Abstract
Salmonella enterica pathogenesis is dependent on its ability to enter and replicate inside host cells. Replication occurs inside the Salmonella-containing vacuole (SCV), a vacuolar compartment that is modified by bacterial effectors secreted through the two type III secretion systems (T3SS-1 and T3SS-2). Type III effectors interact with the host cell endocytic pathway to aid replication. We investigated whether Salmonella effector proteins may also interact with the host’s exocytic pathway. A secreted alkaline phosphatase (SEAP) assay indicated three Salmonella effectors inhibited the secretory pathway, although only Salmonella outer protein B (SopB) was confirmed to block exocytosis using a vesicular stomatitis virus glycoprotein-green fluorescent protein (VSVG-GFP) transport assay. The 4-phosphatase activity of SopB was crucial to its effect on exocytosis. The interaction with the secretory pathway could potentially be important for providing replicating Salmonella with nutrients, contributing membrane material necessary for SCV biogenesis, altering antibacterial peptide/protein secretion or manipulating cell surface proteins important in the host response to infection.
Introduction
Salmonella enterica species are among one of the main bacterial causative agents of gastroenteritis in humans and animals, including livestock, and the cause of the systemic disease Typhoid fever. Recent Salmonella outbreaks in the United States were attributed to contaminated peanut butters, cantaloupes and raw vegetables. Salmonella is therefore a re-emerging zoonotic pathogen, with a considerable public health burden and economic cost to society. Consequently, much research has been conducted to understand Salmonella and its pathogenesis, and develop mechanisms by which to eliminate it and prevent transmission.
A characteristic of Salmonella infection is the uptake of the bacterium into non-phagocytic intestinal epithelial cells.Citation1,Citation2 Genes within a region of the Salmonella chromosome known as Salmonella pathogenicity island-1 (SPI-1) encode a type three protein secretion system (T3SS-1),Citation3,Citation4 which drives bacterial invasion. T3SS-1 translocates SPI-1-encoded effector proteins, and effectors encoded elsewhere in the Salmonella chromosome, into the host cell cytoplasm.Citation5 The coordinated actions of these effector proteins, including SipA, SipC, SopB/SigD, SopE, SopE2 and SptP, modulate the host cell actin cytoskeleton to promote internalisation of Salmonella into the non-phagocytic cell in a characteristic membrane ‘ruffle’.Citation6,Citation7,Citation8,Citation9,Citation10
Upon entrance to the host cell, Salmonella exists within a Salmonella-containing vacuole (SCV).Citation2 Salmonella successively modifies the SCV using effectors secreted by T3SS-1Citation11 and the Salmonella pathogenicity island-2 (SPI-2)-encoded type three secretion system (T3SS-2).Citation12 These effector proteins facilitate interactions of the SCV with the host cell endocytic pathway leading to maturation of the SCV,Citation13,Citation14 and also serve to position the SCV towards the Golgi where replication is initiated.Citation15
For successful invasion and replication inside host cells, Salmonella must spatially and temporally regulate its effector proteins. This is particularly important in the case of effectors, such as SopB, which perform more than one role. Salmonella outer protein B (SopB) is a phosphatidylinositol phosphatase,Citation6,Citation16 being composed of a C-terminus that possesses 4-phosphatase motifsCitation16 and a synaptojanin-like 5-phosphatase domain.Citation17 SopB uses its phosphatidylinositol phosphatase activity to (i) activate the Rho GTPases RhoG and Rho to mediate actin-dependent and myosin II-dependent bacterial invasion respectively;Citation18,Citation19 (ii) modulate the phosphatidylinositol composition of the plasma membrane to allow SCV formation;Citation20,Citation21,Citation22 (iii) modulate the phosphatidylinositol composition of the SCV to allow maturation, in part through the recruitment of host proteins such as Rab5 and Vps34Citation23 and sorting nexins-1 and sorting nexins-3,Citation24,Citation25 and avoidance of SCV-lysosome fusion;Citation26 (iv) activate myosin II to place the SCV in a juxtanuclear position;Citation27 (v) activate serine protein kinase AKT to prevent host cell death via apoptosis;Citation28 and (vi) regulate host cell chloride channel function.Citation29,Citation30,Citation31
The multiple roles of SopB are permitted by regulating its activity through its N-terminal domain and its half-life. Ubiquitination of the N-terminal leads to translocation of SopB from the plasma membrane to the SCV, potentially switching the role of SopB from invasion to intracellular survival.Citation32,Citation33 SopB can also bind Cdc42 through its N-terminal,Citation34,Citation35 and this too appears to be important for SopB localisation to the SCV and its spatial regulation.Citation32,Citation35 Although, only translocated by T3SS-1,Citation36,Citation37 SopB is detected in cells for up to 12 h post-invasion.Citation38 This relatively long half-life for an effector protein allows SopB to extend its role from the early stages of invasion through to the intracellular phase of Salmonella survival.
As the SCV locates to a juxtanuclear position, close to the Golgi, it has been proposed that the SCV may also interact with the host’s exocytic/secretory pathway,Citation15 as this occurs with several intracellular pathogens, e.g., Legionella, Brucella and Chlamydia.Citation39 Kuhle et al.Citation40 found that intracellular Salmonella could indeed recruit secretory vesicles from the trans-Golgi network (TGN) to the SCV in a SPI-2-dependent manner; the SPI-2-secreted effectors SseF, SseG and SifA play pivotal roles in post-Golgi vesicle recruitment.Citation40 The interaction of the SCV with the secretory pathway could potentially be important for providing replicating Salmonella with nutrients and/or to provide membrane material to the growing SCV. However, fusion between secretory vesicles and the SCV was not detected by Kuhle et al., although it has now been shown that secretory carrier membrane protein 3 (SCAMP3), which is typically located on the TGN, is also located on Salmonella-induced filaments and Salmonella-induced SCAMP3 tubules, dynamic structures which extend from the SCV membrane.Citation41 However, it is not yet clear how SCAMP3 is recruited to the SCV and whether the exocytic, endocytic or both pathways are used in this process. Additionally, it has been found that enterohemorrhagic and enteropathogenic Escherichia coli (EHEC and EPEC), gastrointestinal pathogens that like Salmonella utilize a T3SS for pathogenesis, both reduce the efficiency of host cell secretion through effector protein Non-LEE-encoded effector A (NleA) which inhibits coat protein II complex (COPII)-dependent protein export from the endoplasmic reticulum.Citation42 Thus, interaction of bacterial pathogens, like Salmonella, with the host cell secretory system may not necessarily be important for maintenance of their replication niche inside the cell but by manipulating the cell's ability to secrete antibacterial peptides/proteins or by manipulating cell surface expression of proteins important in the host response to infection, be important for their survival once they leave the cell or to their extracellular counterparts.
This study examined how Salmonella may interact with the exocytic pathway during the intracellular phase of survival, specifically looking at whether any Salmonella type III effectors were capable of inhibiting secretion. Three Salmonella effectors were identified with the potential to inhibit the host cell secretory pathway. SopB, as one of these effectors, was studied in further detail to determine its mode of action.
Materials and methods
Bacterial strains
Bacterial strains are listed in Table .Citation10,Citation22,Citation43,Citation44 Salmonella serovar Typhimurium strains were routinely cultured in Luria–Bertani broth. For invasion experiments overnight cultures of Salmonella were diluted 1:30 in Luria–Bertani broth with 0.3 M NaCl and grown for 3 h with aeration on a rotating wheel. Antibiotics were used at the following concentrations: ampicillin at 120 µg/mL, streptomycin at 25 µg/mL, kanamycin at 40 µg/mL.
Table 1 Bacterial strains and plasmids used in this study
Construction of plasmids
Plasmids used in this study are listed in Table . Each Salmonella effector gene was cloned into the eukaryotic expression vector pIRES2-EGFP either by subcloning the gene from another plasmid or by amplifying the gene from the Salmonella genome by polymerase chain reaction using Deep Vent DNA polymerase (NEB). All plasmids were verified by restriction digests and sequencing.
The plasmids carrying sopB mutations were created by performing site-directed mutagenesis using the Pfu Turbo DNA polymerase (Stratagene) as per manufacturer’s instructions. The primer for creating the SopB R468A mutation was 5′ GTA AAA GCG GCA AAG ATC TGA CAG GGA TGA TGG ATT CAG AA and for the K530A mutation 5′ GGG CGG GAA ACA AAG TAA TGG CCA ATT TAT CGC CA. All point mutations were verified by sequencing.
Mammalian cell culture and transfections
HeLa cells (CCL-2; ATCC, Manassas, VA, USA) and 293T cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. All cells were maintained at 37 °C and 5% CO2. Cells were transfected with plasmids by using TransIT-express transfection reagent (Mirus) according to the manufacturer’s instructions. 24 h after transfection, cells were infected with Salmonella as indicated.
Secreted alkaline phosphatase (SEAP) assay
293T cells were plated in 24-well dishes at 1×105 cells/well, allowing three wells per effector. After an overnight incubation, the cells were cotransfected with plasmids encoding corresponding proteins and the plasmid encoding the SEAP. 24 h later, cells were washed, and fresh serum-free tissue culture medium was added. SEAP activity was measured in triplicate wells 7 h later using the Phospha-Light System (Applied Biosciences, Grand Island, NY, USA) following the manufacturer’s instructions. Data are presented as a secretion index, which is the ratio of SEAP activity detected in the culture medium to the cell-associated SEAP activity. Total SEAP inside cells was not affected by coexpression of any of the effector plasmids.
Vesicular stomatitis virus glycoprotein (VSVG) transport assay
293T cells were plated in 100 mm dishes at 6.5×105 cells/dish. After an overnight incubation, cells were cotransfected, using calcium phosphate, with plasmids encoding the indicated proteins and a plasmid encoding a ts045-VSVG-GFP plasmid. 4 h after transfection the cells were washed and placed overnight at 40 °C. Next, 100 µg/mL cycloheximide was added to each dish which was then transferred to 32 °C for the appropriate time period. Cells were then transferred to ice, and washed twice with ice-cold PBS before being collected. Cells were lysed with lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 1:100 protease inhibitor cocktail (Calbiochem, Billerica, MA, USA)) on ice for 10 min, before the lysate was collected in an Eppendorf tube. After protein concentration determination using the Bradford assay (Bio-Rad, Hercules, CA, USA), equal amounts of protein from all samples were treated or mock-treated with Endoglycosidase H (Roche, Indianapolis, IN, USA) according to the manufacturer’s instructions. Sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer was added to the samples, boiled for 10 min and subjected to electrophoresis on an 8% SDS–PAGE gel. Samples were transferred to nitrocellulose membrane for immunoblot analysis using anti-GFP and anti-actin antibodies.
Data analysis
All statistical tests were performed using GraphPad Prism version 6. One-way analysis of variance was performed with three or more data sets, with Dunnett’s multiple comparison test being applied post hoc.
Results
Salmonella effectors inhibit the host cell secretory pathway
To determine whether Salmonella, like EHEC and EPEC which also use a T3SS in their virulence, may inhibit the host cell exocytic pathway during the infection process, known Salmonella effector proteins were screened for inhibition of the secretory pathway. A plasmid encoding a secreted form of human placental alkaline phosphatase (SEAP) was cotransfected into 293T cells with a plasmid encoding a Salmonella effector protein. The ratio of SEAP activity detected in the culture medium to the cell-associated SEAP activity (secretion index) was then measured for each Salmonella effector and for a Legionella effector (Lpg2556) known to inhibit exocytosis and which acted as a positive control (Dr Zhao-Qing Luo, personal communication). Of the 30 Salmonella effectors tested, SopB, PipB2 and SspH2 appeared to affect the secretion of SEAP (Figure ).
Figure 1 Salmonella effectors inhibit host cell exocytosis. A SEAP assay was performed on 293T cells transfected with plasmids encoding the indicated type III effectors. The protein secretion index is displayed. Values are the mean±SD for three independent experiments. Asterisks indicate a statistical significant difference compared to the empty vector.

SopB requires its 4-phosphatase activity to inhibit the secretory pathway
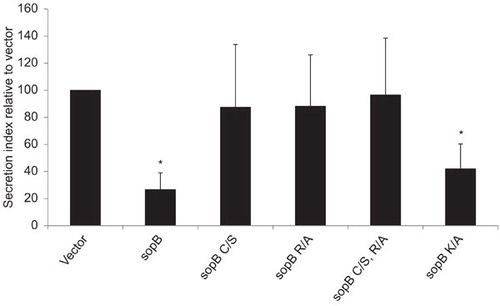
Among the three Salmonella type III effectors tested, SopB appeared to be the most potent in inhibiting SEAP secretion. We decided to test whether the phosphatidylinositol phosphatase activity of SopB was required for this effect. Point mutations were made in catalytic residues known to be essential for the phosphatase activity of SopB. Mutation of the 420 cysteine (C420S; C/S) or mutation of the 468 arginine (R468A; R/A), both of which are conserved between E. coli and Shigella IpgD proteins and human inositol phosphatase sequences, have been shown to be essential for 4-phosphatase activity.Citation6,Citation16 The lysine residue at position 530 lies within the putative synaptojanin-homology domain that is essential for the 5-phosphatase activity of SopB.Citation17 When examined in the SEAP secretion assay, mutation of either C420 or R468 restores SEAP secretion to levels comparable to the empty vector indicating the 4-phosphatase region but not the 5-phosphatase domain is necessary for SopB inhibition of exocytosis (Figure ).
Figure 2 The 4-phosphatase activity is necessary for SopB inhibition of exocytosis. SEAP assays were performed on 293T cells transfected with pEGFP-N2 plasmids encoding the indicated forms of SopB. sopB mutation abbreviations: C460S (C/S), R468A (R/A), and K530A (K/A). The protein secretion index is displayed. Values are the mean±SD for three independent experiments. Asterisks indicate a statistical significant difference compared to vector.
Role of phosphatidylinositol 4-phosphate (PI(4)P) in SopB-mediated inhibition of exocytosis
Since the 4-phosphatase activity of SopB appears critical to its ability to inhibit exocytosis, we investigated whether overexpression of specific phosphoinositide kinases or phosphatases could restore SopB-mediated inhibition of exocytosis, and thereby aid identification of the pathways that may be used to inhibit secretion. Cells were cotransfected with pSEAP, vector or SopB and then one of the phosphoinositide kinases or phosphatases listed (Figure ). Exocytosis in cells coexpressing SopB and phosphatidylinositol 4-kinase type IIβ (PI4K IIβ) was not statistically different to the control cells. PI4K IIβ is a 4-kinase which appears to regulate the PI(4)P synthesis required for several cellular processes, most notably secretion.Citation45 Since SopB relies on its 4-phosphatase activity to inhibit exocytosis it may suggest 4-kinase activity can rescue the SopB-mediated defect. It is also interesting to note that PI4K IIβ significantly increases the secretion of cells transfected with the vector. Thus, it appears PI(4)P levels may be important in the control of exocytosis.
Figure 3 PI4K IIβ prevents SopB-mediated exocytosis inhibition. SEAP assays were performed on 293T cells transfected with pSEAP, either the pEGFP-N2 or pEGFP-N2-SopB plasmids, and one of the phosphoinositol kinase or phosphatases listed. The protein secretion index is displayed. Values are the mean±s.d. for three independent experiments. Asterisks indicate a statistical significant difference compared to cells transfected with vector only.
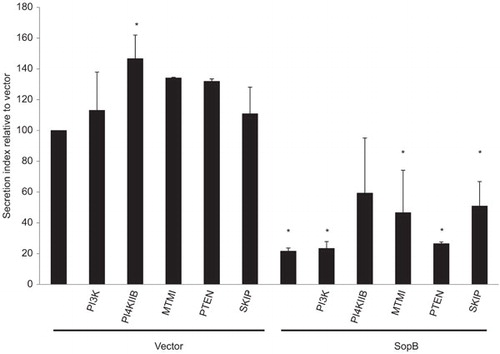
The phosphatidylinositol kinase PI3K had no affect on SopB-mediated inhibition, and therefore it is perhaps no surprise that PTEN, a 3-phosphatase, also had no affect since both enzymes play a role in the interconversion of phosphatidylinositol 4,5-biphosphate (PI(4,5)P2) and phosphatidylinositol (3,4,5)-triphosphate (PI(3,4,5)P3). The PI(3)P phosphatase, MTMI (myotubularin I), and the PI(3)P inhibitors wortmannin and LY294002 were also found to have no effect (data not shown) and therefore PI(3)P does not appear to be involved in exocytosis inhibition which may be expected since PI(3)P is typically associated with early endosomes. The 5-phosphatase, skeletal muscle and kidney enriched inositol phosphatase (SKIP), appeared to in part restore exocytosis but the result was not statistically significant. Since one of the reactions catalyzed by SKIP is the conversion of PI(4,5)P2 to PI(4)P and PI4K IIβ, which also had an effect in this assay, regulates PI(4)P, it is possible that PI(4)P is the key intermediate in SopB-mediated exocytosis inhibition. This is more probable given that PI(4)P is a prominent phosphatidylinositol in the Golgi membrane, particularly the TGN, and plays an important role in the secretory pathway.
As regulation of PI(4)P concentrations at the TGN are used to regulate secretion,Citation46 a FAPP1-PH-GFP probe, specific to PI(4)P,Citation47 was used in HeLa cells during Salmonella infection to determine whether PI(4)P levels were altered and may explain the effect of SopB. No change in PI(4)P levels were detected (data not shown). However, since detecting phosphatidylinositols and their fluctuating concentrations is not always accurate by such probes, we cannot rule out that PI(4)P is one of the mediators of SopB secretion inhibition.
SopB delays VSVG-GFP between the endoplasmic reticulum (ER) and Golgi
To determine at which stage of the secretory pathway SopB may act, we employed a temperature-sensitive variant of vesicular stomatitis virus glycoprotein tagged with green fluorescent protein (ts045-VSVG-GFP). At 40 °C, ts045-VSVG-GFP misfolds and is retained in the ER, but upon shifting cells to 32 °C, the protein refolds and resumes transport to the Golgi and then to the plasma membrane.Citation48,Citation49 Transport of VSVG from the ER to the Golgi is accompanied by a modification of glycosylation on the protein. The high-mannose glycosylation present on the ER form of ts045-VSVG-GFP is sensitive to the glycosidase Endoglycosidase H (Endo H), while glycan modification in the Golgi confers resistance to Endo H digestion.Citation48 By measuring the sensitivity of VSVG to Endo H at certain time periods after a shift from 40 °C to 32 °C the localization of VSVG within the secretory pathway can be determined and whether it is affected by a protein/drug of interest.
To monitor the effect of SopB on VSVG trafficking, 293T cells were cotransfected with ts045-VSVG-GFP and either vector, SopB or SopBc/s. Cells were shifted to 40 °C overnight before cycloheximide was added to inhibit further protein synthesis and cells were shifted to 32 °C for various lengths of time to monitor VSVG transport. The vector- and SopBc/s-transfected cells exhibited similar behaviour, namely, that 1 h after the temperature shift the majority of the VSVG protein is Endo H-resistant and therefore has reached the Golgi (Figure ). In contrast, cells transfected with SopB, show VSVG sensitivity to Endo H up to 2 h after the temperature shift, indicating that, in these cells, some of the VSVG protein remains localized to the ER (). Therefore, in the presence of SopB the transport of VSVG between the ER and Golgi is inhibited or delayed. The phosphatidylinositol phosphatase activity of SopB is important for this delay in transport between the ER and Golgi.
Figure 4 SopB delays transport of VSVG between the ER and Golgi. Immunoblot analysis of VSVG in cells cotransfected with ts045VSVG-GFP and either pEGFP-N2 (control), pEGFP-N2-SopB (SopB) or pEGFP-N2-SopBc/s (SopBc/s) at various time points after the shift to 32 °C. Endoglycosidase H (Endo H)-treated (+) and mock-treated (−) samples are indicated. The mobility of the Endo H-resistant (R; upper band) and -sensitive (S; lower band) forms of the protein is indicated. The boxes in B and C indicate the different Endo H sensitivity pattern exhibited by SopB-transfected cells. Actin immunoblotting is included as a loading control.

Determining whether SopB inhibition of ER to Golgi transport occurs during Salmonella infection
Although SopB has the potential to inhibit transport between the ER and Golgi, this may not occur during Salmonella infection, for instance the spatial regulation of SopB or the presence of another effector protein may inhibit this activity. We therefore sought to determine if the SopB-dependent inhibition of secretion observed in transfected cells was observed during Salmonella infection. We tried to measure the secretion index of the SEAP reporter protein in 293Tcells infected with wild type and sopB mutant Salmonella, however Salmonella proved cytotoxic to 293T cells which prevented accurate SEAP activity readings being taken. We therefore, performed the VSVG transport assay in cells that had been infected with Salmonella. For these experiments, cells were maintained at 40 °C overnight prior to infection and no cycloheximide treatment was performed. Once cells were infected with Salmonella the cells were either immediately shifted to 37 °C, which was found to permit VSVG transport and would not affect the Salmonella, to monitor VSVG transport during infection or were maintained at 40 °C until 2 or 8 h post-invasion before the shift. In each instance wild type and sopB mutant Salmonella exhibited similar behaviour and neither affected the transport of VSVG (Supplementary information Figure S1).
Discussion
For a bacterium to have an intracellular lifestyle, it must have the ability to do two things. First, the bacterium must be able to hide from or combat any anti-microbial defences the host cell possesses. Second, it must utilize the available cellular resources to ensure survival, and if possible, replication. Salmonella takes care of the first objective by using its T3SS-1 and T3SS-2 effectors to modulate the SCV membrane so that it avoids fusing with lysosomes or does so in a controlled fashion.Citation50,Citation51,Citation52 It is currently less clear how Salmonella supports its replication.
It is known that the SCV undergoes a series of interactions with the host endocytic pathwayCitation53,Citation54 and this may in part account for the increase in SCV membrane and the way in which the nutrients required for bacterial replication are obtained. However, other intracellular bacterial pathogens favour the exocytosis pathway to obtain the materials they need for replication. For example, Legionella tethers host-derived transitional ER-derived vesicles to its vacuolar compartment (Legionella-containing vacuole), which subsequently fuse with the Legionella-containing vacuole.Citation55,Citation56 Coxiella employs a similar mechanism,Citation57 while the intermediate Brucella-containing vacuoles of Brucella interact with and subsequently fuse with the actual ER to generate ER-derived, replicative Brucella-containing vacuoles.Citation58,Citation59 There is growing evidence that Salmonella may also utilize the secretory pathway to maintain and expand its replicative niche. The first evidence came in 2003, when it was found that not only do SCV accumulate near to the Golgi apparatus but that inhibition of ADP-ribosylation factor 1, a small GTPase required for the formation of COPI-coated vesicles that traffic between the ER and Golgi, inhibited Salmonella replication.Citation15 Kuhle et al.Citation40 have since found secretory vesicles from the TGN recruited to the SCV in a SPI-2-dependent manner, and a protein, SCAMP3, typically located at the TGN has been identified on Salmonella-induced filaments and Salmonella-induced SCAMP3 tubules.Citation41 However, direct interaction of secretory vesicles with the TGN still eludes visualisation, and thus while it appears likely that Salmonella interacts with the secretory pathway there is little direct evidence to indicate Salmonella, like its bacterial counterparts, uses this route to secure the materials it requires for replication.
The data presented here explores the alternative view that Salmonella may in fact use the secretory pathway in a different manner, that is, to disrupt secretion. In doing so, Salmonella may create a pool of material close to the SCV that could be harvested to support replication. Alternatively, inhibition of exocytosis may prevent particular proteins reaching the cell surface, including those that could alert the immune system to the infection. Several Salmonella effectors were identified that could inhibit exocytosis, as measured by the SEAP assay. Interestingly, one of these effectors was SopB, which through its phosphatidylinositol phosphate activity has been associated with manipulating membrane dynamics to allow fission of the SCV from the plasma membraneCitation20 and determine subsequent interactions of the SCV with host proteins.Citation23,Citation24 The 4-phosphatase activity of SopB was found to play a critical role in the ability of SopB to inhibit exocytosis and one hypothesis that was explored was whether SopB used this activity to manipulate PI(4)P levels at the TGN. PI(4)P accumulates in regions of the TGN and is important to the creation and fission of secretory vesicles.Citation46 No direct evidence was obtained for SopB manipulating PI(4)P levels at the TGN and this is supported by the data obtained from the VSVG transport assay that indicates SopB acts earlier in the secretory pathway, i.e., on the transport pathway from the ER to Golgi. How exactly SopB acts at this stage of the pathway was not determined. It has been reported that SopB affects the production of the cytokines that cause inflammation of the gut, a characteristic of Salmonella infection.Citation7,Citation60,Citation61,Citation62 Further studies are required to test whether SopB also plays a role in the secretion process.
Currently, the only other bacteria found to inhibit exocytosis are EHEC and EPEC, who use effector protein NleA to inhibit COPII-dependent protein export from the endoplasmic reticulum.Citation42 The reason for this inhibition is, as yet, unknown. It is interesting that EHEC, EPEC and Salmonella, which all appear to inhibit exocytosis, all possess a T3SS, and this may perhaps allude to a unique mechanism used by these bacteria to manipulate the host cell. The activity of the EHEC and EPEC effector, NleA, was partially determined after examining its location in the cell after translocation by the T3SS.Citation42,Citation63 NleA was found to colocalize with mannosidase II and thus determined to localize to the Golgi.Citation63 SopB has predominantly been studied at the plasma membrane and early SCV membrane but this does not preclude the ability of SopB to associate with other membranes, such as the ER or Golgi, at other time points, and given that the SCV eventually orientates towards the Golgi, it may not need to, to have an effect on the secretory pathway, especially if its effects are mediated by changes in phosphatidylinositol concentrations. SopB has a relatively long half-lifeCitation38 and thus it could easily have multiple sites of activity during the infectious process, and which may play an important part in allowing Salmonella to successfully transition from its invasion to intracellular phase. This will require further study in the future to determine how exactly SopB affects exocytosis. To conclude, this work may reveal yet another important role of SopB in Salmonella infection and perhaps another layer to the carefully orchestrated manipulation of the cell, and the host, by this bacterium.
Supplementary information Figure S1
Download PDF (473.3 KB)We would like to thank Rajdeep Borman (Department of Biological Sciences, Purdue University, West Lafayette, IN, USA) for help with creating the plasmids carrying sopB mutations, and Heather Piscatelli (Department of Biological Sciences, Purdue University, West Lafayette, IN, USA) for the phosphatidylinositol kinase and phosphatase plasmids. This work was supported by the NIH grant NO AI049978.
Supplementary Information for this article can be found on Emerging Microbes and Infections’s website (http://www.nature.com/EMI).
Supplementary Material
Supplementary Information for this article can be found on the Emerging Microbes & Infections website (http://www.nature.com/emi)
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol1967; 50: 109–136.
- Finlay BB, Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie1988; 70: 1089–1099.
- Galán JE, Curtiss R 3rd. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA1989; 86: 6383–6387.
- Groisman EA, Ochman H. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J1993; 12: 3779–3787.
- Galán JE. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol1996; 20: 263–271.
- Zhou D, Chen LM, Hernandez L, Shears SB, Galán JE. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol Microbiol2001; 39: 248–259.
- Hardt WD, Chen LM, Schuebel KE, Bustelo XR, Galán JE. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell1998; 93: 815–826.
- Kaniga K, Uralil J, Bliska JB, Galán JE. A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol Microbiol1996; 21: 633–641.
- McGhie EJ, Hayward RD, Koronakis V. Cooperation between actin-binding proteins of invasive Salmonella: SipA potentiates SipC nucleation and bundling of actin. EMBO J2001; 20: 2131–2139.
- Zhou D, Mooseker MS, Galán JE. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science1999; 283: 2092–2095.
- Steele-Mortimer O, Brumell JH, Knodler LA, Meresse S, Lopez A, Finlay BB. The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell Microbiol2002; 4: 43–54.
- Hensel M, Shea JE, Waterman SRet al. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol1998; 30: 163–174.
- Beuzon CR, Meresse S, Unsworth KEet al. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J2000; 19: 3235–3249.
- Deiwick J, Salcedo SP, Boucrot Eet al. The translocated Salmonella effector proteins SseF and SseG interact and are required to establish an intracellular replication niche. Infect Immun2006; 74: 6965–6972.
- Salcedo SP, Holden DW. SseG, a virulence protein that targets Salmonella to the Golgi network. EMBO J2003; 22: 5003–5014.
- Norris FA, Wilson MP, Wallis TS, Galyov EE, Majerus PW. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc Natl Acad Sci USA1998; 95: 14057–14059.
- Marcus SL, Wenk MR, Steele-Mortimer O, Finlay BB. A synaptojanin-homologous region of Salmonella typhimurium SigD is essential for inositol phosphatase activity and Akt activation. FEBS Lett2001; 494: 201–207.
- Patel JC, Galan JE. Differential activation and function of Rho GTPases during Salmonella–host cell interactions. J Cell Biol2006; 175: 453–463.
- Hanisch J, Kolm R, Wozniczka M, Bumann D, Rottner K, Stradal TE. Activation of a RhoA/myosin II-dependent but Arp2/3 complex-independent pathway facilitates Salmonella invasion. Cell Host Microbe2011; 9: 273–285.
- Terebiznik MR, Vieira OV, Marcus SLet al. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol2002; 4: 766–773.
- Hernandez LD, Hueffer K, Wenk MR, Galán JE. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science2004; 304: 1805–1807.
- Dai S, Zhang Y, Weimbs T, Yaffe MB, Zhou D. Bacteria-generated PtdIns(3)P recruits VAMP8 to facilitate phagocytosis. Traffic2007; 8: 1365–1374.
- Mallo GV, Espina M, Smith ACet al. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J Cell Biol2008; 182: 741–752.
- Bujny MV, Ewels PA, Humphrey S, Attar N, Jepson MA, Cullen PJ. Sorting nexin-1 defines an early phase of Salmonella-containing vacuole-remodeling during Salmonella infection. J Cell Sci2008; 121( Pt 12) 2027–2036.
- Braun V, Wong A, Landekic M, Hong WJ, Grinstein S, Brumell JH. Sorting nexin 3 (SNX3) is a component of a tubular endosomal network induced by Salmonella and involved in maturation of the Salmonella-containing vacuole. Cell Microbiol2010; 12: 1352–1367.
- Bakowski MA, Braun V, Lam GYet al. The phosphoinositide phosphatase SopB manipulates membrane surface charge and trafficking of the Salmonella-containing vacuole. Cell Host Microbe2010; 7: 453–462.
- Wasylnka JA, Bakowski MA, Szeto Jet al. Role for myosin II in regulating positioning of Salmonella-containing vacuoles and intracellular replication. Infect Immun2008; 76: 2722–2735.
- Steele-Mortimer O, Knodler LA, Marcus SLet al. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J Biol Chem2000; 275: 37718–37724.
- Feng Y, Wente SR, Majerus PW. Overexpression of the inositol phosphatase SopB in human 293 cells stimulates cellular chloride influx and inhibits nuclear mRNA export. Proc Natl Acad Sci USA2001; 98: 875–879.
- Mason D, Mallo GV, Terebiznik MRet al. Alteration of epithelial structure and function associated with PtdIns(4,5)P2 degradation by a bacterial phosphatase. J Gen Physiol2007; 129: 267–283.
- Bertelsen LS, Paesold G, Marcus SL, Finlay BB, Eckmann L, Barrett KE. Modulation of chloride secretory responses and barrier function of intestinal epithelial cells by the Salmonella effector protein SigD. Am J Physiol Cell Physiol2004; 287: C939–C948.
- Knodler LA, Winfree S, Drecktrah D, Ireland R, Steele-Mortimer O. Ubiquitination of the bacterial inositol phosphatase, SopB, regulates its biological activity at the plasma membrane. Cell Microbiol2009; 11: 1652–1670.
- Patel JC, Hueffer K, Lam TT, Galan JE. Diversification of a Salmonella virulence protein function by ubiquitin-dependent differential localization. Cell2009; 137: 283–294.
- Aleman A, Rodriguez-Escudero I, Mallo GV, Cid VJ, Molina M, Rotger R. The amino-terminal non-catalytic region of Salmonella typhimurium SigD affects actin organization in yeast and mammalian cells. Cell Microbiol2005; 7: 1432–1446.
- Rodriguez-Escudero I, Ferrer NL, Rotger R, Cid VJ, Molina M. Interaction of the Salmonella Typhimurium effector protein SopB with host cell Cdc42 is involved in intracellular replication. Mol Microbiol2011; 80: 1220–1240.
- Galyov EE, Wood MW, Rosqvist Ret al. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol1997; 25: 903–912.
- Hong KH, Miller VL. Identification of a novel Salmonella invasion locus homologous to Shigella ipgDE. J Bacteriol1998; 180: 1793–1802.
- Drecktrah D, Knodler LA, Galbraith K, Steele-Mortimer O. The Salmonella SPI1 effector SopB stimulates nitric oxide production long after invasion. Cell Microbiol2005; 7: 105–113.
- Salcedo SP, Holden DW. Bacterial interactions with the eukaryotic secretory pathway. Curr Opin Microbiol2005; 8: 92–98.
- Kuhle V, Abrahams GL, Hensel M. Intracellular Salmonella enterica redirect exocytic transport processes in a Salmonella pathogenicity island 2-dependent manner. Traffic2006; 7: 716–730.
- Mota LJ, Ramsden AE, Liu M, Castle JD, Holden DW. SCAMP3 is a component of the Salmonella-induced tubular network and reveals an interaction between bacterial effectors and post-Golgi trafficking. Cell Microbiol2009; 11: 1236–1253.
- Kim J, Thanabalasuriar A, Chaworth-Musters Tet al. The bacterial virulence factor NleA inhibits cellular protein secretion by disrupting mammalian COPII function. Cell Host Microbe2007; 2: 160–171.
- Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature1981; 291: 238–239.
- Zhou D, Mooseker MS, Galán JE. An invasion-associated Salmonella protein modulates the actin-bundling activity of plastin. Proc Natl Acad Sci USA1999; 96: 10176–10181.
- Martin TF, Loyet KM, Barry VA, Kowalchyk JA. The role of PtdIns(4,5)P2 in exocytotic membrane fusion. Biochem Soc Trans1997; 25: 1137–1141.
- Blagoveshchenskaya A, Cheong FY, Rohde HMet al. Integration of Golgi trafficking and growth factor signaling by the lipid phosphatase SAC1. J Cell Biol2008; 180: 803–812.
- Dowler S, Currie RA, Campbell DGet al. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem J2000; 351( Pt 1): 19–31.
- Bergmann JE. Using temperature-sensitive mutants of VSV to study membrane protein biogenesis. Methods Cell Biol1989; 32: 85–110.
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature1997; 389: 81–85.
- Hashim S, Mukherjee K, Raje M, Basu SK, Mukhopadhyay A. Live Salmonella modulate expression of Rab proteins to persist in a specialized compartment and escape transport to lysosomes. J Biol Chem2000; 275: 16281–16288.
- Drecktrah D, Knodler LA, Howe D, Steele-Mortimer O. Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system. Traffic2007; 8: 212–225.
- McGourty K, Thurston TL, Matthews SA, Pinaud L, Mota LJ, Holden DW. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science2012; 338: 963–967.
- Steele-Mortimer O, Meresse S, Gorvel JP, Toh BH, Finlay BB. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol1999; 1: 33–49.
- Meresse S, Steele-Mortimer O, Finlay BB, Gorvel JP. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J1999; 18: 4394–4403.
- Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol2002; 4: 945–954.
- Robinson CG, Roy CR. Attachment and fusion of endoplasmic reticulum with vacuoles containing Legionella pneumophila. Cell Microbiol2006; 8: 793–805.
- Campoy EM, Zoppino FC, Colombo MI. The early secretory pathway contributes to the growth of the Coxiella-replicative niche. Infect Immun2011; 79: 402–413.
- Celli J, Salcedo SP, Gorvel JP. Brucella coopts the small GTPase Sar1 for intracellular replication. Proc Natl Acad Sci USA2005; 102: 1673–1678.
- Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med2003; 198: 545–556.
- Chen LM, Hobbie S, Galán JE. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science1996; 274: 2115–2118.
- Figueiredo JF, Lawhon SD, Gokulan Ket al. Salmonella enterica Typhimurium SipA induces CXC-chemokine expression through p38MAPK and JUN pathways. Microbes Infect2009; 11: 302–310.
- Bruno VM, Hannemann S, Lara-Tejero M, Flavell RA, Kleinstein SH, Galan JE. Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog2009; 5: e1000538.
- Gruenheid S, Sekirov I, Thomas NAet al. Identification and characterization of NleA, a non-LEE-encoded type III translocated virulence factor of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol2004; 51: 1233–1249.