Abstract
Plasmodium vivax is a common cause of imported malaria in the USA, second only to P. falciparum. We present a case of P. vivax malaria in a child returning from India. P. vivax was initially diagnosed by standard methodology and detected retrospectively by use of broad-range PCR and electrospray ionization mass spectrometry using a panel of primers designed to detect vector-borne pathogens. This is the first reported case of P. vivax detection using PCR and electrospray ionization mass spectrometry.
Emerging Microbes & Infections (2012) 1, e48; doi:10.1038/emi.2012.49
Case report
A 12-year-old male presented on 13 August 2010 with a nine day history of fever. He had recently spent 6 weeks in India, where he did not take any malaria chemoprophylaxis or use a mosquito net. He developed a 105.7 °F fever on 5 August on the flight home, which recurred every morning between 6:00 and 11:00 a.m., ranged from 104 °F to 105 °F, and was associated with headaches. He had treated his fevers only with Tylenol. Additionally, he presented with a five day cough, and one episode of diarrhea and three episodes of emesis in the two days prior to presentation. The day after his flight home, the patient saw his primary care physician; a complete blood count and liver panel were ordered (). A malaria smear was also ordered and was negative for Plasmodium and Babesia. A second complete blood count and a liver panel were performed upon patient presentation on 13 August (). Urinalysis revealed small bilirubin, trace ketones and protein 30, but was otherwise negative. The patient was negative by Monospot test, as well as for influenza A and B, and had had a negative purified protein derivative test 3 years prior. Negative results were also obtained for antibodies (IgM and IgG) to Chickungunya and Dengue fever and for Ehrlichia PCR. The patient had evidence of mosquito bites. Blood films () demonstrated the diagnostic features of Plasmodium vivax infection: all parasite forms were present (trophozoite, schizont, and gametocyte); not all red blood cells were infected, unlike P. falciparum infections; and the schizonts were larger than uninfected red blood cells (), and merozoites and Schuffner’s dots were present, both characteristic of P. vivax.
Figure 1 Blood film slides. Slides are taken from a blood film performed on 14 August and are similar to slides prepared on 13 and 16 August. Schizonts are larger than uninfected red blood cells and Schuffner’s dots are present, both characteristic of P. vivax.
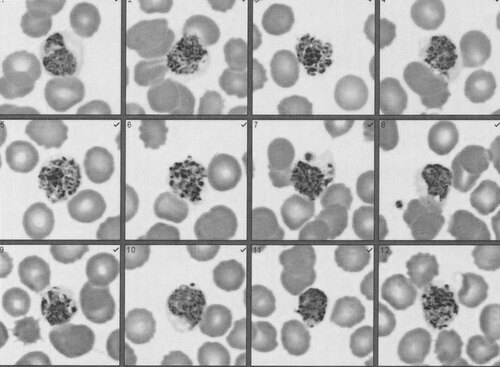
Table 1 Laboratory test results
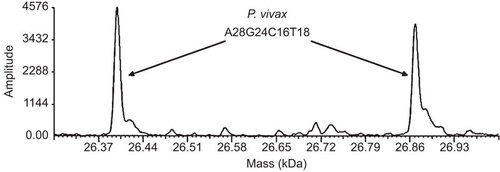
Several months subsequent to treatment, remnant whole-blood and plasma specimens collected the day of presentation were de-identified and sent to Ibis Biosciences for analysis with the Vector-Borne BPN assay using the PLEX-ID (Abbott Molecular, Des Plaines, IL, USA). This technology employs broad-range PCR and electrospray ionization mass spectrometry (PCR/ESI-MS) and has been used to detect a range of vector-borne organisms.Citation1,Citation2,Citation3,Citation4 Specifically, PCR/ESI-MS involves generation of short PCR amplicons, followed by electrospray mass spectrometry to determine the mass of the amplicon. Based on the amplicon mass, the numbers of each of the four nucleotides in the amplicon, the basecount, can be calculated and compared to a database of basecounts to identify the microorganism. This assay, which is not available for diagnostic use, is designed to detect species of Anaplasma, Babesia, Bartonella, Borrelia, Ehrlichia, Francisella and Rickettsia.Citation5 Nucleic acids were extracted using a modified version of a previously published procedure.Citation5 Modifications consisted of the following: homogenized lysates were incubated in lysis buffer for 1 h at 30 °C prior to loading on the Kingfisher Flex; isopropanol was used instead of ethanol in Wash 1 buffer; specimens were washed twice with Wash I buffer and twice with Wash II buffer; and the temperature of the initial binding step on the Kingfisher was changed to 30 °C for 22.5 min. Ten microliters of the resulting 200 µL extracts was used in each well of the Vector-Borne BPN assay plate. Negative controls included water extractions; all controls were negative. A novel base count, A28 G24 C16 T18, was detected in both specimen extracts () with primer pair INV4855, designed to target the Babesia β-tubulin gene. To identify the detection, initially thought to be a species of Babesia, the specimen extracts were amplified with 4 sets of primers targeting the 18S rDNA, ITS1, ITS2 and hsp70 genes of Babesia spp.Citation6 using the Platinum Taq High Fidelity kit (Invitrogen, Carlsbad, CA, USA). The reactions were performed on an MJ Thermocycler with the following PCR cycle: 95 °C for 2 min, followed by eight cycles of 95 °C for 15 s, 50 °C for 45 s and 68 °C for 90 s with the 50 °C annealing temperature increasing 0.6 °C each cycle. The PCR was then continued for 37 additional cycles of 95 °C for 15 s, 60 °C for 15 s and 68 °C for 60 s. The cycle ended with a final extension of 4 min at 72 °C and a 4 °C hold. PCR products were cloned with the ZeroBlunt TOPO PCR kit (Invitrogen) according to the manufacturer’s instructions, and then sequenced with SP6 and T7 promoter primers by SeqWright (Houston, TX, USA). Sequences were obtained only for the ITS2 region. Six of the ITS2 clones tested had sequences bearing 97.3% (397/408) identity with Plasmodium vivax (GenBank accession number AF316892.1).
Figure 2 Mass spectra for Plasmodium vivax. Mass spectra of the forward and reverse strands of the amplicon produced by primer pair INV4855, which was designed to target the β-tubulin gene of Babesia spp. The base count A28 G24 C16 T18 represents the number of adenines, guanines, cytosines, and thymines found in the forward strand of the amplicon.
Based upon the initial clinical findings in August 2010, the patient was diagnosed with vivax malaria and treated with Malarone (atovaquone-proguanil 250/100 mg) for 3 days, and then received 14 days of primaquine (15 mg base per day). The patient had a complete recovery.
Comments
Malaria is an important cause of illness in travelers returning to the USA from endemic countries. P. vivax is the second most common etiological agent of malaria after P. falciparium and, although vivax malaria is not as severe as that caused by P. falciparum, P. vivax is more geographically widespread, particularly in temperate climates.Citation7 2010 saw a significant upswing in the number of imported malaria cases in the USA, with the highest reporting levels seen since 1980.Citation8 Of the 1479 cases of imported malaria from all infecting species, 216 (15%) were acquired in India, a 57% increase in the number of imported cases acquired in India over 2009.Citation8 Among the 1366 cases of imported malaria in the USA for which the etiological agent and the region of acquisition were known, infections caused by P. vivax comprised 87% of the infections acquired in Asia.Citation8 P. vivax infection also displays a seasonal transmission cycle;Citation7 in 2010, the incidence of imported P. vivax cases peaked in August, with a secondary peak in November.Citation8
This case marks the first report of malaria detection using the PCR/ESI-MS technique with the PLEX-ID. This technique has previously been used to rapidly detect and identify nucleic acids from pathogens in specimens collected from patients diagnosed with various infectious diseases.Citation2,Citation9,Citation10,Citation11 The particular assay used in this report, the Vector-Borne BPN assay, was designed to detect and identify common vector-borne pathogens from the USA and Europe,Citation5 including Babesia. While not specifically designed to target Plasmodium, the broad-range primer, INV4855, designed to target the related Babesia β-tubulin gene, amplified P. vivax and produced a unique basecount signature allowing for the identification of the organism. Bioinformatic analysis has since determined that primer pair INV4855 can also detect and differentiate P. falciparum and P. knowlesi. That there is conservation in β-tubulin gene between Babesia and Plasmodium species is not surprising, as both genera are members of the class Aconoidasida.
There are several advantages of using PCR/ESI-MS over traditional means of malaria detection. The technique offers a rapid molecular method for the identification of pathogen DNA from human specimens,Citation12 which does not require the screening of multiple blood films to identify infected red blood cells and provides a molecular basis for species differentiation rather than one based on visual inspection of parasite forms. Additionally, the Vector-Borne BPN assay was able to detect P. vivax DNA in a plasma specimen, indicating that sample types other than whole blood can be used to detect active malaria infection. Finally, the use of the Vector-Borne BPN assay in particular offers the advantage of being able to screen for multiple vector-borne pathogens simultaneously, which proved useful in this case for differentiating an imported P. vivax infection from infection with domestic vector-borne pathogens associated with high fevers, such as Babesia microti and Ehrlichia chaffeensis.
This work was supported in part by a National Institutes of Health grant 2R44AI077156-02.
- Crowder CD, Matthews HE, Schutzer S et al.Genotypic variation and mixtures of Lyme Borrelia in Ixodes ticks from North America and Europe. PLoS ONE2010;5: e10650.
- Eshoo MW, Crowder CD, Li H et al.Detection and identification of Ehrlichia species in blood by use of PCR and electrospray ionization mass spectrometry. J Clin Microbiol2010;48: 472–478.
- Ecker JA, Massire C, Hall TA et al.Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J Clin Microbiol2006;44: 2921–2932.
- Muddiman DC, Anderson GA, Hofstadler SA, Smith RD. Length and base composition of PCR-amplified nucleic acids using mass measurements from electrospray ionization mass spectrometry. Anal Chem1997;69: 1543–1549.
- Crowder CD, Matthews H, Rounds MA et al.Detection of heartworm infection from canine blood by PCR and electrospray ionization mass spectrometry. J Am Vet Res2012;76: 854–859.
- Blaschitz M, Narodoslavsky-Gfoller M, Kanzler M, Stanek G, Walochnik J. Babesia species occurring in Austrian Ixodes ricinus ticks. Appl Environ Microbiol2008;74: 4841–4846.
- Carlton JM, Sina BJ, Adams JH. Why is Plasmodium vivax a neglected tropical disease? PLoS Negl Trop Dis2011;5: e1160.
- Mali S, Kachur SP, Arguin PM. Malaria surveillance—United States, 2010. MMWR Surveill Summ2012;61: 1–17.
- Eshoo MW, Crowder CC, Rebman AW et al . Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS ONE2012;7: e36825.
- Chen KF, Blyn L, Rothman RE et al.Reverse transcription polymerase chain reaction and electrospray ionization mass spectrometry for identifying acute viral upper respiratory tract infections. Diagn Microbiol Infect Dis2011;69: 179–186.
- Hall TA, Sampath R, Blyn LB et al.Rapid molecular genotyping and clonal complex assignment of Staphylococcus aureus isolates by PCR coupled to electrospray ionization-mass spectrometry. J Clin Microbiol2009;47: 1733–1741.
- Ecker DJ, Sampath R, Li H et al.New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev Mol Diagn2010;10: 399–415.