Abstract
Shigella flexneri is a leading cause of bacterial dysentery in developing countries. Among the 15 known serotypes, four (1b, 3a, 3b and 4b) contain a group 6 epitope due to an acetyl group connected to the O-2 position of rhamnose III on the tetrasaccharide structure of the lipopolysaccharide. O-acetyltransferase encoded by a bacteriophage, Sf6, mediates the acetylation reaction. We found that the oac gene in serotype 1b strains was very different from that in serotypes 3a, 3b and 4b strains and is herein after referred to as oac1b which shares with oac 88%–89% identity at the DNA level and 85% identity at the protein level. Considering that S. flexneri strains of serotypes 1–5 share a recent common ancestry, the divergent oac1b is more likely to have been obtained from outside S. flexneri than to have undergone rapid divergence from the oac gene in the other serotypes (3a, 3b and 4b) within S. flexneri. The cloned oac1b gene was found to perform the same acetylation function as oac. Analysis of the genomic regions flanking oac1b showed that it was present in a prophage on the chromosome and the organizational structure is different from that of phage Sf6. Additionally, phage conversion assay showed that serotype 1b cannot be generated by infecting serotype 1a strains with Sf6. We conclude that oac1b was carried by a non-Sf6 phage and is uniquely present in serotype 1b.
Introduction
Shigella flexneri is the major pathogen responsible for bacterial dysentery in developing countries.Citation1 It is estimated that there are 164.7 million cases of shigellosis worldwide annually, resulting in 1.1 million deaths, most of which are children under the age of 5 years.Citation1 A more recent study estimated approximately 125 million annual shigellosis cases and 14 000 related deaths in Asia.Citation2 In China, S. flexneri is the most common Shigella spp., accounting for up to 80% of shigellosis cases.Citation3,Citation4,Citation5
Based on the O-antigen structure of the lipopolysaccharide (LPS), S. flexneri is currently divided into 15 serotypes.Citation5,Citation6 With the exception of serotype 6, all share a common polysaccharide backbone comprised of repeating tetrasaccharide units (N-acetylglucosamine–rhamnose–rhamnose–rhamnose). Serologically, serotypes 1b, 3a, 3b and 4b strains may cross-react with group 6 antisera. Structural analysis also revealed that the LPS tetrasaccharide backbone in serotypes 1b, 3a, 3b and 4b all contain an acetyl group connected to the O-2 position in the rhamnose III, and this acetylation results in the appearance of the group 6 epitope.
Currently, it is generally believed that O-antigen acetylation is mediated by an O-acetyltransferase (Oac) encoded by the oac gene carried by the temperature bacteriophage Sf6.Citation7,Citation8 In 1975, Gemski et al.Citation9 first reported that phage Sf6 could be isolated from a S. flexneri serotype 3a strain. Clark et al.Citation7 and Verma et al.Citation8 independently identified the oac gene from phage Sf6, which is 1002 bp in size, encoding a protein of 333 amino acids. Sequence comparison showed that Oac shares homology with a variety of proteins involved in O-acetylation.Citation10,Citation11 Oac is an integral membrane protein with 10 transmembrane segments, and Oac function is associated with residues within cytoplasmic and periplasmic loops.Citation12 Residues R73, and R75R76 within cytoplasmic loop 3 are critical to the Oac function.Citation12 The oac gene cloned from phage Sf6 was shown to be capable of converting serotype X, Y, la and 4a to 3a, 3b, lb and 4b, respectively.Citation7
In this study, we amplified and sequenced the oac gene from 36 serotype 1b, 3a, 3b and 4b strains, and found that the serotype 1b strains possess a divergent oac gene, herein named oac1b. We further characterized the gene and genetic organization of the regions flanking oac1b to show that oac1bwas not introduced by the phage Sf6, but by a potential novel phage.
Materials and methods
Bacterial strains and culture conditions
The S. flexneri strains used for oac gene analysis in this study were shown in . All strains were identified biochemically using the Dade Behring MicroScan WalkAway 40 (Dade Behring, Hessen, Germany). Serotypes were confirmed using two commercial serotyping antiserum kits: the antisera made by Denka Seiken (Tokyo, Japan) and the monoclonal antibodies against S. flexneri (Reagensia AB, Stockholm, Sweden). Ampicillin sensitive S. flexneri strains 03XZ014 (serotype Y), NCTC9725 (serotype 4a), 05004 (serotype 1a) and 04SH03 (serotype X) were used as hosts for oac gene functional analysis. S. flexneri strains 03HL12 (serotype 3a) and 019 (serotype 1a)Citation13 were used as hosts for phage Sf6 and SfI induction, respectively. Four serotype X strains (014, 51580, 04SH03 and 062 ), 11 serotype Y strains (036, 035, 51581, 017, 03XZ014, 038, 043, 064, 065, 025 and 026), 12 serotype 1a strains (51571, 019, GS30, HB31, 080, SX25, QH20, SX12, 05004, HN184, QH37 and AH93), 4 serotype 4a strains (NCTC9725, NCTC8296, NCTC7885 and 004) and 2 serotype 3b strains (110 and 061) were used for Sf6 infection experiments. All the Chinese strains were isolated from diarrheal patients. Other strains were obtained from National Collection of Type Cultures (NCTC). All strains were generally grown at 37 °C in Luria broth (LB) with agitation, or on LB agar.
Table 1 Properties of S. flexneri serotype 3a, 3b, 4b and 1b strains analyzed in this study
Oligonucleotide primers, PCR and DNA sequencing
Primers used in this study were listed in . All primers were synthesized by Sangon Biotech (Shanghai, China). Unless otherwise stated, PCR amplification was performed using a standard protocol with the following thermal cycling profile: 94 °C for 5 min followed by 30 cycles of 94 °C for 30 s, 55 °C for 50 s and 72 °C for 5 min, on a SensoQuest LabCycler (SensoQuest, Germany). Walking PCR was performed using the Genome Walking PCR Kit (TaKaRa, Kyoto, Japan) according to the manufacturer's protocol. PCR products were either sequenced directly or cloned into the pMD20T TA cloning vector (TaKaRa, Japan) for sequencing.
Table 2 Primers used in this study
oac gene sequencing and functional analysis
The full length of the oac gene was amplified using primer pair O1 (). DNA and deduced protein sequence comparison was performed using BLASTn or BLASTp at NCBI (http://www.ncbi.nlm.nih.gov/). Additionally, the fragment (nt 2540–3799 of accession NO. JF450728) carrying the oac gene, together with the putative promoter and terminator regions (nts 2615–2643 and 3683–3698 of accession NO. JF450728, respectively) was amplified using primer pair O2 (). Purified products were cloned into pMD20T (Ampr) vector and transformed into S. flexneri strains 03XZ014 (serotype Y), NCTC9725 (serotype 4a), 05004 (serotype 1a) and 04SH03 (serotype X), respectively. Transformation was performed by electroporation using a Bio-Rad Gene Pulsar (BioRad, Hercules, CA, USA). Transformants were identified by PCR amplification of oac gene and serotyping by slide agglutination with both monovalent antisera (Denka Seiken, Japan) and monoclonal antibodies against S. flexneri (Reagensia AB, Sweden).
Characterization of the regions flanking the oac1b gene
Based on known arrangements of Sf6 genome in its host strains,Citation14 primer pairs O3, O4 and O5 (), which are complementary to sequences of yfdC, oac and tsp genes, respectively, were designed for PCR identification of the chromosomal regions flanking oac gene in serotypes 3a, 3b and 4b strains. In order to identify the regions flanking oac1b in serotype 1b, we first performed PCR walking starting from gene oac1b, and then used Illumina Solexa sequencing on the whole genome of strain 1997020. Genomic DNA was extracted from broth culture using a Wizard Genomic DNA Purification kit (Promega, Madison, WI, USA) with methods as described in their manual. A paired-end library was constructed, and the average length of insert was about 500 bp. Reads were generated with Illumina Solexa GA IIx (Illumina, San Diego, CA, USA) and re-assembled into scaffolds using SOAPdenovo (Release 1.04). Fragments carrying the gene oac1b and flanking sequence were extracted. Open reading frames (ORFs) were determined using the ORF Finder program, which is accessible through the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/gorf/gorf.html), and conformed to the codon usage table for Escherichia coli. Searches for homologous DNA and protein sequences were conducted with the BLAST software against the non-redundant GenBank database (http://www.ncbi.nlm.nih.gov/blast/blast/). Based on the DNA sequence of the oac1b carrying fragment in 1997020 (accession NO. JN377795), a series of primers were designed and used for overlapping PCR to confirm the genetic structures in the other 19 serotype 1b strains.
Phages conversion assay
Phages Sf6 and SfI used in this study were induced, isolated and purified from S. flexneri strains 03HL12 (serotype 3a) and 019 (serotype 1a), respectively, using the methods as described previously.Citation13 Phage infection and identification of lysogens were performed essentially according to the methods for phage λ with the following modifications.Citation15 Firstly, S. flexneri host strains were inoculated in LB and incubated for 3 h at 37 °C with shaking. Cells were collected by centrifugation when OD600 reached 1.2 and resuspended in MgSO4 (10 mM). Then 100 µl purified phage particles were added into 200 µl competent cells at a ratio of 1 phage to 100 cells. After further incubation at 37 °C for 20 min, 3 ml of semisolid agar (LB with 0.7% (w/v) agar) were added and mixture was laid on the Brain-Heart solid medium, and then incubated at 37 °C. The area of turbid growth was streaked for single colony isolation and serotype identification.
Nucleotide sequence accession number
The nucleotide sequences obtained in this study have been published in GenBank (accession NOs. JF450698–JF450729 and JN377795).
Results
A new oac1b gene was identified from S. flexneri serotype 1b strains, which was divergent from the oac genes in serotypes 3a, 3b and 4b strains
Serotypes 1b, 3a, 3b and 4 are known to contain an O-acetyl group and thus carry an oac gene. We sequenced the oac gene from 36 S. flexneri strains to determine its diversity. The oac genes in serotype 3a, 3b and 4b strains were highly homologous; 6 strains (HB05, 51575, NCTC8522, NCTC8598, NCTC8336 and 51577) had an identical sequence, whereas the remaining 10 strains were nearly identical, but differed by one base (334, A→G) from the other six strains (). The oac gene from phage Sf6,Citation9 which was derived from an S. flexneri serotype 3a strain was identical to the former group of strains.
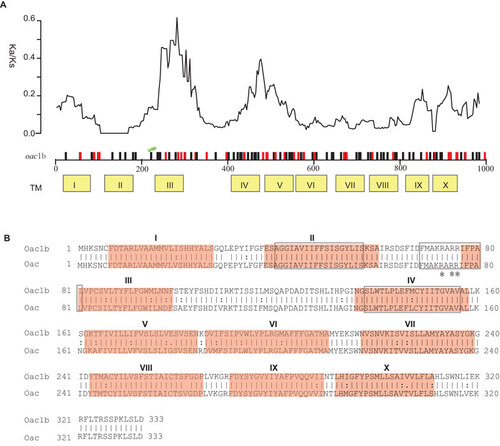
Sequences of the oac genes amplified from all 20 S. flexneri serotype 1b strains were also highly homologous to each other. All except three strains were identical with the remaining three strains (07GS73, 09GS70 and 09GS119) differing by one base (783, C→T) (). Surprisingly, the oac gene in serotype 1b was significantly different from that in serotype 3a, 3b and 4b strains (). There are 110 base changes in the 1002 bp gene, of which, 72 and 38 are synonymous and non-synonymous changes respectively. The distribution of these changes along the gene is shown in . We calculated the ratio of synonymous and non-synonymous substitution rates (Ka/Ks) using a sliding window. We found that the ratios in 2 regions are much higher than the rest of the gene (), suggesting that these regions may have been subjected to diversifying selection. The three major regions (amino acid residues 40–57, 69–81 and 138–156) conserved among the inner membrane trans-acylase family proteinsCitation10 show very low Ka/Ks ratio (). The residues R73 and R75R76, known to be critical for Oac function,Citation12 are also conserved ().
Figure 1 Comparison of protein Oac1b and Oac. (A) Plot of variation between Oac1b and Oac. Nucleotide differences were plotted across the horizontal axis with synonymous and non-synonymous mutations plotted as black and red vertical lines. Ticks and numbers across the horizontal axis are base positions. Transmembrance (TM) segments as predicted by Verma et al. are shown in yellow boxes below the gene. The critical residues for Oac function are indicated by green stars. The ratio of non-synonymous and synonymous substitution rate (Ka/Ks) across the gene using a sliding window of 90 bases (30 codons) and overlap of 3 bases (1 codon) is shown above the gene. (B) Pair-wise list the amino acid sequences of protein Oac1b and Oac. Lines represent amino acid residues that are identical, whereas dots represent amino acids that are similar. TM segments are indicated in red color. The three major regions conserved among the inner membrane trans-acylase family proteins are boxed. The three critical residues for Oac function are marked by asterisk.
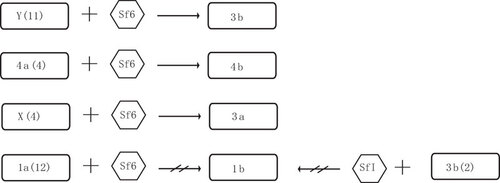
The serotype 1b strains used in this study were isolated from different countries, different regions, at different times (), and belonged to at least two different sequence types based on multilocus sequence typing of 15 house keeping genes (Sun et al, unpublished data), while the other serotypes were also from diverse sources. Thus, we conclude that the divergent oac gene is specific to serotype 1b and hence, we named it oac1b.
Oac1b has the same enzymatic function as Oac
To determine whether Oac1b performs the same O-acetylation function as the Oac, oac1b from strain 1997020, together with its promoter and terminator elements were amplified and cloned into TA vector pMD20T and transformed into 03XZ014 (serotype Y), NCTC9725 (serotype 4a), 05004 (serotype 1a) and 04SH03 (serotype X), respectively. All the transformants were found to be capable of agglutinating with S. flexneri group 6 antisera, and these serotype Y, X, 4a and 1a strains were converted into serotypes 3b, 3a, 4b and 1b respectively. The agglutination intensity of the transformants showed no difference from that of serotype 3a, 3b, 4b and 1b clinical isolates. These results suggest that Oac1b possesses Oac activity, mediating the acetylation of the O-antigen in these serotypes.
The oac1b gene was located in a chromosomal region characteristic of phage origin but different from the Sf6 phage genome
In order to determine whether the oac1b gene is also carried by a Sf6 phage, we performed PCR on all the 20 serotype 1b strains using primer pairs O3 and O4 () designed on the Sf6 genome sequence, and all PCR amplifications were negative, suggesting the region is very different. We then used genome walking PCR starting from the oac1b gene and obtained two fragments about 3.1 kb and 2.3 kb up- and down-stream of the oac1b gene respectively from serotype 1b strain 1997020. The 5451 bp sequence encodes six complete ORFs and two incomplete ORFs (). Upstream of oac1b are two housekeeping genes (torR and torT) and an IS element (IS1), whereas downstream of oac1b are three genes of high identity to phage or prophage genes, which are non-homologous to any of the genes in phage Sf6 (), suggesting the presence of a novel prophage. In order to obtain the entire sequence of the prophage, the whole genome of strain 1997020 was sequenced using Illumina Solexa sequencing technology. A total of 4 637 796 reads were generated to reach about 110-fold coverage and these were assembled de novo into 280 contigs (>1000 bp). A contig of 30 kb carrying oac1b and flanking regions was identified and a total of 38 ORFs (including one pseudogene (ORF36)) were able to be predicted by the ORF finder (Supplementary Table S1 and ) (accession NO. JN377795).
DNA and protein level analyses of the 38 ORFs found that ORF1 and ORF2 subsume genes torR and torT, while ORF36, ORF37 and ORF38 are genes ycmA, yccZ and ept, respectively, which are present in the genomes of sequenced S. flexneri strains 301, 2457t and 2002017. The sequence between ORF3 to ORF35 has non-homologus sequences among torT and ycmA in the genomes of 301, 2457t and 2002017 (). The insertion of ORF3 to ORF35 has been accompanied by deletion of torS to part of the ORF36 (ycmA), which has its 5′ 935-bp region truncated, thereby resulting in a pseudogene ().
Figure 2 Genomic structure of the oac1b region. (A) Comparison of chromosomal regions flanking O-acetyltransferase gene oac1b in serotype 1b strains, and oac in serotype 3a, 3b and 4b strains and serotype-converting phage Sf6. Regions sharing >85% sequence identity are indicated by shaded boxes. Genes coding the same function are shown in the same color. Key primers used in this study are marked by arrows. (B) Genomic structure of regions flanking O-acetyltransferase gene oac1b in serotype 1b strain 1997020 and comparison with relevant regions of sequenced S. flexneri strain 301, 2457t and 2002017. The details of ORFs in strain 1997020 are listed in Supplementary Table S1. Genes share high homologies are shown in the same color. (C) Comparison of the genomic structure of oac1b-carrying prophage in serotype 1b strain 1997020 with prophage genomes in Salmonella enterica serovar Paratyphi B strain SPB7 and E. coli O127:H6 strain E2348/69. Genes sharing >40% identity at amino acid level between the strains are marked by color, red, >80% identity; yellow, 60%–80%; blue, 40%–60%.
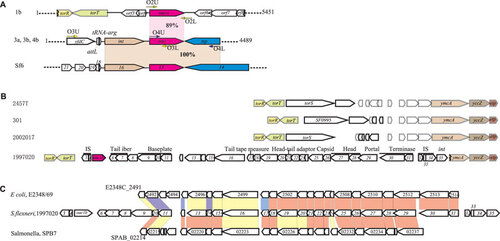
BLASTp analysis found that most of the proteins encoded by the 33 ORFs (ORF3–ORF35) are similar to bacteriophage proteins except for 9 ORFs, whose functions are unknown. These ORFs are ORF6 (tail fiber), ORF7 (tail protein), ORF9 (baseplate protein), ORF11 (baseplate assembly protein), ORF17 (tail tape measure protein), ORF18 (structural protein), ORF21 (head-tail adaptor), ORF23 (structural protein), ORF25 (capsid), ORF27–ORF28 (head), ORF29 (portal), ORF30 (terminase large subunit) and ORF31 (terminase small subunit) (Supplementary Table S1 and ). Two putative insertion sequences, IS1 (ORF3 and ORF4) and IS911 (ORF32–ORF34), were located downstream torT and ORF31, respectively (Supplementary Table S1 and ). The sequences of IS1 and IS911 are identical to the IS sequences found in the S. flexneri genomes. However, there are many copies of IS1 and IS911 in the genome, making it difficult to draw any inference of their origins. It should be noted that an IS911 is also present in the Sf6 genome. However, that IS was located in the Nin region but not the virion head domain as was found here. ORF35 is an integrase sharing 99% amino acid identity with an integrase of phage HK022 (Supplementary Table S1) and may have played a role in the integration of the bacteriophage. These data clearly indicate that this segment of DNA carrying oac1b originated from a phage. Since no phage genes for recombination, immunity, replication and lysis were found, this sequence represents an incomplete prophage genome. Attempts to induce the phage from all 20 serotype 1b strains available in our collection were unsuccessful using conditions described by Mavris et al.Citation16 We also performed overlapping PCR amplification to show that the genomic organization of this prophage region is similar among the serotype 1b strains.
Apart from two regions of homology—the gene oac and IS911 as described above, the DNA sequence and gene organization of oac1b-carrying prophage is entirely different from that of phage Sf6 and contains no remnants of Sf6 phage genes (). Thus, we can conclude that the oac1b-carrying prophage in serotype 1b strains had a non-Sf6 phage origin. Sequence analysis also indicates that this prophage remnant is not homologous to any of other known bacteriophages. However, continuous similarity of genes was found with prophage regions of Salmonella enterica serovar Paratyphi B strain SPB7 and E. coli O127:H6 strain E2348/69, respectively, as shown in .
Current studies show that all the serotype-converting phages integrate into host chromosome at two conserved positions, tRNA-thrW for SfI, SfII, SfIV, SfV and SfXCitation17 and tRNA-argW for Sf6.Citation14 Once integrated, the int and O-antigen modification genes are located at opposition ends of the phage DNA, ending with attL and attR sites.Citation17 To confirm that the Sf6 was inserted into the tRNA-argW in non-serotype 1b strains, a fragment of 4489 bp (accession NO. JF450729) including the oac gene was obtained from strain 03HL12 (serotype 3a) by PCR using primer pairs O5 (). The sequence and gene structure downstream tRNA-argW were identical to that of Sf6 and as expected from the structural organization of Sf6 (). The insertion site of the presumable oac1b-carrying phage is unusual. It has apparently inserted between gene torT and ycmA, resulting in the deletion of genome region between gene torT and ycmA, including part of ycmA (1–935 bp) ().
Phage Sf6 is unable to infect and convert serotype 1a strains
We induced the Sf6 phage from serotype 3a strain 03HL12 and used it to infect the four serotypes (X, Y, la and 4a) that are expected to be convertible based on O-antigen structure. All four serotype X strains and 11 serotype Y strains tested can be converted into 3a and 3b, respectively, as shown previously.Citation7,Citation8,Citation9 Interestingly, four serotype 4a strains were also converted to serotype 4b, which is contrary to previous reports that serotype 4a cannot infected by Sf6.Citation7 However, all 12 serotype 1a strains tested cannot be infected and converted into serotype 1b by phage Sf6 (). Similar phenomenon was also observed by Clark et al.Citation7 Serotype 3b strains contain only an O-acetyl group connected to the O-2 position in the rhamnose III of the tetrasaccharide of the O-antigen. Theoretically, serotype 3b can be converted to serotype 1b by adding a glucosyl group to the N-acetylglucosamine of tetrasaccharide, which is mediated by the gtrI genes carried by phage SfI. We tested two serotype 3b strains by infecting them with phage SfI, but no serotype 1b convertants were found (). The non-conversion cannot be attributed to the phage since the same stock was previously used successfully to infect serotype X strains which was converted to serotype 1d.Citation13
Discussion
The divergent oac1b is more likely to have been obtained from outside S. flexneri than to have undergone rapid divergence from the oac gene in the other serotypes (3a, 3b and 4b) within S. flexneri. Previous studies have shown that S. flexneri strains of serotypes 1 to 5 arose as an independent lineage from within E. coli recently and there is very low level of variation in house keeping genes.Citation18 The virulence plasmid carried by these strains also showed high levels of similarity among the serotype 1–5 strains.Citation19 Thus, it seems less probable that oac1b was evolved from the oac gene within S. flexneri given the high level of divergence.
Clark et al.Citation7 had previously showed that the oac gene cloned from Sf6 was capable of converting Y, X, 4a and 1a to 3b, 3a, 4b and 1b, respectively. Thus the oac and oac1b genes are functionally interchangeable despite the high level of sequence variation.
The host range for Sf6 was proposed to be restricted to strains with a group 3;4 antigen of the O-polysaccharide chain which is presumably recognized by the phage tail protein TSP.Citation20 Hydrolysis by TSP of the 1,3-α-linkage between rhamnose II and rhamnose III exposes the host cell membrane to phage DNA to allow entry into host and complete lysogenic conversion.Citation21,Citation22 Since all four serotypes (X, Y, la and 4a) carry group 3;4 antigen, there must be additional antigenic difference that render serotype 1a resistant to Sf6 infection.
The putative insertion site of the likely oac1b-carrying phage appeared to be unusual. It had apparently inserted between gene torT and ycmA, resulting in the deletion of genome region between gene torT and ycmA including 936 bp of the ycmA gene. The tor operon which encodes the trimethylamine N-oxide respiratory system is apparently nonfunctional in S. flexneri as torD, torA and torS are known pseudogenes in S. flexneri 2a strains Sf301, 2457T and 2002017. ymcA, which encode a putative outer membrane lipoprotein, which is highly conserved among Shigella and E. coli. The effect of inactivation of the ycmA gene in 1b strains is not clear. It should be noted that neither the tRNA genes, nor the att site sequence, was found in this region of 1b strains.
Our data clearly indicated that the DNA carrying oac1b originated from a phage; however, since no phage genes for recombination, immunity, replication and lysis were found, this sequence appears to represent an incomplete prophage genome. Our attempts to induce the phage, using conditions described above, from all of the 20 serotype 1b strains available in our collection, proved to be unsuccessful. Additionally overlapping PCR amplification showed that the genomic organization of this prophage region is similar among all the serotype 1b strains.
Of significance, we found that the oac1b gene mediating the O-antigen acetylation in the S. flexneri serotype 1b strains was highly divergent different from the oac gene in serotype 3a, 3b and 4b strains and phage Sf6. We have shown that oac1b was likely part of a prophage and had a non-Sf6 phage origin. In comparison to the Sf6-like genomic structure in serotype 3a, 3b and 4b strains, the organization of the prophage carrying oac1b in serotype 1b chromosome is rather unique. The ancestral phage was apparently inserted between genes torT and ymcA, however, with no typical phage attachment sites found. Sf6 infection experiments showed that the LPS of serotype 1a must contain additional changes to render it resistant to Sf6 infection. This study extends the current understanding of serotype conversion in S. flexneri and helps identify mechanisms that give rise to Shigella serotype evolution and diversity.
Supplementary information
Download MS Word (113.5 KB)This work was supported by grants (2011CB504901, 2011SKLID203, 2008SKLID106 and YB20098450101) from the Ministry of Science and Technology, and State Key Laboratory for Infectious Disease Prevention and Control, China.
Notes
The source of M1349Citation18 is not known but not isolated from China.
- Kotloff KL, Winickoff JP, Ivanoff B et al.Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ1999;77: 651–666.
- Bardhan P, Faruque AS, Naheed A, Sack DA.Decrease in shigellosis-related deaths without Shigella spp.-specific interventions, Asia. Emerg Infect Dis2010;16: 1718–1723.
- von Seidlein L, Kim DR, Ali M et al.A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med2006;3: e353.
- Wang XY, Tao F, Xiao D et al.Trend and disease burden of bacillary dysentery in China (1991–2000). Bull World Health Organ2006;84: 561–568.
- Ye C, Lan R, Xia S et al.Emergence of a new multidrug-resistant serotype X variant in an epidemic clone of Shigella flexneri. J Clin Microbiol2010;48: 419–426.
- Clemens JD, Kotloff KL, Kay B. Generic protocol to estimate the burden of Shigella diarrhoea and dysenteric mortality. Geneva: World Health Organization, 1999.
- Clark CA, Beltrame J, Manning PA.The oac gene encoding a lipopolysaccharide O-antigen acetylase maps adjacent to the integrase-encoding gene on the genome of Shigella flexneri bacteriophage Sf6. Gene1991;107: 43–52.
- Verma NK, Brandt JM, Verma DJ, Lindberg AA.Molecular characterization of the O-acetyl transferase gene of converting bacteriophage SF6 that adds group antigen 6 to Shigella flexneri. Mol Microbiol1991;5: 71–75.
- Gemski P Jr, Koeltzow DE, Formal SB.Phage conversion of Shigella flexneri group antigens. Infect Immun1975;11: 685–691.
- Slauch JM, Lee AA, Mahan MJ, Mekalanos JJ.Molecular characterization of the oafA locus responsible for acetylation of Salmonella typhimurium O-antigen: oafA is a member of a family of integral membrane trans-acylases. J Bacteriol1996;178: 5904–5909.
- Cheon HG, Hanna PE.Effect of group-selective modification reagents on arylamine N-acetyltransferase activities. Biochem Pharmacol1992;43: 2255–2268.
- Thanweer F, Tahiliani V, Korres H, Verma NK.Topology and identification of critical residues of the O-acetyltransferase of serotype-converting bacteriophage, SF6, of Shigella flexneri. Biochem Biophys Res Commun2008;375: 581–585.
- Sun Q, Lan R, Wang Y et al.Genesis of a novel Shigella flexneri serotype by sequential infection of serotype-converting bacteriophages SfX and SfI. BMC Microbiol2011;11: 269–274.
- Casjens S, Winn-Stapley DA, Gilcrease EB et al.The chromosome of Shigella flexneri bacteriophage Sf6: complete nucleotide sequence, genetic mosaicism, and DNA packaging. J Mol Biol2004;339: 379–394.
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. New York: Cold Spring Harbor Laboratory, 1989.
- Mavris M, Manning PA, Morona R.Mechanism of bacteriophage SfII-mediated serotype conversion in Shigella flexneri. Mol Microbiol1997;26: 939–950.
- Allison GE, Verma NK.Serotype-converting bacteriophages and O-antigen modification in Shigella flexneri. Trends Microbiol2000;8: 17–23.
- Pupo GM, Lan R, Reeves PR.Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc Natl Acad Sci U S A2000;97: 10567–10572.
- Lan R, Lumb B, Ryan D, Reeves PR.Molecular evolution of large virulence plasmid in Shigella clones and enteroinvasive Escherichia coli. Infect Immun2001;69: 6303–6309.
- Lindberg AA, Wollin R, Gemski P, Wohlhieter JA.Interaction between bacteriophage Sf6 and Shigella flexner. J Virol1978;27: 38–44.
- Chua JE, Manning PA, Morona R.The Shigella flexneri bacteriophage Sf6 tailspike protein (TSP)/endorhamnosidase is related to the bacteriophage P22 TSP and has a motif common to exo- and endoglycanases, and C-5 epimerases. Microbiology1999;145: 1649–1659.
- Freiberg A, Morona R, van den Bosch L et al.The tailspike protein of Shigella phage Sf6. A structural homolog of Salmonella phage P22 tailspike protein without sequence similarity in the beta-helix domain. J Biol Chem2003;278: 1542–1548.