagent method, high efficiency of purification, small volume of waste deposits, the nontoxic nature of solvents and non requirement of any additional safety measures (CitationBeyaz et al., 2009). The utilized sorbents after sorption can be utilized as pigments, polishing pastes, catalysts and agglomerating additives (CitationLaurent et al., 2008; CitationMahzuz et al., 2009). The magnetic sorbent characterization includes particle size, crystalline phases, surface area, point of zero charge (pHpzc), and saturation magnetization (CitationYang et al., 2008). Several authors have attempted to resource this magnetic sorbent in order to test its sorption feasibility of arsenate in solutions.
2.1 Characterization of magnetic sorbents
The widely used methods for the magnetic sorbent characterization are as follows: Atomic Absorption Spectroscopy (AAS), Differential Scanning Calorimetry (DSC), Electrical Conductivity measurement (CitationOliveira et al., 2003), Fourier Transform Infrared spectroscopy (FTIR), Gel Permeation Chromatography (GPC), Powder X-ray Diffraction (XRD) Technique, Scanning Electron Microscopy (SEM) (CitationSlavov et al., 2010), Transmission Electron Microscopy (TEM), Thermogravimetric Analysis (TGA) (CitationSahoo et al., 2001; CitationMahmoudi et al., 2008; CitationZheng et al., 2009). XRD spectrum is extensively used for the identification of the crystallographic characteristics of the produced matter and phase transparency. Mineralogy obtained by XRD specify the presence of metal oxides. The BET surface area can be determined using an ASAP analyzer. Physicochemical characterization helps us to determine the metal content, particle size distribution, shape, mineralogy, surface area, and charge of the adsorbents. SEM is used to determine the texture, surface morphology, porosity and particle size of the materials. The saturation magnetization of the adsorbent could be measured using Superconducting Quantum Interference Device (SQID). The pH at which the particles in suspension have a net charge of zero and complete immobility in the electric field is the isoelectric point or the point of zero charge (PZC) (CitationNikolaidis et al., 2002). The determination of PZC in the adsorption study is considered as highly significant (CitationSmiciklas et al., 2000). Small Angle X-ray Scattering (SAXS) and Dynamic Light Scattering (DLS) are the methods employed for the determination of particle size and its distribution through the material of analysis. SAXS can be used to analyze dispersions, powders and clumps of solids. DLS is restricted to analysis of the dilute solutions. SAXS provides information about particles and aggregates from a single scattering experiment in the absence of high vacuum (Gil et al., 2009). TEM provides direct images and local information on size and shape of sorbents. The combination of SAXS and DLS methods leads to better-quality information in consideration to shape and size of sorbents in dispersions or powders (CitationPabisch et al., 2012). BET theory (Brunauer–Emmet–Teller) allows obtaining data on the size of non-agglomerated, dense particles (CitationKammler et al., 2004). Every technique has its specific advantages: TEM delivers direct images, SAXS (CitationKammler et al., 2004) is able to measure powders, solids and also particles in solution, DLS is a fast and cheap method, BET gives the size of nanoparticles as well as the determination of phases within a sample or the specific surface. Powder XRD and Electronic Diffraction patterns (ED) are used to determine the crystal structure and characterization of the bulk Fe3O4 nanoparticles. The surface coverage of magnetite particles and their elemental analysis could be characterized using Energy Dispersive X-ray spectroscopy (EDAX) (CitationAnuradha Jabasingh et al., 2015). Raman and Fourier Transform Infrared (FTIR) spectroscopy have been used to determine the infrared spectrum of absorption or emission of a magnetite nanoparticles (CitationGalindo-Gonzalez et al., 2009; CitationSlavov et al., 2010). FTIR spectra yield information on the chemical bonds between the Fe3O4 core and the organic surface coverage (CitationMaity and Agrawal, 2007). Vibrating Sample Magnetometer (VSM) is used to determine the particle intrinsic coercivity (Hc) and Saturation magnetization (CitationBeyaz et al., 2009).The relaxation behavior of the magnetic materials in liquid suspensions could be measured by Magnetic Relaxometry (MRX) (CitationDavydov et al., 2015). FTIR spectra yield information on the chemical bonds between the Fe3O4 core and the organic surface coverage (CitationGleich and Weizenecker, 2005). The desorption binding heat of the covering layers of adsorbates and the weight of adsorbate adhered to the magnetic particle surface are evaluated by thermogravimetric and differential methods of thermal (CitationSahoo et al., 2001; CitationMahmoudi et al., 2008). Studies have proved the extreme sensitivities of the surface properties of oxides in relation to the pH variations in the surrounding environment. The application of these magnetic particles as adsorbents are of great importance and this is facilitated by the determination of the zeta-potential and electrophoretic mobility of the particles (CitationGalindo-Gonzalez et al., 2009; CitationSlavov et al., 2010).
2.2 Magnetic metal oxide structures for arsenic removal
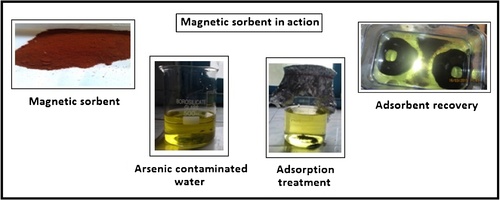
Many metal oxide structures have been used for the removal of arsenic from waste waters. . shows the magnetic sorbent in action. The metal oxide structures are activated alumina, iron oxides, titanium oxide, zirconium oxide, cerium oxide, manganese oxide, magnesium oxide, copper oxide, and binary metal oxides. In contrast, the binary oxides are found to be superior to single metal oxides, during the removal of heavy metal ions from wastewater streams. Magnetic separation using nano- and micro- sized magnetic adsorbents supported onto inorganic compounds, including silica, zeolites, carbon, graphene, and onto organic compounds including polysaccharides, macromolecules, biomolecules and polymers are proven highly effective for treating water polluted by microorganisms, viruses and heavy metals. Micron-sized magnetic iron oxide particles have been employed for this purpose (CitationYavuz et al., 2009). The magnetic sorbents are mostly a combination of magnetic iron oxides and sorbent material. The very commonly employed sorbent material is activated carbon, clay, zeolite, polymer and biopolymer (CitationOliveira et al., 2008, Citation2002, Citation2003; CitationLim and Chen, 2007; CitationChen et al., 2009). These magnetic sorbents were used to remove heavy metals, dyes, oils, and volatile organic compounds (CitationYavuz et al., 2006; CitationYantasee et al., 2007; CitationZhou et al., 2009; CitationSafarik et al., 1995; CitationOrbell et al., 1997; CitationBourlinos et al., 2001) from surface water, process water and ground water.
Iron oxide/activated carbon magnetic composite was extensively synthesized and studied by CitationYao et al. (2014). The procedures adopted were a modification of an existing method proposed by CitationOliveira et al. (2002). The prepared composite exhibited a moisture content and ash content of 1.2% and 10.3% respectively. The iodine value, hardness in percentage and the density of the composite were 1029 mg/g, 96.2%, and 480 g/L respectively. In this study, the composite was prepared by rinsing the activated carbon with de-ionized water and 0.001 mol/L HCl solution followed by modification with 10% HNO3 for 12 h at the room temperature, repeated washing with de-ionized water and finally oven-drying at 85 °C for 24 h. The iron oxide/activated carbon magnetic composite sorbent was then synthesized from the suspension of the prepared activated carbon in a 400 mL solution of FeCl3 and FeSO4 at 70 °C by adding 100 mL, 5 mol/L NaOH solution dropwise in order to precipitate the iron oxides. The obtained material was then washed with de-ionized water until the rinsing water became neutral. The sorbent was finally dried at 100 °C for 8 h and stored in polystyrene bottles for further usage (CitationYao et al., 2014). The adsorption studes and their results revealed the removal percentage of arsenate to be over 95% at conditions of 5.0 g/L adsorbent dosage, initial pH range 3.0–8.0, and contact time of 1 h.
Novel fabricated copper ferrite, CuFe2O4, a low cost sorbent was manufactured from printed circuit board (PCB) sludge by a blend of acid leaching, ferrite process and chemical exchange. The glimpse of preparation of copper ferrite was reported earlier (CitationTu et al., 2010). Acid leaching was conducted with 500 g of the industrial sludge comprising the constituents of PCB. Diluted sulfuric acid was added for extracting copper from solid to solution. Fe powder was employed to substitute Cu2+ by chemical exchange in liquid. Ferrite process was employed to ensure that the supernatant fulfill the properties of the standard effluent. This green low-cost sorbent was collected by magnetic separation method followed by repeated washing to render neutrality. The sorbent was dried at 50 °C and stored for further tests. The maximum As sorption capacity of the copper ferrite was 45.66 mg/g at pH 3.7 and this decreased with pH due to enhanced electrostatic repulsion between As(V) and sorbent surface. Montmorillonite–Cu(II)/Fe(III) oxides magnetic composite was prepared by co-precipitation method (CitationPeng et al., 2006). This composite was used to remove humic acid from water and exhibited a favorable adsorption capacity. The exhausted montmorillonite–Cu(II)/Fe(III) oxides were regenerated at 300 °C and the result confirmed the high catalytic activity of copper ferrite. The magnetic composite adsorbents were also found to be good for some heavy metals. Magnetic metal oxide composite adsorbent (powder CuFe2O4 and MnO–Fe2O3) was prepared by a simple co-precipitation method from environmentally friendly and low cost materials by Wu et al. (Citation2004, Citation2005). These sorbents were found to possess a relatively high surface area, smaller particle size, and porous structure. The results demonstrated that both the CuFe2O4 and MnO–Fe2O3 adsorbents had a high adsorption rate for the azo dye, namely, Acid Red B (ARB) and the maximal sorption capacities are 86.8 and 105.3 mg/g, respectively. The regeneration procedures were highly significant over many cycles in this case. Graphene oxide (GO) was prepared from natural graphite in another research (CitationHummers and Offeman, 1958). In this procedure, 1.0 g NaNO3, 1.0 g graphite and 40 mL H2SO4 were mixed and stirred in a three neck flask in an acid bath, 6 g KMnO4 was added slowly to the bath. The solution was transferred to a 35 °C water bath with continuous stirring for ∼1 h. 80 mL Milli-Q water was added and the solution was stirred for another 30 min at 90 °C. This was followed by adding 150 mL Milli-Q water and 6 mL H2O2 (30%). When the color of solution turned from dark brown to yellow, the solution was filtered and rinsed with 100 mL Milli-Q water. Further treatment was carried out using was further treated with 3 mol/L HNO3, for the introduction of hydrophilic functional groups (epoxy (C–O–C), hydroxyl (OH) and carboxyl (COOH)) at the surface of GO. The filter was vacuum dried and brown GO powder was thus obtained. This procedure was followed by the synthesis of MGO composites by co-precipitation of FeCl3·6H2O and FeCl2·4H2O in the presence of GO. Mixed solution of FeCl3 and FeCl2 was added to the GO solution, and ammonia solution was added quickly to precipitate Fe2+/Fe3+ ions for the synthesis of magnetite (Fe3O4) particles. The black dark colored solution was filtered and rinsed with Milli-Q water/ethanol followed by drying in vacuum at 70 °C. The MGO composites were used for the removal of As(V).
Fe3O4 nanoparticles can also be used as highly effective and economically practical sorbents. In comparison to bare Fe3O4 particles, surface functionalized particles have been extensively used for the removal of toxic metal ions. Surface engineered Fe3O4 nanoparticles have been prepared using carboxyl-, amine- and thiol-functionalized Fe3O4 nanoparticles (ethylenediamine, succinic acid, 2,3-dimercapto succinic acid). Depending upon the surface functionality (SH, COOH, NH2), these magnetic nano-sorbents were found to capture metal ions either by forming chelate complexes, by ion exchange process and by electrostatic interaction. It was observed that these surface engineered Fe3O4 nanoparticles had a strong affinity for the sorption of As3+ from wastewater (CitationSingh et al., 2011). The removal efficiency of As3+ by carboxyl-functionalized Fe3O4 was 91%, and that of amine-functionalized Fe3O4 and thiol-functionalized Fe3O4 was found to be 95% and 97%, respectively, at slightly above neutral pH. The functional groups present on the surface of magnetic nanoparticles were responsible for the creation of a large number of active sites.
Magnetic nanoparticles remain the spotlight as they were triumphant for the separation of toxic metal ions from different sources. CitationFeng et al. (2012) reported super paramagnetic high surface area Fe3O4 nanoparticles for arsenic removal (CitationFeng et al., 2012). CitationMayo et al. (2007) studied the effect of nanocrystalline magnetite size on arsenic removal. Remediation of organic and inorganic arsenic contaminated groundwater was carried out using a nanocrystalline TiO2 based adsorbent by CitationJing et al. (2009). Magnetic multi-granule nanoclusters (MGNCs) were investigated to successfully remove arsenic from aqueous environment. The researchers, herein, have extensively studied the magnetic coercivity (Hc), measured in Oersted (Oe) or ampere/meter(A/m) of the magnetic multi-granule nanoclusters and its effect on the magnetic saturation. Magnetic coercivity refers to the measure of the ability of a ferromagnetic material to endure an external magnetic field devoid of becoming demagnetized. In this case, the magnetic saturation (MS) values were measured at a temperature of 300 K and a magnetic coercivity value of 70 kOe. The magnetic saturation values in emu/g (electromagnetic unit per gram) were found to be 73.9, 80.3, and 84.6 emu/g for MGNC’s of 100, 200, and 400 nm, respectively. The high MS value of multi-granule nanocluster (MGNC) samples at 100 nm was comparative to the MS values reported for Fe3O4 nanoparticles (CitationLee et al., 2014). MGNC’s used were stable ferro magnets in aqueous solution and their multi-granular structure allowed them to get homogeneously dispersed, hence displaying an increased number of surface functional groups (–OH). The research thus revealed the removal of the toxic heavy metal, arsenic from the contaminated groundwater containing 0.6 mg/L of arsenate using 1.0 g of 100 nm MGNCs. This limit was found to comply with the WHO permissible arsenic limit of 10 μg/ L in drinking water. Studies were conducted on the preparation and application of a magnetic composite (Mn3O4/Fe3O4) for removal of As(III) from aqueous solutions (CitationSilva et al., 2012). The blend of an active high surface area sorbent (Mn3O4) with a magnetic phase (Fe3O4) allowed efficient As(III) removal and solid/liquid separation. γ-Fe2O3 nanoparticles were prepared by a wet chemical synthetic method whereby 860 mg of FeCl2·4H2O and 1400 mg of FeCl3 was dissolved in 170 mL of deionized water. This was followed by adding 10 M NaOH under intensive stirring. The color change was observed from orange to dark brown after the addition of NaOH. Subsequent stirring for 1 h, in water bath at 90 °C, allowed the separation of products by a simple hand magnet having a magnetic induction of ∼0.3 T (CitationKilianova et al., 2013).
2.3 Mechanism of magnetic sorbent action during water treatment
Several research findings reported the mechanism behind the removal of heavy metals by the magnetic sorbents. Such mechanisms, for removal of arsenic from the solution phase to the sorbents, as reported by various researchers are discussed in detail, in this section. In order to understand the mechanism of the adsorption of metal ions by nanoparticles, a number of methods have been investigated, including as infrared (IR) spectroscopy (CitationAbollino et al., 2003; CitationLefèvre et al., 2008), X-ray diffraction (XRD) (CitationLo-Irene and Chen, 2005), X-ray photoelectron spectroscopy (XPS) (CitationLee and Anderson, 2005) and extended X-ray absorption fine structure (EXAFS) spectroscopy (CitationSingh et al., 2011). The basis of discussion includes, ion exchange (CitationLo-Irene and Chen, 2005), physical adsorption (CitationLee and Anderson, 2005), surface complexation (CitationShen et al., 2009), electrostatic interaction (CitationLiu et al., 2008) and hard/soft acid-base interaction (CitationPearson, 1963). Though arsenic removal by adsorption mechanism is relatively intricate, potential removal mechanisms can be assumed based on the batch experiments and adsorbent characteristics. The mechanisms and relative rates by which arsenic is removed from solution and incorporated into the sorbents has been the core area of interest. The kinetically stable bonds created with the sulfur and carbon inorganic compounds are the primary reasons for arsenic toxicity (CitationWebb, 1966). The most important aspect is that the compounds which are formed are thermodynamically unstable but persistent. Many thousands of these arsenic compounds include, phenylarsenic acid [C6Hs AsO(OH)2], phenyl- and diphenyl-diarseno-compounds, Cacodylic acid [(CHa) EAsOOH], methane arsonic acid [CH3AsO(OH)2], lewisite [CHaCH=CHAsC12]. Most of these compounds are rapidly hydrolyzed, degraded or oxidized, nevertheless some persist in water. For example, Trimethylarsine, (CH3)3As, which is formed by microorganisms from inorganic arsenic compounds (CitationBird et al., 1948).
Arsenic is unquestionably more soluble in hydrocarbons than water, which may account for its toxicity and for its accumulation in certain organisms. Organic arsenicals synthesized by organisms or formed by reaction with constituents of organisms can affect the distribution of arsenic in the water bodies and their inherent sediments. The most important reactions resulting in removal of arsenic from the solution phase include adsorption onto clays or co-precipitation into metal ion precipitation. Arsenate species consistently co-precipitate with or adsorb onto hydrous iron oxides (CitationLa Peintre, 1954). Iron ores are always observed enriched with arsenic, due to the steady adsorptive capacity of the hydrous iron oxides, while arsenic is nearly absent from manganese ores (CitationShnyukov, 1963). The fact that iron oxide has a positive surface charge in most geological environments and preferentially adsorbs anions has been cited as an explanation of such distributions of arsenic (CitationGoldschmit and Peters, 1934). Arsenite, As+3, species may also be present in surface waters if the Eh (the activity of electrons or the oxidation potential) is less than about 0.1 V or if oxidation to arsenic, As+5 is incomplete. Arsenious acid species will adsorb or coprecipitate with iron oxide in a manner similar to arsenic acid (CitationFerguson and Gavis, 1972).
In a desorption study, experiments were conducted using six different 0.1 M acid and salt solutions (H3PO4, Na2SO4, Na3PO4, H2SO4, HCl, HNO3). Copper ferrite was firstly reacted with 10 mg/L As(V) solution at pH 3.7. In this case, the mechanism of desorption was divided in two types in this study due to the change of electric charge on CuFe2O4 surface caused chiefly by pH variation and due to the competition of ions in the systems (CitationSinha et al., 2011). Studies have indicated that the electric repulsion would also hinder the adsorption of anionic As(V) species at pH > pHpzc (7.3) for copper ferrite. This phenomenon was consistent with the observations of substantial decrease in As(V) adsorption at high pH. This could also be understood as high As(V) removal efficiency at pH < 7.1. According to thse authors, the reason for the absence of desorption under 0.1 M HNO3 and HCl could be attributed to the strong affinity between positive charged copper ferrite and anionic As(V) species present at acidic conditions. It was rather difficult for NO3 and Cl− to desorb anionic As(V) species from the copper ferrite (CitationYao et al., 2012). CitationSinha et al. (2011) made an attempt to remove the arsenic from water using various adsorbents, including magnetic ion exchange resins (MIEX), hydrous iron oxide particles (HIOP), granular ferric hydroxide (GFH), activated alumina (AA), sulfur modified iron (SMI), and iron oxide-coated microsand (IOCM). For MIEX, the primary mechanism for arsenic removal was by ion exchange, whereas negatively charged As(V) (as HAsO42− or H2AsO4−) was removed by exchanging Cl−. Surface complexation adsorption onto hydrous iron oxide surfaces was the major mechanism for aresenate removal for HIOPs (CitationSinha et al., 2011; CitationYao et al., 2014; CitationChen et al., 2014). For GFH and AA, the primary mechanism was by site-specific adsorption on the adsorbent surfaces and inner pores. One hypothesized mechanism for arsenic removal by SMI was via reduction and surface precipitation, in which zero-valent iron (Fe0) oxidizes to Fe3+ while reducing arsenate.
The primary mechanism for arsenate removal by IOC-M was assumed to be surface facilitated adsorption onto the iron-oxide sand coating (CitationSinha et al., 2011). In general, the negatively surface-charged particles form a chelate complex with metal ions above their point of zero charge. For example, the negatively-charged carboxylate ions (COO–) of carboxyl-functionalized particles have a strong coordinative affinity in forming chelate complexes towards metal ions (Mn+) at pH > pHpzc (CitationRostamnia et al., 2013). The chelation tendency of carboxylate ions at higher pH values is expected, as at lower pH values, the chelation sites would be occupied with H+ (the chelation sites are neutral, and were released at a higher pH), thereby originating the desired chelation. Also, at lower pH values, H+ ions were adsorbed onto the surface of particles, resulting in a net positive charge. A convinced amount of metal ions could be observed to be adsorbed by the carboxyl-functionalized nanoparticles at pH < pHpzc. This was suggested due to the ion exchange that was happening likely at pH < pHpzc. The affinity of metal ions to Fe3O4 is higher than that of H+ ions, thus metal ions were found to replace the adsorbed H+ ions from the Fe3O4 surface by an ion exchange mechanism (CitationLo-Irene and Chen, 2005). CitationLiu et al. (2008) observed the adsorption of metal ions by ion exchange as relatively slow, when compared to surface complexation, since the organic molecules present on the surface of the particles may cause steric hindrance towards the adsorption of metal ions.
During the adsorption mechanism of metal ions by amine functionalized particles, the protonation of amine groups (–NH3+) and the surface complexation of metal ions (–NH2Mn+) were found to occur simultaneously on the surface of particles at pH < pHpzc. And, only a few –NH2 sites are available for the adsorption of metal ions through complexation initiated by the transition –NH2 groups to –NH3+. Moreover, the electrostatic repulsion between Mn+ and the particles increased with the formation of more –NH3+. However, the surface of amine-functionalized nanoparticles is negatively charged, due to the formation of –NH2OH– at higher solution pH (pH > pHpzc) (CitationDinhthao et al., 2013). This could increase the adsorption of metal ions through electrostatic attraction between –NH2OH– and Mn+ but reduces the adsorption of metal ions through complexation. Thus, the adsorption of metal ions by amine-functionalized nanoparticles increases with an increase of the pH of the solution.
For the thiol-functionalized nanoparticles, the adsorption mechanism of metal ions involved two surface reactions, as follows: the strong metal–sulfur complexation and weak electrostatic interaction (CitationYantasee et al., 2007). The thiol group that contains a soft donor atom (sulphur) on the surface of nanoparticles reacted with heavy metal ions directly to form stable metal-sulphur complexes through chelation and this acted in addition to the metal-sulfur complexation. There existed a non-specific adsorption of metal ions by thiol-functionalized nanoparticles through a less selective electrostatic interaction chiefly made available between the metal ions and the oppositely charged surface functional groups. This selective electrostatic interation was found to be active at an assured distance from the surface (CitationYantasee et al., 2007). The removal efficiency of metal ions was a combined effect of the presence of organic ligands on the surface and that of the competing adsorbates. The type and concentration of the ligand and metal ion, the pH of the solution and the type of adsorbent were found to determine the bonding factor of the organic ligands on metal ions (CitationLunge et al., 2014). In complex systems with multiple adsorbates, competition occurs among the adsorbates for surface sites. By and large, the degree of competition is reliant on the type and concentration of the competing ions, the affinity of the surface for the adsorbate and the number of surface sites (CitationPabisch et al., 2012). The adsorption process, using the advantages of magnetic separation, leads to a swift and inexpensive removal. presents detailed information regarding the magnetic sorbents and their synthesis methods as studied by various researchers for the removal of arsenic ions from aqueous streams.
Table 1 List of magnetic sorbents employed for the removal of Arsenic ions from aqueous streams.
3 Desorption studies
The reversible nature of most adsorption processes, demands the regeneration of adsorbents by suitable desorption processes with less maintenance cost, trouble-free operation and high efficiency. Regeneration of adsorbents in water treatment is one of the crucial aspects as it commands the economy of water treatment technology. Several reagents were used for the regeneration of magnetic sorbents by various researchers worldwide. Some of them include, perchlorate, sodium bisulfate solution, and nitric acid for the regeneration of Fe3O4 nanoparticles after Pd(II), Pt(IV), and Rh(III) adsorption (CitationUheida et al., 2006). The authors also reported desorption as pH dependent. Nearly 98% Cr(VI) was recovered by using 0.01 M NaOH, with no change in adsorption capacity during the regeneration of γ-Fe2O3 coated with δ-FeOOH nanoparticles used in the sorption of Cr(VI) [130]. Many researchers have employed HCl for the desorption studies including 90% desorption of Fe3O4/Sphaerotilus natans (CitationGuan et al., 2007), regeneration of nanosized iron-oxide-coated quartz (IOCQ) (CitationMostafa et al., 2010), regeneration of polymeric hybrid nanoparticles sorbent (ZrPS-001) with 6.0 M HCI solution (CitationZhang et al., 2008). KCl solution of different molarity were employed for Cr(VI) desorption from magnetite nanoparticles (CitationNamdeo and Bajpai, 2008). NaOH was used as a potential solvent for desorption of heavy metals from various magnetic sorbents by various researchers. CitationHu et al. (2007) described suitability of 0.01 M NaOH for the desorption of Cr(VI) from Jacobsite nanoparticle, with 98.9% as desorption efficiency. Arsenic was desorbed from activated carbon impregnated with nano zero valent iron adsorbents using 0.1 M NaOH solution (CitationZhu et al., 2009a, Citation2009b, Citation2009c). 0.1 M NaOH was employed for the regeneration of titanium dioxide nanoparticles for the uptake of selenium ions from aqueous solution (CitationZhang et al., 2009). Some researchers have employed a solvent mixture for desorption. A mixture of nitric acid and methanol was used for three adsorption/desorption cycles for 2,4-dinitrophenylhydrazine immobilized on sodium dodecylsulfate-coated nanoalumina during the desorption of cadmium and zinc ions, a binary NaOH–NaCl solution being used for regenerating HFO-201 loaded with selenite (CitationPan et al., 2010), 4% NaOH and 2% NaCl solutions were employed for the regeneration of hydrated ferric oxide nanoparticles after removal of phosphate (CitationMartin et al., 2009).
The results of effective reusability of MION (magnetic iron oxide nanoparticles)-tea adsorbent showed the percentage recovery of As(III) as 65.0% using 0.001 M NaOH and 76.0%, using 0.1 M NaOH respectively (CitationLunge et al., 2014). According to CitationShan and Tong (2013); when maghemite-magnetic core and Fe–Mn binary oxide (FMBO), were combined, resulting in Mag-Fe-Mn particles, the ideal circumstance would be an extreme alkaline solution favouring As desorption (CitationShan and Tong 2013). To recover the reduced Mn, NaClO was introduced to oxidize Mn (CitationLi et al., 2012). This observation also indicated the safety of the usage of hybrid adsorbents, as they do not release Fe or Mn into the treated water, and remain stable during cyclic runs without significant component loss. The disruption percentage was maintained above 87%, even after five cycles of desorption. In a study where, magnetic nanoparticles impregnated chitosan beads (MICB) were synthesized, chitosan template was used as sorbent and the regeneration of the MICB was carried out by initially saturating the orbent with As(V) and As(III) followed by shaking the adsorbent with initial arsenic concentration of 1 mg/L, at pH 6.8 ± 0.2, adsorbent dosage of 1 g/L for 24 h (CitationWang et al., 2014). Regeneration of arsenic saturated adsorbents was achieved using sodium hydroxide solution (0.1 mol/L). After a single adsorption-desorption cycle, the MICB was found to be maintained at 99.5% for As(V) and at 97.9% for As(III), respectively as compared to the original adsorption capacity. After five cycles of reuse, the regenerated MICB retained about 88.2% of the original adsorption capacity for As(V) and 76.02% of the original adsorption capacity for As(III). This indicates the good reusability of the adsorbent for arsenic removal. The study is an indication that arsenic adsorbed MICB could be regenerated efficiently with 0.1 mol/L sodium hydroxide solution.
Magnetic honeycomb briquette cinders and its calcined products including MHBC, MHBC (A) and MHBC (N) were used for the removal of As(III) in a fixed-bed column (CitationBaig et al., 2014). For desorption, NaOH (10%) solution of 200 bed volume was employed with the same operating procedures by collecting the effluent samples after certain intervals. The exhausted IOH (iron oxide hydroxide nanoparticle) with flower like morphology were regenerated with eluents like dilute HCl and dilute NaOH (CitationRaul et al., 2014). Desorption efficiency of IOH nanoparticle was studied using 0.2 M, 0.5 M HCl and 0.2 M, 0.5 M NaOH as eluents and all the regeneration experiments were carried out at room temperature. The study revealed NaOH, as the best eluent as it had 75% desorption efficiency, whereas HCl showed a 54% desorption efficiency. Evaluation on the potential arsenic adsorption by synthesized magnetite (Fe3O4) nanoparticles was of remarkable facet (CitationMayo et al., 2007), whereby a lucid spotlight is made on the effect of Fe3O4 particle size on the adsorption and desorption behavior of As(III) and As(V). Desorption studies were conducted with 20 nm and 300 nm Fe3O4 at pH 6.1, by adding As-free electrolyte to the As-exposed Fe3O4 nanoparticles.
Desorption of As(V) using acid and salt solutions revealed that the desorption rate decreased in the order of H3PO4 > Na3PO4 > H2SO4 > Na2SO4 > HCl > HNO3. Desorption experiments were conducted under the conditions of CuFe2O4 dosage 0.05 g, 10 mL desorption reagent and 30 min of desorption time. The solid/solution ratio (W/V) used here was 5 g L−1. The initial solution pH for 0.1 M Na2SO4, 0.1 M Na3PO4, 0.1 M HNO3, 0.1 M HCl, 0.1 M H2SO4, 0.1 M H3PO4 were observed at 10.87, 11.97, 1.47, 1.45, 1.41, 1.69, respectively. The suspensions were shaken for 30 min and the copper ferrite solids were then separated from the solutions using a magnet (CitationYao et al., 2012). The management of the used sorbents and recovered pollutants is one of the most important aspects leading to the awareness about pollutant hazards and nanotoxicology. The used and exhausted sorbents could be used in the manufacturing of bricks, stones and for manufacturing various commodities. And the recovered organic contaminants should be treated as the priority pollutants and should be safely disposed.
4 Application of arsenic elimination from life water streams
This section describes the day-to-day practical applications of Arsenic elimination from water courses and groundwater wells that are contaminated by As pollution. The technology based on the application of magnetic hetero-structures to aid arsenic elimination from life water streams are currently applied conveniently at the household and industries for the removal of arsenic from contaminated tube well waters. The last few years witnessed the development of small scale arsenic removal technologies in most of the arsenic contaminated regions of the world. These arsenic removal technologies based on the magnetic heterostructures are field tested and adopted in most research based programs in some regions of arsenic affected aquifers. Some of the arsenic affected aquifers across the globe are found in Red River Delta in Vietnam (CitationBerg et al., 2006, Citation2007), Shanxi, Jilin, and Xinjiang Province in China (CitationYu et al., 2007), West Bengal in India (CitationBhattacharya et al., 1997), most of the regions in Bangladesh (CitationAhmed et al., 2004), Great Hungarian plain in Hungary (CitationVarsányi and Fodré Zs Bartha, 1991), Romania (CitationTudorache et al., 2011) Chaco-Pampean Plain in Argentina, North-West Argentina (CitationSmedley et al., 2001a, Citation2001b), Lagunera District in Mexico and Western U.S.A. (CitationCamacho et al., 2011). In some regions of the world, Ronphibun District in Thailand (CitationWilliams et al., 1996), Lavrion Peninsula in Greece (CitationTsagarakis et al., 2002; CitationDaskalaki and Voudouris, 2006), South–West England (CitationAston et al., 1975), Ashanti Region in Ghana (CitationNyarko, 2001), Zimbabwe (CitationJonnalagadda and Nenzou, 1996), South Africa (CitationRamudzuli and Horn, 2014), Halifaz in Nova Scotia (CitationGibbons and Gagnon, 2010), Zimapan Valley (CitationArmienta et al., 2001), British Columbia (CitationWilson et al., 2008) and Fairbanks in Alaska (CitationHarrington et al., 1978; CitationNSCEP, 2003), the ground water is polluted with arsenic due to the rigorous mining operations. Geothermal waters of the Wairaki in New Zealand (CitationRobinson et al., 1995), Kyushu in Japan, Kamchatka of Inner Mongolia (CitationSmedley et al., 2001a, Citation2001b), Antofagasta in Chile (CitationSmith et al., 2000) and Northcentral Mexico (CitationDel Razo et al., 1990) are found to be naturally contaminated with arsenic. This section gives an insight into various technologies that are adopted in most of these regions based on the magnetic heterostructures. Water treatment with magnetic adsorbents synthesized using Ferric Chloride, FeCl3 and Ferric Sulfate Fe2(SO4)3·7H2O are effective in removing arsenic from water. They are proved to be effective over a wider range of pH. The magnetic sorbents are added and dissolved in water under efficient stirring for one to few minutes resulting in the rapid formation of flocs which are easily settable flocs by electrostatic attachment. Arsenic is also adsorbed onto coagualted flocs. The process of sedimentation and filtration removes the Fe-As complex, which is formed as an end product. In most regions, iron coagulation using magnetic heterostructures, are capable of achieving efficient arsenic removal in a wider pH range between 6.0 and 8.5.
Numerous projects have been developed in the arsenic contaminated regions of the world, to minimize the effects of arsenic toxicity. These projects are based on the application of these magnetic heterostructures and other chemical processes. The Bucket Treatment Unit (BTU), developed by DPHE-Danida Project in Bangladesh is based on the principles of coagulation, co-precipitation and adsorption onto magnetic heterostructures. The DPHE-Danida project in Bangladesh distributed several thousands BTU units in rural areas of Bangladesh for the arsenic elimination. The units were reported to have very good performance in arsenic removal at all the tested conditions (CitationSarkar et al., 2000). Though these units have a limitation on the reduction of arsenic concentration to the desired level of 0.05 mg/L, these discrepancies could be attributed to the poor mixing, variable water quality, and pH of groundwater in different locations of Bangladesh. Another modified method employing ferric chloride reduced the arsenic contents of treated water below 20 ppb and never exceeded 37 ppb from the tube well water, where the arsenic concentrations varied between 375 to 640 ppb. Thus, BTU was found to be a promising technology for arsenic removal at household level and at various industries at a small cost. An additional widely adopted method was the Stevens Institute Technology. Quick assessment showed that the technology was effective in reducing arsenic levels to less than 0.05 mg/L in case of 95% of the samples tested (CitationBAMWSP, 2001). Bangladesh Council of Scientific and Industrial Research (BCSIR), was responsible for the development and implementation of the BCSIR Filter Unit, for the removal of arsenic through the application of coagulation and co-precipitation processes with magnetic heterostructures, followed by sand filtration (CitationSperlich et al., 2005). The most appreciated project, the DPHE-Danida Arsenic Mitigation Pilot Project installed Fill and Draw Units with an effective tank capacity of 600 L for the commercial and drinking purpose. The experimental units installed by DPHE-Danida project is serving the clusters of families and educational institutions in Bangladesh. These arsenic removal units are attached to the tube wells in approximately all the regions of Bangladesh.
One of the most adopted technology in West Bengal, India and Bangladesh is the Granular Ferric Hydroxide (AdsorpAs®) based arsenic removal units installed by M/S Pal Trockner(P) Ltd., India and Sidko Limited, Bangladesh. The proponents of the unit allege to have very elevated arsenic removal capability and produces non-toxic spent granular ferric hydroxide. The Read-F Arsenic Removal Unit is promoted by Shin Nihon Salt Co., Ltd., Japan for the arsenic removal in Bangladesh. Read-F displays high selectivity for arsenic ions under a broad range of conditions and effectively adsorbs both arsenite and arsenate without the need for pretreatment (CitationSNSCL, 2000; CitationAhmed et al., 2000). Apart from these, many indigenous filters based on magnetic heterostructure have been developed and employed as arsenic adsorbents. They are Sono 3-Kolshi Filter (CitationKhan et al., 2000), Granet Home-made Filter and Chari Filter. The Sono 3-Kolshi filter uses zero valent iron fillings. The unit has been found to be very effective in removing arsenic but the media is prone to a containment and growth of microorganism (CitationBAMWSP, 2001). The Garnet home-made filter and Chari filter contained magnetic heterostructure based bricks and sand as filtering media and works on the principles of air oxidation and adsorption onto brick and flocs for arsenic removal from groundwater. The Shapla arsenic filter, the Kanchan TM developed by Massachusetts Institute of Technology (MIT), the Environment and Public Health Organisation (ENPHO) and the Rural Water Supply and Sanitation Program (RWSSSP) of Nepal and the SAFI arsenic filter are examples of a household arsenic removal filter based on nano zero-valent iron coated bricks. These are developed and promoted by the International Development Enterprises (IDE).
The SAFI filter comprises chemically treated active porous composite materials like kaolinite and iron oxide on which hydrated ferric oxide is deposited by sequential chemical and heat treatment. In these filters, surface complexation occurs, and arsenic is rapidly adsorbed onto the surface of the magnetic ferric hydroxide particles. The arsenic loaded iron particles are then flushed into the sand layer below. Cartridge Filters based on the magnetic heterostructures have shown promising results in some of the arsenic contaminated sites of Bangladesh. They are Chiyoda Arsenic Removal Unit from Japan and Coolmart Water Purifier from Korea. Chiyoda Arsenic Removal Unit had a potential to treat arsenic contaminated water to meet the WHO guideline value of 10 μg/L, when the feed water arsenic concentration was 300 μg/L. The Coolmart Water Purifier had a limited potential with the ability to treat only 20 L of water with an effluent arsenic content of 25 μg/L (CitationAhmed et al., 2000). Such magnetic heterostructures based on the granular ferric hydroxides are being used in full scale systems in Germany, and similar materials have been developed in Canada and the United States. These materials generally have high removal efficiency and capacity. Nevertheless, the users of these systems often feel lethargic, and do not or vert rarely follow the instructions for operation of the unit, despite their simplicity. However, it should be explicitly stated that the arsenic removal efficiency did not appear to have been affected much by these irregularities adopted during the treatment methods.
Removal of arsenic from groundwater using naturally occurring iron oxides in rural regions of Mongolia was carried out by a magnet drum separator. The device operates in a continuous mode, and is able to process 1 Gal of water in 5 min (CitationCao et al., 2007). The water flow and the trajectory of the magnetic particles in the device have been simulated numerically to guide the design and operation of the device (CitationYu et al., 2007). Both experimental results and numerical modeling indicate that the magnetic particles can be efficiently separated from water and recovered by the magnet drum, and the concentrations of both the arsenic, the particles, and the iron in the treated water satisfy the WHO’s drinking water guideline value. The collaboration and partnership with research institutions and universities in China and Mongolia, including (i) Shenyang Institute of Environmental Sciences (China), (ii) Tsinghua University (China), and (iii) the National University of Mongolia (Mongolia) led to its everyday applications for the household and industrial purposes, since its inception in 1998. The entire treatment process could be completed by a single operator either in the household or industry, without the use of electricity or gasoline. This contributes to the less operational cost for producing water to be less than 0.05 cent/Gal water. The water, extracted from a nominal 50-m-depth, in Onemana, New Zealand is thermal in nature, having a low pH and moderate to high mineral content. The arsenic content was detected to be at a level of up to 26 ppb. Three years of regular analysis of drinking water treated by ADI International's MEDIA G2® in Onemana, New Zealand confirm that the iron-based adsorptive filter media system consistently reduced arsenic levels from 26 parts per billion (ppb) in raw water to a level below 5 ppb. The arsenic-contaminated water flows downward through the filter bed, where the adsorbent media attracts and binds arsenic ions to the media substrate through a process of chemisorption. This is tested and used in full-scale water treatment applications in New Zealand. The MEDIA G2® that incorporates the magnetic heterostructures was proved to have an arsenic reduction potential from 1200 ppb to less than 5 ppb. In most applications, the treated water contained less than 2 ppb arsenic. The Northern Testing Laboratories and Delta Industrial Services Inc., developed a portable water treatment system and tested on the Taiga woodlands wells, where the arsenic content was 0.237 mg/L in Fairbanks, Alaska (CitationHarrington et al., 1978). This water treatment system was named as the CampWater™ Porta-5. The portable system uses ozonation and cartridge filtration units employing the magnetic heterostructures. Most of the listed practical applications of these magnetic heterostructures have been employed for potable, ground and industrial water fields.
5 Conclusions
The magnetic sorbent particle is a promising alternative treatment technology for the recovery and rejuvenation of As contaminated drinking water. The review clearly demonstrates the high sorption capacity of magnetic heterostructures for the arsenic elimination. This possible higher recovery has favoured the application of these magnetic sorbents to water systems with arsenic levels less than 1500 ppb. The review gives a clear focus on most real life situations encountered in the arsenic contaminated regions around the world. In these regions, the magnetic heterostructures have shown promising results leading to arsenic elimination. This has been implemented through the incorporation and integration of magnetic heterostructures into large-scale water purification systems. These systems are designed based on the specific application of water for various (potable, ground and industrial) purposes. Research is ongoing in this aspect and most scientists and technologists around the globe are in the process of utilizing the monodispersed character and super paramagnetic behavior of these magnetic sorbents for the development of large scale water treatment units with higher arsenic elimination potential from various water reservoirs to make water safe for the living earth.
Acknowledgments
The comments and recommendations of the anonymous reviewers and the Editor, Rameen Abdelhady are greatly acknowledged.
References
- O.AbollinoM.AcetoM.MalandrinoC.SarzaniniE.MentastiAdsorption of heavy metals on Na–montmorillonite. Effect of pH and organic substancesWater Res.37200316191627
- K.AhmadReport highlights widespread arsenic contamination in BangladeshLancet3582001133135
- K.M.AhmedP.BhattacharyaM.A.HasanS.H.AkhterS.M.M.AlamM.A.H.BhuyianM.B.ImamA.A.KhanO.SracekArsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: an overviewAppl. Geochem.192004181200
- F.AhmedM.A.JalilM.A.AliM.D.HossainA.B.M.BadruzzamanAn overview of arsenic removal technologies in BUETM.F.AhmedBangladesh Environment2000Bangladesh Poribesh Andolon177188
- B.AnQ.LiangD.ZhaoRemoval of arsenic(V) from spent ion exchange brine using a new class of starch-bridged magnetite nanoparticlesWater Res.45201119611972
- S.Anuradha JabasinghD.LalithG.PavithraSorption of chromium(VI) from electroplating effluent onto chitin immobilized Mucor racemosus sorbent (CIMRS) impregnated in Rotating Disk Contactor bladesJ. Ind. Eng. Chem.2320157992
- S.Anuradha JabasinghG.PavithraResponse surface approach for the biosorption of Cr6+ ions by Mucor racemosusClean-Soil, Air, Water.382010492499
- S.Anuradha JabasinghS.Sheeba VarmaOptimization and kinetic studies of nickel treatment in the electroplating effluent with activated carbon prepared from rice huskInd. Chem. Eng.522010230247
- S.Anuradha JabasinghC.Valli NachiyarOptimization and kinetics of nickel ion adsorption from electroplating effluent onto activated carbon prepared from Anas platyrhyncha egg shellAdsorpt. Sci. Technol.282010125136
- S.Anuradha JabasinghC.Valli NachiyarImmobilization of Aspergillus nidulans AJSU04 cellulase on modified activated carbon: sorption and kinetic studiesJ. Therm. Anal. Calorim.1092012193202
- M.A.ArmientaG.VillaseñorR.RodriguezL.K.OngleyH.MangoThe role of arsenic-bearing rocks in groundwater pollution at Zimapán Valley, MéxicoEnviron. Geol.402001571581
- S.R.AstonI.ThorntonJ.S.WebbArsenic in stream sediments and waters of South West EnglandSci. Total Environ.41975347358
- ATSDRToxicological Profile for Arsenic2000US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registryp.428
- S.A.BaigJ.ZhuL.TanX.XueC.SunX.XuInfluence of calcination on magnetic honeycomb briquette cinders composite for the adsorptive removal of As(III) in fixed-bed columnChem. Eng. J.257201419
- BAMWSPRapid Assessment of Household Level Arsenic Removal Technologies, Phase-I and Phase-II, Final Report2001DFID, WaterAid Bangladesh, WS Atkins International Limited
- K.BanerjeeG.L.AmyM.PrevostS.NourM.JekelP.M.GallagherC.D.BlumenscheinKinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxide (GFH)Water Res.42200833713378
- K.A.BarakatNew trends in removing heavy metals from industrial wastewaterArab. J. Chem.42011361377
- M.BergS.LuziP.K.T.TrangP.H.VietW.GigerD.StubenArsenic removal from groundwater by household sand filters-comparative field study, model calculations, and health benefitsEnviron. Sci. Technol.40200655675573
- M.BergC.StengelP.T.K.TrangP.H.VietM.L.SampsonM.LengS.SamrethD.FredericksMagnitude of arsenic pollution in the Mekong and Red River Deltas—Cambodia and VietnamSci. Total Environ.3722007413425
- M.BergH.C.TranT.C.NguyenH.V.PhamR.SchertenleibW.GigerArsenic contamination of groundwater and drinking water in Vietnam: a human health threatEnviron. Sci. Technol.35200126212626
- S.BeyazH.KockarT.TanriseverSimple synthesis of superparamagnetic magnetite nanoparticles and ion effect on magnetic fluidsJ. Optoelectron. Adv. Mater.12009447450
- P.BhattacharyaD.ChatterjeeG.JacksOccurrence of arsenic-contaminated groundwater in alluvial aquifers from delta plains, eastern India: options for safe drinking water supplyWater Resour. Dev.1319977992
- M.L.BirdF.ChallengerP.T.CharltonJ.O.SmithStudies on biological methylations. The action of moulds on inorganic and organic compounds of ArsenicBiochem. J.4319487883
- P.BoseA.SharmaRole of iron in controlling speciation and mobilization of arsenic in subsurface environmentWater Res.36200249164926
- A.B.BourlinosA.SimopoulosN.BoukosD.PetridisMagnetic modification of the external surfaces in the MCM-41 porous silica: synthesis, characterization, and functionalizationJ. Phys. Chem. B105200174327437
- P.BrandhuberG.AmyArsenic removal by a charged ultrafiltration membrane- influences of membrane operating conditions and water quality on arsenic rejectionDesalination1402001114
- L.M.CamachoM.GutierrezM.T.Alarcon- HerreraS.DengOccurrence and treatment of arsenic in groundwater and soil in northern Mexico and southwestern USAChemosphere832011211225
- A.M.CaoJ.D.MonnellC.MatrangaJ.M.WuL.L.CaoD.GaoHierarchical nanostructured copper oxide and its application in arsenic removalJ. Phys. Chem. C1115020071862418628
- S.ChakravartyV.DurejaG.BhattacharyyaS.MaityS.BhattacharjeeRemoval of arsenic from groundwater using low cost ferruginous manganese oreWater Res.362002625632
- C.J.ChenL.I.HsuC.H.TsengY.M.HsuehH.Y.ChiouEmerging epidemics of arseniasis in AsiaW.R.ChappellC.O.AbernathyR.L.CalderonArsenic Exposure and Health Effects1999Elsevierp.113
- L.ChenH.XinY.FangC.ZhangF.ZhangX.CaoC.ZhangX.LiApplication of metal oxide heterostructures in arsenic removal from contaminated waterJ. Nanomater.2014110
- R.ChenC.ZhiH.YangY.BandoZ.ZhangN.SugiurD.GolbergArsenic(V) adsorption on Fe3O4 nanoparticle-coated boron nitride nanotubesJ. Colloid. Interface Sci.3592011261268
- X.Q.ChenK.F.LamQ.J.ZhangB.C.PanM.ArrueboK.L.YeungSynthesis of highly selective magnetic mesoporous adsorbentJ. Phys. Chem. C113200998049813
- T.S.Y.ChoongT.G.ChuahY.RobiahF.L.Gregory KoayI.AzniArsenic toxicity, health hazards and removal techniques from water: an overviewDesalination2172007139166
- C.M.ChouH.L.LienDendrimer-conjugated magnetic nanoparticles for removal of zinc(II) from aqueous solutionsJ. Nano Res.13201120992107
- H.J.CuiJ.K.CaiH.ZhaoB.YuanC.L.AiM.L.FuFabrication of magnetic porous Fe–Mn binary oxide nanowires with superior capability for removal of As(III) from waterJ. Hazard. Mater.27920142631
- L.DambiesE.GuibalA.RozeArsenic(V) sorption on molybdate-impregnated chitosan beadsColloids Surf. A: Phys. Chem. Eng. Asp.17020001931
- P.DaskalakiK.VoudourisThe impacts of the irrational water resources management on the groundwater quality of Greece2nd International Conference, Water Science and Technology, Integrated Management of Water RecoursesAthens, Hellas, AQUA2006
- V.V.DavydovE.N.VelichkoA.Yu.KarseevNuclear-magnetic mini-relaxometer for liquid and viscous media controlSci. Technol. J. Infor. Technol. Mech. Optics152015115121
- L.M.Del RazoM.A.ArellanoM.E.CebrianThe oxidation states of arsenic in well-water from a chronic arsenicism area of northern MexicoEnviron. Pollut.641990143153
- V.U.DinhthaoL.I.XiangC.WangEfficient adsorption of As(V) on poly(acrylo-amidino ethylene amine) nanofiber membranesChin. Sci. Bull.58201317021707
- L.FengM.CaoX.MaY.ZhuC.HuSuperparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removalJ. Hazard. Mater.217–2182012439446
- J.F.FergusonJ.GavisA review of the arsenic cycle in natural watersWater Res.6197212591274
- C.Galindo-GonzalezJ.M.FeinbergT.KasamaL.C.GontardM.PósfaiI.KósaJ.D.G.DuranJ.E.GilR.J.HarrisonR.E.Dunin-BorkowskiMagnetic and microscopic characterization of magnetite nanoparticles adhered to clay surfacesAm. Mineral.94200911201129
- M.K.GibbonsG.A.GagnonAdsorption of arsenic from a Nova Scotia groundwater onto water treatment residual solidsWater Res.44201057405749
- B.GleichJ.WeizeneckerTomographic imaging using the nonlinear response of magnetic particlesNature435200512141217
- Goldschmit, V.M., Peters, C., 1934. The geochemistry of Arsenic. Nachr Ges Wiss Geottingen. Math Phys Kl NF Fachgruppe I, 11–22.
- X.H.GuanY.C.QinY.H.QinR.YinM.J.SunRemoving Cr(VI) by composite biosorbent of nano FeO4/Sphaerotilus natansHuan Jing Ke Xue28200720962100
- W.E.HalterH.R.PfeiferArsenic(V) adsorption onto α-Al2O3 between 25 and 70 °CAppl. Geochem.162001793802
- Y.HanX.WuY.MaL.GongF.QuH.FanPorous SnO2 nanowire bundles for photocatalyst and Li ion battery applicationsCrystEngComm13201135063510
- J.M.HarringtonJ.P.MiddaughD.L.MorseJ.HousworthA survey of a population exposed to high concentrations of arsenic in well water in Fairbanks, AlaskaAm. J. Epidemiol.1081978377385
- J.HindmarshO.R.McLetchieL.P.M.HeffernanO.A.HayneH.A.EllenbergerR.F.McCurdyH.J.ThiebauxElectromyographic abnormalities in chronic environmental arsenicalismClin. Chemist. Chem. Toxicol. Metals11977287
- J.HlavayK.PolyakDetermination of surface properties of iron hydroxide-coated alumina adsorbent prepared for removal of arsenic from drinking waterJ. Colloid Interface Sci.28420057177
- J.HuI.M.C.LoG.ChenPerformance and mechanism of chromate(VI) adsorption by d-FeOOH-coated maghemite (c-Fe2O3) nanoparticlesSep. Purif. Technol.5820077682
- M.HuS.ZhangB.PanW.ZhangL.LvQ.ZhangHeavy metal removal from water/wastewater by nanosized metal oxides: a reviewJ. Hazard. Mater.211–2122012317331
- S.J.HugO.LeupinIron-catalyzed oxidation of arsenic(III) by oxygen and by hydrogen peroxide: pH-dependent formation of oxidants in the Fenton reactionEnviron. Sci. Technol.37200327342742
- W.S.HummersR.E.OffemanPreparation of graphitic oxideJ. Am. Chem. Soc.80195813391341
- IARCArsenic and arsenic compounds (Group 1)IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans1987 Supplement 7
- IPCSEnvironmental health criteria on arsenic and arsenic compoundsEnvironmental Health Criteria Series, No. 224. Arsenic and Arsenic Compoundssecond ed.2001WHOGenevap.521
- B.JiaW.JiaY.MaX.WuF.QuSnO2 core–shell microspheres with excellent photocatalytic propertiesSci. Adv. Mater.42012702707
- J.Q.JiangRemoving arsenic from groundwater for the developing world—a reviewWater Sci. Technol.4420018995
- C.JingX.MengE.CalvacheG.JiangRemediation of organic and inorganic arsenic contaminated groundwater using a nanocrystalline TiO2 based adsorbentEnviron. Pollut.157200925142519
- S.B.JonnalagaddaG.NenzouStudies on arsenic rich mine dumps: III. Effect on the river waterJ. Environ. Sci. Health. Part A: Environ. Sci. Eng. Toxicol.31199625472555
- H.K.KammlerG.BeaucageR.MuellerS.E.PratsinisStructure of flame-made silica nanoparticles by ultra-small-angle X-ray scatteringLangmuir20200419151921
- A.H.KhanS.B.RasulA.K.M.MunirM.AlauddinM.HabibuddowlahA.HussamOn two simple arsenic removal methods for groundwater of BangladeshM.F.AhmedBangladesh Environment—20002000Bangladesh Poribesh Andolon151173
- M.KilianovaR.PrucekJ.FilipJ.KolařikL.KvitekA.PanačekJ.TučekR.ZbořilRemarkable efficiency of ultrafine superparamagnetic iron(III) oxide nanoparticles toward arsenate removal from aqueous environmentChemosphere93201326902697
- J.J.KoekkoekW.KimV.DegirmenciH.XinR.RyooE.J.M.HensenCatalytic performance of sheet-like Fe/ZSM- 5 zeolites for the selective oxidation of benzene with nitrous oxideJ Catalysis29920138189
- J.J.KoekkoekH.XinQ.YangC.LiE.J.M.HensenHierarchically structured Fe/ZSM-5 as catalysts for the oxidation of benzene to phenolMicroporous Mesoporous Mater.1452011172181
- S.KongY.WangQ.HuA.K.OlusegunMagnetic nanoscale Fe–Mn binary oxides loaded zeolite for arsenic removal from synthetic groundwaterColloids Surf. A: Physicochem. Eng. Asp.4572014220227
- V.KumarN.TalrejaD.DevaN.SankararamakrishnanA.SharmaN.VermaDevelopment of bi-metal doped micro- and nano multi-functional polymeric adsorbents for the removal of fluoride and arsenic(V) from wastewaterDesalination28220112738
- M.La PeintreSolubilization par les eaux naturelles de l'arsenic lie au fer dans lea roches sedlaentariesCoot. Rend. Acad. Sci.2391954359360
- S.LaurentD.ForgeM.PortA.RochC.RobicL.Vander ElstR.N.MullerMagnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations and biological applicationsChem. Rev.108200820642110
- S.LeeP.R.AndersonEXAFS study of Zn sorption mechanisms on hydrous ferric oxide over extended reaction timeJ. Colloid Interface Sci.28620058289
- S.H.LeeJ.ChaK.SimJ.K.LeeEfficient removal of arsenic using magnetic multi- granule nanoclustersBull. Korean Chem. Soc.352014605609
- G.LefèvreJ.F.KneppersM.FédoroffSorption of uranyl ions on titanium oxide studied by ATR-IR spectroscopyJ. Colloid Interface Sci.32720081520
- V.LenobleO.BourasV.DeluchatB.SerpaudJ.C.BollingerArsenic adsorption onto pillared clays and iron oxidesJ. Colloid Interface Sci.25520025258
- O.X.LeupinS.J.HugOxidation and removal of arsenic(III) from aerated groundwater by filtration through sand and zero-valent ironWater Res.39200517291740
- C.LiH.ZhangD.JiangQ.YangChiral catalysis in nanopores of mesoporous materialsChem. Commun.432007547558
- Q.LiX.T.XuH.CuiJ.PangZ.B.WeiZ.SunJ.ZhaiComparison of two adsorbents for the removal of pentavalent arsenic from aqueous solutionsJ. Environ. Manage.98201298106
- X.LiY.YangQ.YangOrgano-functionalized silica hollow nanospheres: synthesis and catalytic applicationJ. Mater. Chem.1201315251535
- S.F.LimJ.P.ChenSynthesis of an innovative calcium–alginate magnetic sorbent for removal of multiple contaminantAppl. Surf. Sci.253200757725775
- S.F.LimY.M.ZhengS.W.ZouJ.P.ChenUptake of arsenate by an alginate-encapsulated magnetic sorbent: process performance and characterization of adsorption chemistryJ. Colloid Interface Sci.33320093339
- J.F.LiuZ.S.ZhaoG.B.JiangCoating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in waterEnviron. Sci. Technol.42200869496954
- H.M.C.Lo-IreneG.H.ChenFast removal and recovery of Cr(VI) using surface-modified jacobsite nanoparticlesLangmuir2120051117311179
- S.LungeS.SinghA.SinhaMagnetic iron oxide (Fe3O4) nanoparticles from tea waste for arsenic removalJ. Magn. Magn. Mater.35620142131
- M.MahmoudiA.SimchiM.ImaniA.S.MilaniP.StroeveOptimal design and characterization of superparamagnetic iron oxide nanoparticles coated with polyvinyl alcohol for targeted delivery and imagingJ. Phys. Chem. B11220081447014481
- H.M.A.MahzuzR.AlamM.M.AlamR.BasakM.S.IslamUse of arsenic contaminated sludge in making ornamental bricksInt. J. Environ. Sci. Technol.62009291298
- D.MaityD.C.AgrawalSynthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous mediaJ. Magn. Magn. Mater.30820074655
- B.D.MartinS.A.ParsonsB.JeffersonRemoval and recovery of phosphate from municipal wastewaters using a polymeric anion exchange bound to hydrated ferric oxide nanoparticlesWater Sci. Technol.60200926372645
- Y.MasueR.H.LoeppertT.A.KramerArsenate and arsenite adsorption and desorption behavior on coprecipitated aluminum:iron hydroxidesEnviron. Sci. Technol.412007837842
- H.MatsunagaT.YokoyamaR.J.EldridgeB.A.BoltoAdsorption characteristics of arsenic(III) and arsenic(V) on iron(III)-loaded chelating resin having lysine-Nα, Nα-diacetic acid moietyReact. Funct. Polym.291996167174
- J.T.MayoC.YavuzS.YeanL.CongH.ShipleyW.YuJ.FalknerA.KanM.TomsonV.L.ColvinThe effect of nanocrystalline magnetite size on arsenic removalSci. Technol. Adv. Mater.820077175
- X.MengG.P.KorfiatisS.BangK.W.BangCombined effects of anions on arsenic removal by iron hydroxidesToxicol. Lett.1332002103111
- D.MohanC.U.PittmanJr.Arsenic removal from water/wastewater using adsorbents—a critical reviewJ. Hazard. Mater.1422007153
- M.G.MostafaY.H.ChenJ.S.JeanC.C.LiuH.TengAdsorption and desorption properties of arsenate onto nano-sized iron-oxide-coated quartzWater Sci. Technol.622010378386
- G.S.MurugesanM.SathishkumarK.SwaminathanArsenic removal from groundwater by pretreated waste tea fungal biomassBioresour. Technol.972006483487
- M.NamdeoS.K.BajpaiInvestigation of hexavalent chromium uptake by synthetic magnetite nanoparticlesElectron. J. Environ. Agric. Food. Chem.7200830823094
- J.C.NgD.JohnsonP.ImrayB.ChiswellM.MooreSpeciation of arsenic metabolites in the urine of occupational workers and experimental rats using an optimised hydride cold-trapping methodAnalyst1231998929933
- J.C.NgA.A.SeawrightL.QiC.M.GarnettB.ChiswellM.R.MooreTumors in mice induced by exposure to sodium arsenate in drinking waterW.R.ChappellC.O.AbernathyR.L.CalderonArsenic Exposure and Health Effects1999Elsevierp.217
- N.P.NikolaidisG.M.DobbsJ.A.LackovicArsenic removal by zero-valent iron: field, laboratory and modeling studiesWater Res.37200214171425
- NSCEPAlaska, Case Study Arsenic Treatment Technologies Fairbanks, AK2003National Service Center for Environmental Publications
- B.J.B.NyarkoDetermination of arsenic in some water bodies, untreated ore and tailing samples at Konongo in the Ashanti region of Ghana and its surrounding towns and villages by instrumental neutron activation analysisJ. Radioanal. Nucl. Chem.2492001581585
- L.C.A.OliveiraD.I.PetkowiczA.SmaniottoS.B.C.PergherMagnetic zeolites: a new adsorbent for removal of metallic contaminants from waterWater Res.3820083699
- L.C.A.OliveiraR.V.R.A.RiosJ.D.V.FabrisK.GargR.M.SapagR.M.LagoActivated carbon/iron oxide magnetic composites for the adsorption of contaminants in waterCarbon40200221772183
- L.C.A.OliveiraR.V.R.A.RiosJ.D.V.FabrisK.GargR.M.SapagR.M.LagoClay-iron oxide magnetic composites for the adsorption of contaminants in waterAppl. Clay Sci.222003169177
- J.D.OrbellL.GodhinoS.W.BiggerT.M.NguyenL.N.NgehOil spill remediation using magnetic particlesJ. Chem. Edu.74199714461448
- S.PabischB.FeichtenschlagerG.KickelbickH.PeterlikEffect of interparticle interactions on size determination of zirconia and silica based systems—a comparison of SAXS DLS, BET, XRD and TEMChem. Phys. Lett.52120129197
- B.PanL.XiaoG.NieB.PanJ.WuL.LvW.ZhangS.ZhengAdsorptive selenite removal from water using a nano-hydrated ferric oxides (HFOs)/polymer hybrid adsorbentJ. Environ. Monit.122010305310
- R.G.PearsonHard and soft acids and basesJ. Am. Chem. Soc.85196335333539
- F.PengT.LuoL.QiuY.YuanAn easy method to synthesize graphene oxide–FeOOH composites and their potential application in water purificationMater. Res. Bul.48201321802185
- X.PengZ.LuanH.ZhangMontmorillonite–Cu(II)/Fe(III) oxides magnetic material as adsorbent for removal of humic acid and its thermal regenerationChemosphere632006300306
- J.B.PiY.KumagaiG.F.SunH.YamauchiT.YoshidaH.IsoA.EndoL.Y.YuK.C.YukiT.MiyauchiN.ShimojoDecreased serum concentrations of nitric oxide metabolites among Chinese in an endemic area of chronic arsenic poisoning in Inner MongoliaFree Rad. Biol. Med.2820001137
- M.RahmanO.AxelsonDiabetes mellitus and arsenic exposure: a second look at case-control data from a Swedish copper smelterOccup. Environ. Med.521995773
- M.R.RamudzuliA.C.HornArsenic residues in soil at cattle dip tanks in the Vhembe district, Limpopo Province, South AfricaS. Afr. J. Sci.110201417
- P.K.RaulR.R.DeviI.M.UmlongA.J.ThakurS.BanerjeeV.VeerIron oxide hydroxide nanoflower assisted removal of arsenic from waterMater. Res. Bull.492014360368
- B.E.ReedR.VaughanL.JiangHg and Pb removal by Fe-oxide impregnated activated carbonJ. Environ. Eng.1262000869873
- B.RobinsonH.OutredR.BrooksJ.KirkmanThe distribution and fate of arsenic in the Waikato River System North Island, New ZealandChem. Speciat. Bioavailab.719958996
- S.RostamniaA.NuriH.XinA.PourjavadiS.H.HosseiniWater dispersed magnetic nanoparticles (H2O–DMNPs) of gamma-Fe2O3 for multicomponent coupling reactions: a green, single-pot technique for the synthesis of tetrahydro-4H chromenes and hexahydroquinoline carboxylatesTetrahedron Lett.54201333443347
- I.SafarikM.SafarikovaV.BuricovaSorption of water soluble organic dyes on magnetic poly(oxy-2,6-dimethyl-1,4-phenylene)Collect. Czech. Chem. Commun.6019951448
- Y.SahooH.PizemT.FriedD.GolodnitskyL.BursteinC.N.SukenikG.MarkovichAlkyl phosphonate/phosphate coating on magnetite nanoparticles: a comparison with fatty acidsLangmuir17200179077911
- Sarkar, A., Thogersen, Choudhury, Rahaman, Akhter, Choudhury, 2000. Bucket Treatment unit for arsenic removal, in: Water, Sanitation and Hygiene: Challenges of the Millennium, Pre-prints of the 26 WEDC Conference, Dhaka, Bangladesh, pp. 308–310.
- C.ShanM.TongEfficient removal of trace arsenite through oxidation and adsorption by magnetic nanoparticles modified with Fe–Mn binary oxideWater Res.47201334113421
- Y.F.ShenJ.TangZ.H.NieY.D.WangY.RenL.ZuoPreparation and application of magnetic Fe3O4 nanoparticles for wastewater purificationSep. Purif. Technol.682009312329
- E.F.ShnyukovArsenic in the Cimmerian Iron Ores of the Azov-Black Sea regionGeochemistry (Geokhimiya)19638793
- G.C.SilvaF.S.AlmeidaA.M.FerreiraV.S.T.CiminelliPreparation and application of a magnetic composite (Mn3O4/Fe3O4) for removal of As(III) from aqueous solutionsMater. Res.152012403408
- S.SinghK.C.BarickD.BahadurSurface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogensJ. Hazard. Mater.192201115391547
- S.SinghK.C.BarickD.BahadurFe3O4 embedded ZnO nanocomposites for the removal of toxic metal ions, organic dyes and bacterial pathogensJ. Mater. Chem. A1201333253333
- A.SinhaN.R.JanaFunctional, mesoporous, superparamagnetic colloidal sorbents for efficient removal of toxic metalsChem. Commun.48201292729274
- S.SinhaG.AmyY.YoonN.HerArsenic removal from water using various adsorbents: magnetic ion exchange resins, hydrous iron oxide particles, granular ferric hydroxide, activated alumina, sulfur modified iron, and iron oxide-coated microsandEnviron. Eng. Res.162011165173
- L.SlavovM.V.AbrashevT.MerodiiskaC.GelevR.E.VandenbergheI.Markova- DenevaI.NedkovRaman spectroscopy investigation of magnetite nanoparticles in ferrofluidsJ. Magn. Magn. Mater.322201019041911
- P.L.SmedleyD.G.KinniburghA review of the source, behaviour and distribution of arsenic in natural watersAppl. Geochem.172002517568
- P.L.SmedleyH.B.NicolliD.M.J.MacdonaldA.J.BarrosJ.O.TullioHydrochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, ArgentinaAppl. Geochem.172001259284
- P.L.SmedleyM.ZhangG.ZhangZ.LuoArsenic and other redox-sensitive elements in groundwater from the Huhhot Basin, Inner MongoliaR.CiduProc. WRI-102001Swets & ZeitlingerLisse581584
- I.D.SmiciklasS.K.MilonjicP.PfendtS.RaicevicThe point of zero charge and sorption of cadmium(II) and strontium(II) ions on synthetic hydroxyapatiteSep. Purif. Technol.182000185194
- A.H.SmithA.P.ArroyoD.N.G.MazumderM.J.KosnettA.L.HernandezM.M.SmithL.E.MooreArsenic-induced skin lesions among Atacamero people in northern Chile despite good nutrition and centuries of exposureEnviron. Health Perspect.1082000617620
- SNSCLReport on Performance of Read-F Arsenic Removal Unit (ARU)2000Shin Nihon Salt Co., Ltd. October
- A.SperlichA.WernerA.GenzG.AmyE.WorchM.JekelBreakthrough behavior of granular ferric hydroxide (GFH) fixed-bed adsorption filters: modeling and experimental approachesWater Res.39200511901198
- W.TangQ.LiS.GaoJ.K.ShangArsenic(III,V) removal from aqueous solution by ultrafine α-Fe2O3 nanoparticles synthesized from solvent thermal methodJ. Hazard. Mater.1922011131138
- O.S.ThirunavukkarasuT.ViraghavanK.S.SuramanianO.ChaalalM.R.IslamArsenic removal in drinking water-impacts and novel removal technologiesEnergy Sources272005209219
- K.P.TsagarakisN.V.ParanychianakisA.N.AngelakisAqualibrium-country report greeceAqualibrium, European Water Management Between Regulation and Competition2002European Commission, Directorate-General for ResearchBrussels
- C.H.TsengC.P.TsengH.Y.ChiouY.M.HsuehC.K.ChongC.J.ChenEpidemiologic evidence of diabetogenic effect of arsenicToxicol. Lett.13320026976
- W.P.TsengEnvironmental Effects and dose-response relationships of skin cancer and blackfoot disease with arsenicHealth Perspect.191977109119
- Y.J.TuC.K.ChangC.F.YouJ.C.LouRecycling of Cu powder from industrial sludge by combined acid leaching, chemical exchange and ferrite processJ. Hazard. Mater.1812010981985
- A.TudoracheC.MarinI.A.BadeaL.Vl̆adescuDetermination of arsenic content of some Romanian natural mineral groundwatersEnviron. Monit. Assess.17320117989
- T.TurkI.AlpArsenic removal from aqueous solutions with Fe-hydrotalcite supported magnetite nanoparticleJ. Ind. Eng. Chem.202014732738
- T.TuutijärviJ.LuM.SillanpääG.ChenAs (V) adsorption on maghemite nano- particlesJ. Hazard. Mater.166200914151420
- A.UheidaM.IglesiasC.FontasM.HidalgoV.SalvadoY.ZhangM.MuhammedSorption of palladium(II), rhodium(III), and platinum(IV) on Fe3O4 nanoparticlesJ. Colloid Interface Sci.3012006402408
- USEPAArsenic in Drinking Water2011US Environmental Protection AgencyWashington, DC
- M.VaclavikovaG.P.GalliosS.HredzakS.JakabskyRemoval of arsenic from water streams: an overview of available techniquesClean Technol. Environ. Policy1020088995
- I.VarsányiA.Fodré Zs BarthaArsenic in drinking water and mortality in the Southern Great PlainHung. Environ. Geochem. Health1319911422
- B.WangY.ShiD.XueLarge aspect ratio titanate nanowire prepared by monodispersed titania submicron sphere via simple wet-chemical reactionsJ. Solid State Chem.180200710281037
- H.X.WangH.Q.YangH.R.LiuY.H.YuH.C.XinA mesoporous silica nanocomposite shuttle: pH-triggered phase transfer between oil and waterLangmuir29201366876696
- J.WangF.QuX.WuSynthesis of ultra-thin ZnO nanosheets: photocatalytic and superhydrophilic propertiesSci. Adv. Mater.5201310521059
- J.WangW.XuL.ChenX.HuangJ.LiuPreparation and evaluation of magnetic nanoparticles impregnated chitosan beads for arsenic removal from waterChem. Eng. J.25120142534
- L.WangGeneral aspectsL.WangEndemic Arsenism and Blackfoot Disease [in Chinese]1997Xinjiang Public Health and Scientific Technology Publication HouseUrumqi, Xinjiangp.12
- Y.WangG.MorinG.Ona-NguemaF.JuillotG.CalasG.E.BrownDistinctive arsenic(V) trapping modes by magnetite nanoparticles induced by different sorption processesEnviron. Sci. Technol.45201172587266
- J.L.WebbEnzyme and Metabolic Inhibitorsvol. II1966Academic PressNew York Ch. 7, p. 29
- J.A.WilkieJ.G.HeringAdsorption of arsenic onto hydrous ferric oxide: effects of adsorbate/adsorbent ratios and co-occurring solutesColloids Surf. A: Physicochem. Eng. Asp.107199697110
- M.WilliamsF.FordyceA.PaijitprapaponP.CharoenchaisriArsenic contamination in surface drainage and groundwater in part of the southeast Asian tin belt Nakhon SiThammarat Province, southern ThailandEnviron. Geol.2719961633
- J.WilsonH.SchreierS.BrownArsenic in Groundwater in the Surrey–Langley Area2008Institute for Resources & Environment, University of British Columbia Arsenic Groundwater Report
- D.WuH.ZhuC.ZhangL.ChenNovel synthesis of bismuth tungstate hollow nanospheres in water-ethanol mixed solventChem. Commun.46201072507252
- R.C.WuJ.H.QuY.S.ChenMagnetic powder MnO–Fe2O3—a novel material for the removal of azo-dye from waterWater Res.392005630638
- R.C.WuJ.H.QuH.HeRemoval of azo-dye Acid Red B (ARB) by adsorption and combustion using magnetic CuFe2O4 powderAppl. Catal. B4820044956
- Y.WuJ.ZhangY.TongX.XuChromium(VI) reduction in aqueous solutions by Fe3O4-stabilized Fe0 nanoparticlesJ. Hazard. Mater.172200916401645
- H.XinJ.LiuF.FanZ.FengG.JiaQ.YangC.LiMesoporous ferrosilicates with high content of isolated iron species synthesized in mild buffer solution and their catalytic applicationMicroporous Mesoporous Mater.1132008231239
- H.XinJ.ZhaoS.XuJ.LiW.ZhangX.GuoE.J.M.HensenQ.YangC.LiEnhanced catalytic oxidation by hierarchically structured TS-1 ZeoliteJ. Phys. Chem. C.114201065536559
- H.C.XinX.P.LiL.ChenY.HuangG.R.ZhuX.B.LiOrganosilane surfactant-directed synthesis of mesoporous zeolitesEnergy Environ. Focus220131840
- H.C.XinC.H.LiuS.C.ZhangL.Q.WangS.G.LiOxidation of CO over supported La–Sr–Cu mixed oxide catalystsChin. J. Cat.252004727730
- C.YanL.NikolovaA.DadvandC.HarnageaA.SarkissianD.F.PerepichkaD.XueF.RoseiMultiple NaNbO3/Nb2O5 heterostructure nanotubes: a new class of ferroelectric/semiconductor nanomaterialsAdv. Mater.22201017411745
- C.YanD.XueNovel self-assembled MgO nanosheet and its precursorsJ. Phys. Chem. B10920051235812361
- Q.YangD.HanH.YangC.LiAsymmetric catalysis with metal complexes in nanoreactorsChemistry3200812141229
- W.YantaseeC.L.WarnerT.SangvanichR.S.AddlemanT.G.CarterR.J.WiacekG.E.FryxellC.TimchalkM.G.WarnerRemoval of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticlesEnviron. Sci. Technol.41200751145119
- J.T.YaoF.Y.ChenK.C.ChienL.W.ShanS.C.TingArsenate adsorption from water using a novel fabricated copper ferriteChem. Eng. J.198–1992012440448
- S.YaoZ.LiuZ.ShiArsenic removal from aqueous solutions by adsorption onto iron oxide/activated carbon magnetic compositeJ. Environ. Health Sci. Eng.12201458
- C.T.YavuzJ.Y.MayoW.W.YuA.PrakashJ.C.FalknerS.YeanL.CongH.J.ShipleyA.KanM.TomsonD.NatelsonV.L.ColvinLow-field magnetic separation of monodisperse Fe3O4 nanocrystalsScience3142006964967
- C.T.YavuzA.PrakashJ.T.MayoV.L.ColvinMagnetic separations: from steel plants to biotechnologyChem. Eng. Sci.64200925102521
- J.YoonG.AmyJ.ChungJ.SohnY.YoonRemoval of toxic ions (chromate, arsenate, and perchlorate) using reverse osmosis nanofiltration, and ultrafiltration membranesChemosphere772009228235
- G.YuD.SunY.ZhengHealth effects of exposure to natural arsenic in groundwater and coal in China: an overview of occurrenceEnviron. Health Perspect.1152007636642
- M.ZawM.T.EmettArsenic removal from water using advanced oxidation processesToxicol. Lett.1332002113118
- F.ZhangJ.LanZ.ZhaoY.YangR.TanW.SongRemoval of heavy metal ions from aqueous solution using Fe3O4–SiO2–poly(1,2-diaminobenzene) core-shell sub-micron particlesJ. Colloid Interface Sci.3872012205212
- F.S.ZhangH.ItohPhotocatalytic oxidation and removal of arsenite from water using slag-iron oxide-TiO2 adsorbentChemosphere652006125131
- L.ZhangM.FangNanomaterials in pollution trace detection and environmental improvementNano Today52010128142
- L.ZhangN.LiuL.YangQ.LinSorption behavior of nano-TiO2 for the removal of selenium ions from aqueous solutionJ. Hazard. Mater.170200911971203
- M.ZhangG.PanD.ZhaoG.HeXAFS study of starch-stabilized magnetite nanoparticles and surface speciation of arsenateEnviron. Pollut.159201135093514
- Q.ZhangB.PanB.PanW.ZhangK.JiaQ.ZhangSelective sorption of lead, cadmium and zinc ions by a polymeric cation exchanger containing nano-Zr(HPO3S)2Environ. Sci. Technol.42200841404145
- Y.ZhangM.YangX.HuangArsenic(V) removal with a Ce(IV)-doped iron oxide adsorbentChemosphere512003945952
- J.ZhaoY.L.ZhangP.P.SuZ.X.JiangQ.H.YangC.LiPreparation of Zn–Co–O mixed-metal oxides nanoparticles through a facile coordination polymer based processRSC Adv.3201340814085
- J.ZhengQ.ZengY.ZhangY.WangJ.MaX.ZhangW.SunR.LiHierarchical porous zeolite composite with a core-shell structure fabricated using β-zeolite crystals as nutrients as well as coresChem. Mater.22201060656074
- Y.M.ZhengS.F.LimJ.P.ChenPreparation and characterization of zirconium-based magnetic sorbent for arsenate removalJ. Colloid Interface Sci.33820092229
- L.ZhouY.WangZ.LiuQ.HuangCharacteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheresJ. Hazard. Mater.16120099951002
- H.J.ZhuY.F.JiaX.WuH.WangRemoval of arsenite from drinking water by activated carbon supported nano zero-valent ironHuan Jing Ke Xue30200916441648
- H.J.ZhuY.F.JiaX.WuH.WangRemoval of arsenic from water by supported nano zero-valent iron on activated carbonJ. Hazard. Mater.172200915911596
- H.J.ZhuY.F.JiaS.H.YaoX.WuS.Y.WangRemoval of arsenate from drinking water by activated carbon supported nano zero-valent ironHuan Jing Ke Xue30200935623567