Abstract
To explore early biomarkers for establishing more sensitive safety evaluation assays in preclinical settings that determine the potential risks during the application of microbicide candidates, three representative microbicide candidates (cellulose sulphate, nonoxynol-9 and tenofovir), whose safety profiles have been well established in clinical trials, were included to gauge the sensitivities of different assays. Both mouse models and cell lines were employed to determine the sensitivities. The recruitment of immune cells at topical mucosal sites and the upregulation of HIV receptor/coreceptors in vitro were identified as highly sensitive biomarkers of the impact of microbicide candidates. Our data suggest that different evaluations/assays have their inherent sensitivities, and at least one assay from each sensitivity level should be included in the safety evaluation algorithm.
Introduction
Microbicides, topically applied antimicrobial products, may represent one of the most promising preventive interventions for protecting women and men who have sex with men from the acquisition of human immunodeficiency virus (HIV)-1.Citation1 However, phase 2B/3 efficacy trials, including those for nonoxynol-9 (N9), C31G, BufferGel, cellulose sulphate (CS), Carraguard and PRO2000, indicate that the tested substances have failed to significantly reduce HIV infection incidence, or even increased the risk of HIV acquisition, such as N9 and CS.Citation2 The failure of those microbicide candidates has highlighted the inadequacies of the current preclinical evaluation systems and has also encouraged researchers to investigate the potential mechanisms accounting for the enhancement of HIV infection and to explore more stringent preclinical assessments for microbicide safety.
The safety concerns related to microbicide candidates on topical mucosa include not only the destruction of epithelial barriers, but also the aberrant activation of localized immunity.Citation1,Citation3 Inflammatory cytokines elicited by the microbicide candidates are frequently used as biomarkers to evaluate the activation of topical mucosal immunity.Citation4,Citation5 However, the complexity of the microenvironment in the vagina and the varied baseline production of inflammatory cytokines in different physiological conditionsCitation6 render it difficult to determine topical immune status, and a feasible model with high sensitivity for evaluating the impact of microbicide candidates on local mucosal immunity remains to be established and validated.
In a previous study,Citation7 we characterized the soluble selectin levels in vaginal fluids during cervicovaginal inflammation induced by irritating compounds in a murine model, and we observed that E-selectin and P-selectin correlated better than monocyte chemotactic protein-1 and interleukin (IL)-6 with the duration and severity of mucosal inflammation triggered by detergent-based pro-inflammatory compounds including N9, benzalkonium chloride and sodium dodecyl sulphate. The study demonstrated that soluble adhesion molecules might be used as biomarkers of mucosal inflammation in addition to pro-inflammatory cytokines and chemokines. However, our previous work only characterized surfactant-based compounds, which cause epithelial damage, and we do not know whether soluble forms of selectins, which most likely arise by shedding or cleavage from the cell membrane, are suitable markers for other compounds that are toxic through other mechanisms.
In the present study, three microbicide candidates, N9, CS and tenofovir (TFV), which represent surfactant-based toxic compounds, non-surfactant based toxic compounds and safe compounds, respectively, were employed to investigate the effect of microbicide candidates on the recruitment of immune cells at topical mucosal sites and to establish a new safety evaluation system.
Our data demonstrated that the administration of CS and N9 but not TFV resulted in the induction of topical mucosal inflammation; however, expression of soluble adhesion molecule did not increase significantly following CS treatment, a substance that does not cause obvious epithelial damage. Intriguingly, we observed that the recruitment of immune cells, including HIV-targeted cells at, topical mucosal sites in vivo and the up-regulation of activation markers and homing receptors in vitro could occur in an earlier phase compared with the increase of cytokines and the disruption of epithelial barrier. Therefore, these immune responses might serve as sensitive and earlier biomarkers for the safety evaluation of microbicide candidates.
Materials and methods
Reagent
N9 and CS were purchased from Sciencelab.com, Inc. (Houston, TX, USA). TFV was purchased from Molekula Limited (Gillingham, Dorset, UK).
For the in vitro cell experiments, the reagents described above were dissolved in sterile phosphate-buffered saline (PBS) (pH=7.4) at the indicated concentrations. For mouse vaginal application, the dissolved reagents were formulated with 1.5% hydroxyethyl cellulose (HEC) (Sigma-Aldrich, St Louis, MO, USA). All of the formulated gels were adjusted to pH 4.5 and applied intravaginally.
Cell culture
A human colorectal epithelial cell line, Caco-2, was kindly provided by Dr Paul Zhou (Institute Pasteur of Shanghai) and was cultured in Dulbecco’s modified Eagle medium. The human T-cell leukaemia cell-line Jurkat and the human acute monocytic leukaemia cell-line THP-1 were purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences and cultured in RPMI 1640. All cell cultures were supplemented with 10% foetal bovine serum, 2 mmol/L L-glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin at 37 °C/5% CO2.
In situ immunofluorescent assay for tight junction proteins
Caco-2 cells were cultured in 24-well plates for the formation of intact epithelial layers. After 6 h of treatment with HEC, N9 or CS at the indicated concentrations, the culture supernatant was discarded, and the epithelial cell layers were fixed with 3% paraformaldehyde for 30 min at room temperature. The fixed cells were further permeabilized with 0.05% Triton X-100 for 5 min on ice and then subjected to staining for the tight junction protein occludin with 10 µg/mL rabbit anti-occludin antibodies (Invitrogen, Grand Island, NY, USA) and subsequently with Alexa Fluor 488-goat anti-rabbit IgG secondary antibodies (1∶1000 dilution; Invitrogen). The final images were visualized by using an Axiovert 200 inverted fluorescent microscope (Carl Zeiss Inc., Oberkochen, Germany).
Apoptosis assay
Annexin V binding assays were performed using a PE Annexin V Apoptosis Detection Kit I (BD Biosciences, San Diego, CA, USA). Early apoptotic epithelial cells (Caco-2) were observed with flow cytometry and the use of fluorescein-labelled Annexin V. Analysis was conducted on ≥10 000 gated viable lymphocytes based on fluorescence minus one controls.
Safety evaluation in in vivo mouse model
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Shanghai Public Health Clinical Center. Six- to eight-week-old pathogen-free outbred BALB/c female mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). All mice were hormonally synchronized with 2 mg of medroxyprogesterone acetate (Sigma-Aldrich) 5 days before the experiments.Citation8 Mice were separately treated with 40 µL of gel formulated with the indicated microbicide candidates (40 mg/mL or 0.4 mg/mL N9, 10 mg/mL TFV, 60 mg/mL or 0.6 mg/mL CS or placebo (1.5% HEC), delivered intravaginally with a single dose. The mouse cervicovaginal lavages (CVLs) were collected at the indicated time point post-application by washing the mouse cervicovaginal area with 200 µL of sterile saline. The CVLs were stored at −80 °C for subsequent cytokine quantification assays.Citation8 12 h after the treatment, the mice were sacrificed, and their vaginal tissues were collected for histological examination as described below. To determine the recruitment of immune cells at the topical mucosal sites, the mice were killed at the same time points as for the cytokine quantification assay after receiving one dose of the indicated microbicide candidates, and then the vaginal tissues were collected for preparation of single mononuclear cells (MNCs) as described below.
Histopathological examination
Formalin-fixed excised vaginal tissues were embedded within paraffin and transversely sectioned with a microtome. The slides were stained with haematoxylin–eosin and subjected to a blind evaluation for epithelial cell disruption and inflammatory responses.
Cytokine quantification assay
Cytokines from CVLs were determined by using a mouse Th1/Th2/Th17 cytokine Cytometric Bead Array kit (BD Biosciences) and were analysed using a FACAria flow cytometer (BD Biosciences).
Determination of soluble E- and P-selectins by enzyme-linked immunosorbent assay (ELISA)
Soluble selectins in mouse CVL were measured using ELISAs run on 1∶3 diluted mouse CVL. Soluble E- and P-selectin ELISA kits were purchased from Boster Biological Technology, Ltd (China).
Preparation of genital tract mononuclear cells
For isolation of MNCs, the uterus and genital tract, including the cervix, were removed. The tissues from five mice in the same group were pooled and disrupted with scissors (approximately 1–3 mm slices) and then incubated with RPMI 1640 containing 10% bovine calf serum and 0.5 mg/mL collagenase type V (Sigma-Aldrich) at 37 °C with fresh medium replacement every 30 min three times. The digested tissues were centrifuged at 600 g at 25 °C for 20 min in a 40%/75% discontinuous Percoll gradient (GE Healthcare Life Sciences, Chalfont St Giles, UK). Cells residing between the 75% and 40% Percoll layers were recovered as MNCs and resuspended in complete RPMI 1640 at 4 °C for subsequent use.
In vivo and in vitro evaluation of recruitment of immune cells and activation
For in vivo evaluation, the isolated MNCs from mouse tissues were stained with surface antibodies conjugated to different fluorochromes for 30 min at 4 °C, including anti-CD3-Pacific blue, anti-CD4-APC/Cy7, anti-CD8-FITC, anti-CD11c-PE/Cy7, anti-CD335 (NKp46)-PE, anti-CD14-PerCP/Cy5.5 and anti-TCRγδ-APC for cell subset phenotyping.
For in vitro evaluation, CS-treated Jurkat T cells or THP-1 cells were stained with anti-human monoclonal antibodies for evaluating the expression of HIV entry receptors (anti-CD4-PE, anti-CXCR4-APC, anti-CCR5-APC-Cy7, anti-α4-PE/Cy5 and anti-β7-PE) and activation markers (anti-CD38-PE-Cy7, anti-CD69-APC-Cy7, anti-CD86-PE-Cy5 and anti-HLA-DR-Pacific blue) by flow cytometry at indicated time points (0 h, 0.5 h, 1 h, 3 h and 6 h). All antibodies were purchased from Biolegend Inc. (San Diego, CA, USA). Analysis was conducted on ≥10 000 gated viable lymphocytes based on fluorescence minus one controls.
Statistical analysis
Data are presented as the mean±standard deviation (SD). The statistical significance between different groups was calculated with the t-test using GraphPad Prism version 5.0 software (San Diego, CA, USA). Values of P<0.05 were considered as statistically significant.
Results
Soluble adhesion molecules were not suitable as biomarkers for evaluating ‘non-surfactant’ microbicides such as CS
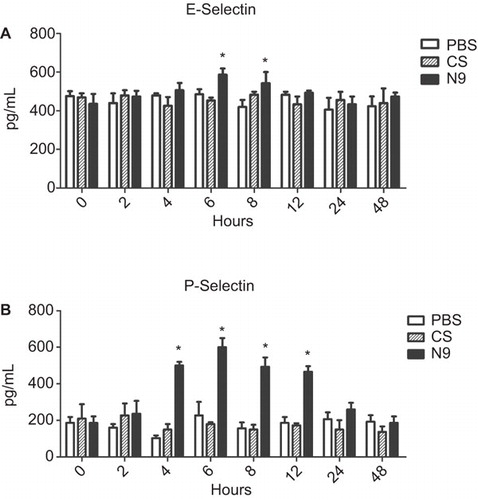
In the present study, we evaluated the soluble P- and E-selectin levels in vaginal fluids induced by CS, a failed microbicide candidate, which increased the risk of HIV acquisition without causing obvious epithelial damage (). As seen in , CS did not induce significant upregulation of the soluble selectins levels in vaginal fluids compared with PBS-treated controls (P>0.05), whereas the positive control, N9, still induced significant levels of soluble selectin (P<0.05). These data demonstrated that these soluble adhesion molecules might not be suitable markers for compounds that do not produce obvious epithelial damage and inflammation.
Figure 2 Dynamics of soluble selectins in CVLs after single application of N9 or CS. Mice were collected for CVL and killed at indicated time points following a single application of 40 mg/mL N9, 60 mg/mL CS or PBS alone. Five mice were set for each time point of each treatment. Soluble factors were quantified by ELISA using CVL supernatant. For both of E- and P-selectins, there was no difference between the CS treatment group and controls at all time points. In contrast, for the treatment of N9, soluble E-selectin and P-selectin were rapidly increased and persisted at significantly increased levels over 12 h. Experiments were repeated two times and produced similar results. Data are represented as the mean±SD for individual mice of one representative experiment. *P<0.05: N9 and CS treatment groups versus corresponding PBS control.
In the present study, we focused our subsequent studies on the direct investigation of the recruitment of immune cells, including HIV-targeted cells in vaginal mucosal tissues instead of soluble adhesion molecules in vaginal fluids, and tried to establish a new system for evaluating the safety profile of microbicide candidates, including not only surfactant-based compound but also other compounds that are toxic through other mechanisms.
Immune cells were recruited to topical mucosal sites earlier than detectable cytokine secretion in CVLs after a single application
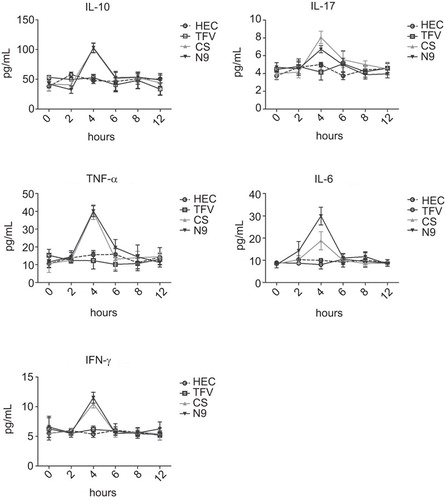
Three representative microbicide candidates, N9, CS and TFV, were employed to investigate the effect of microbicide candidates at topical mucosal sites. A previous report observed that N9 could induce the increase of inflammatory cytokines in CVLs, including IL-1β, tumour-necrosis factor-α and IL-6.Citation4,Citation5,Citation6 Considering the possible engagement of natural killer (NK), Th17 and regulatory T cells at the topical mucosal sites, we added interferon-γ, IL-17A and IL-10 into our cytokine panel and evaluated the cytokine production in CVLs after the administration of microbicide candidates. Four groups of mice were treated with a single dose of 40 mg/mL N9, 60 mg/mL CS, 10 mg/mL TFV and 1.5% HEC gel control, respectively. The dynamic production of cytokines was monitored at different time points using a Cytometric Bead Array as described previously.Citation8 The results indicated that following treatment with both 40 mg/mL N9 and 60 mg/mL CS, the dynamic secretions of the five pro-inflammatory cytokines in CVL were very similar to the pattern observed with monocyte chemotactic protein-1, as reported in our previous work,Citation7 which increased only transiently and then peaked at 4 h post-treatment, followed by a sharp decline and immediate return to baseline values at the later time points. In contrast, 10 mg/mL TFV gel has been proven to be a safe component in microbicide formulation in clinical usage and did not induce any enhancement of cytokine production as compared to HEC gel ().
Figure 3 Dynamics of inflammatory cytokines in CVLs after treatment with microbicide candidates. Inflammatory cytokines secreted in CVLs were quantified at different time points after a single intravaginal application of 1.5% HEC placebo, 10 mg/mL TFV, 60 mg/mL CS and 40 mg/mL N9. The X-axis indicates the hours after the administration of microbicide candidate gels. The Y-axis indicates the production of corresponding inflammatory cytokines. The data represent the mean±SD for individual mice of one representative experiment.
We next investigated the recruitment of immune cells at the topical mucosal sites. As exemplified in , we used CD3/CD4/CD8 to define T-cell subsets, γδ T-cell receptor-specific antibodies to define γδ T cells, NKp46 receptors to define NK cells, and CD14 and CD11c for monocytes and DC cells, respectively. As seen in , the recruitment of immune cells, including HIV-targeted cells, responded earlier than pro-inflammatory cytokines. Significant changes were detectable as early as 2 h (compared to 4 h for cytokines) after a single dose treatment of 40 mg/mL N9 and 60 mg/mL CS and peaked by the 4 h post-treatment (except CD8+ T cells, the change of which were detectable at 4 h post-treatment and peaked at 4 (N9) or 6 h (CS) post-treatment). In contrast, 10 mg/mL TFV did not induce any more recruitment of immune cells as compared with HEC control ().
Figure 4 The recruitment of immune cells at topical mucosal sites by intravaginal application of microbicide candidates. (A) Examples to define different immune cells. CD3/CD4/CD8 were used to define T-cell subsets, γδ T-cell receptor-specific antibodies were used to define γδ T cells, as was the NKp46 receptor for NK cells and CD14 and CD11c for monocytes and DC cells, respectively. (B) The recruitment of different immune cells was displayed after a single treatment of 1.5% HEC placebo (HEC), 10 mg/mL TFV, 60 mg/mL CS and 40 mg/mL N9. The cells were then collected from vaginal tissues at the indicated time points post-treatment. At 2 h post-treatment, significant recruitment was observed for all immune cells except CD8+ T cells after the application of CS and N9 (P<0.05). Recruitment peaked at 4 h post-treatment and then gradually returned to the baseline. In contrast, TFV did not induce any enhanced recruitment of immune cells compared to the placebo control. The data are represented as the mean±SD for individual mice of one representative experiment.

Recruitment was more sensitive than cytokine secretion in determining mild degree stimulation
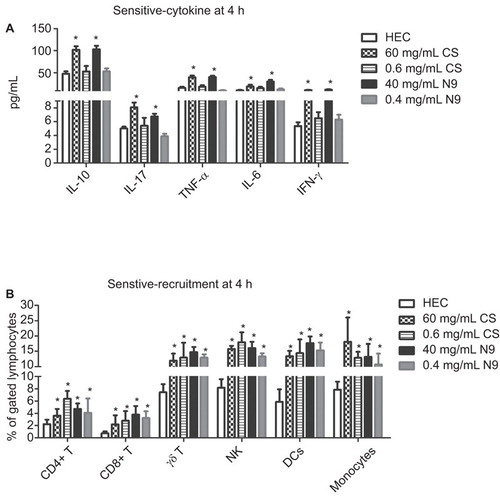
To compare the sensitivity of the recruitment of immune cells with pro-inflammatory cytokines in determining the safety of microbicide candidates, both high and low dose of N9 (40 mg/mL and 0.4 mg/mL in HEC) or CS (60 mg/mL and 0.6 mg/mL) were administered intravaginally, and analysed at 4 h post-treatment, at which the secretion of both pro-inflammatory cytokines and recruitment of immune cells reached their peaks. Compared with HEC alone gel, a dose of 40 mg/mL N9 and 60 mg/mL CS were sufficient to induce significant increases of all five cytokines we tested, whereas their 100-fold dilution formulations (0.4 mg/mL N9 and 0.6 mg/mL CS) could not trigger significant increases of these cytokines in CVLs (). However, low-dose N9 and CS both induced significant increases of immune cell recruitment (P<0.05) () as well as the high-dose formulations. These data demonstrate that recruitment of immune cells was more sensitive than the secretion of pro-inflammatory cytokines in CVLs, especially for determining mild degrees of stimulation that might enhance the infection of pathogens without causing obvious epithelial damage and inflammation.
Figure 5 Recruitment of immune cells is more sensitive than secretion of pro-inflammatory cytokines in the indication of inflammation induced by N9 or CS. In three independent experiments, five groups of mice (five mice per group) were treated intravaginally with a single dose of 15 mg/mL HEC placebo gel, 40 mg/mL N9, 60 mg/mL CS, and their 100-fold dilutions (0.4 mg/mL N9 and 0.6 mg/mL CS), respectively. CVL and vaginal tissues were collected 4 h after application. (A) In regard to the secretion of pro-inflammatory cytokines, there were no differences between the low dose of CS (or N9) treatment group and the control group, whereas significant increases were observed between the high-dose groups and the control. (B) In regard to the recruitment of immune cells in local tissues, both high-dose and low-dose N9 or CS significantly upregulated the per cent of immune cells indicated in the figure. Data are represented as the mean±SD for individual mice of one representative experiment. *P<0.05.
In vitro evaluation model
Although the in vivo model described above could determine the safety profile for a microbicide candidate, we further investigated how much a cell line model in vitro could mimic and thereby potentially replace the in vivo model. We employed the disruption of the integrity of the cell layer and induced apoptosis as surrogates to determine the potential damage of microbicide candidate to the epithelial barrier. As shown in , when we inspected the influence of microbicide candidates on the integrity of a Caco-2 cell layer by staining the tight junction protein occludin in situ, we observed that the density of occludin in a low-dose CS (0.6 µg/mL)-treated Caco-2 cell layer (, upper-right) was similar to that in the PBS-treated cell layer (, upper-left), whereas it was lower in the Caco-2 cell layer exposed to a high dose of CS (60 µg/mL) (, bottom-left), which indicates a decrease of tight junctions between Caco-2 cells after the administration of high-dose CS, and the dissociation of cell junctions occurred in the epithelial cell layer. The dissociation or even disruption was further enhanced when the cell layer was treated with N9 (, bottom-right), suggesting that the application of microbicide candidate could lead to damage to the tight junctions between epithelial cells, and the occludin staining assay could be used to evaluate this damage.
Figure 6 The integrity of Caco-2 epithelial layers and apoptosis induced by microbicide candidates. (A) The occludin immunofluorescence staining for the tight junctions in Caco-2 epithelial layers. After 6 h of co-incubation of Caco-2 cells with microbicides, cell integrity was inspected using occludin fluorescent antibodies. The results revealed that the tight junctions in the epithelial layers remained intact following treatments with PBS (left up) and low-dose CS (0.6 µg/mL) (right up), whereas high-dose CS (60 µg/mL) (left bottom) caused slight destruction of epithelial layers, and 40 µg/mL N9 (right bottom) destroyed the integrity of the epithelial layer. Scale bars=25 µm. (B) The apoptosis of Caco-2 epithelial cells induced by CS. Different cell populations were displayed in the upper panel. The viable cells had low PE and low 7-AAD signals. The apoptotic cells had high PE and low 7-AAD signals, and the necrotic cells had high PE and high 7-AAD signals. After 6 h of co-incubation, low-dose CS (0.6 µg/mL) produced similar apoptosis compared with that of PBS, whereas high-dose CS (60 µg/mL) resulted in a significant increase of apoptotic cells but not cell necrosis (up and bottom panel). Data represent the means±SD from triplicate experiment results (bottom panel). A t-test was performed between the microbicide candidate-treated group and the PBS-treated group. *P<0.05. 7-ADD, 7-aminoactinomycin D; PE, phycoerythrin.

To explore a quantitative assay to determine the potential damage to the epithelial barrier, we employed a Caco-2 apoptosis assay after treatment with the microbicide candidates. Flow cytometry data indicated that apoptotic epithelial cells (Caco-2) but not necrotic cells were greatly increased from the original 8.0% to 30.8% after co-incubation of Caco-2 cells with 60 µg/mL CS for 6 h, whereas no significant difference was observed in the cells treated with a low dose of CS (0.6 µg/mL) as compared with the cells treated with PBS (), indicating that the apoptosis assay could be used to quantify the effect of microbicide candidates on epithelial cells.
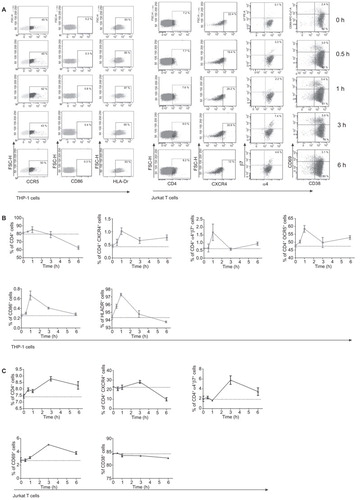
Because our in vivo data suggested that the recruitment of immune cells into topical sites is highly sensitive to microbicide candidate treatment, we tested whether this observation hold true in other cell lines and low-dose CS was employed. The upregulation of activation markers and homing receptors, including HIV-1 receptor/coreceptors, was used as sensitive surrogates to determine the potential recruitment of immune cells to the topical sites. Two cell lines were employed in this assay, including THP-1 cells as a macrophage-derived cell line to represent an innate immunity cell line and Jurkat cells as a representative T-cell line. HIV receptor/coreceptors, including CD4, CXCR4, CCR5, α4β7, and activation markers, including CD86 and HLA-Dr for THP-1 cells and CD69 and CD38 for Jurkat cells, were quantified at different time points, including 0.5 h, 1 h, 3 h and 6 h after the treatment with low-dose CS (). Unexpectedly, upregulation was observed for all surface markers in the THP-1 cells, which peaked at 1 h after the treatment and then decreased (), indicating that the THP-1 cells are capable of rapidly responding to exogenous stimulation. A similar pattern was observed in Jurkat cells (); however, two differences should be noticed. The first difference is that CD38 did not increase after microbicide treatment. The second difference is that all up-regulation peaked at 3 h instead of 1 h in THP-1 cells, suggesting that Jurkat T cells responded slower than THP-1 cells. Interestingly, the enhanced expression of HIV-1 receptor/coreceptors, including CD4, CXCR4, α4β7 and CCR5, was observed for both THP-1 and Jurkat cells, indicating the potential recruitment of HIV-1 infection targets after the administration of low-dose CS.
Figure 7 Upregulation of HIV-1 receptor/coreceptor and activation markers on cell lines after the treatment of low-dose CS. (A) Representative experiments were shown for different surface markers and for different time points after the application of low-dose CS. The expression of HIV receptors (CD4, CCR5, CXCR4, α4β7) and activation markers (HLA-Dr, CD38, CD69) on THP-1 cells or Jurkat T cells at different time points were tested. The left panel shows the kinetics of surface markers for THP-1 cells, and the right panel shows the surface markers for Jurkat T cells. (B, C) The corresponding statistical results for THP-1 and Jurkat T cells, separately. Upon stimulation with low-dose CS, the expression of HIV receptors and activation markers (except CD38) were upregulated and peaked by 1 h post-treatment for the THP-1 cell line and 3 h post-treatment in the Jurkat T-cell line. The data represent the means±SD from triplicate experiment results.
Discussion
The clinical failure of several microbicide candidates due to safety concerns has emphasized the importance of safety evaluation in preclinical testing. Several important evaluations have been taken into consideration by current approaches, including the integrity of the cell layer by measuring the drop of transepithelial electrical resistance in a transwell culture model,Citation9 cell toxicity by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay,Citation4,Citation10,Citation11 influence on the bacterial microenvironment by semiquantitative vaginal culture assay,Citation12,Citation13 inflammatory irritation and colposcopic abnormalities in a rabbit vaginal irritation models,Citation4 and inflammatory cytokine production at the topical sites in mouse models.Citation14 However, there are still some limitations to these evaluation approaches,Citation5,Citation6,Citation7,Citation8 and researchers continue to explore more sensitive assays to improve the examination of the safety profiles of microbicide candidates.
In previous study, we identified some adhesion molecules, such as E- and P-selectin as biomarkers for evaluation of surfactant-based compounds.Citation7 Surfactant-based compounds may promote HIV-1 transmission by eroding the protective mucosal epithelial layers and inducing the inflammatory reaction. Other microbicide candidates, such as polyanionic-based compounds (e.g., CS), displayed better tolerance on vaginal epithelium and flora; however, they still trigger nuclear factor-κB activation through some unknown mechanism and induce release of pro-inflammatory cytokines and chemokines capable of recruiting immune cells, including HIV host cells.Citation9,Citation15,Citation16 Following the treatment of surfactant compounds, shedding soluble E-/P-selectins, vascular adhesion molecule-1, CD14 and/or myeloperoxidase could be released into the vaginal secretions through damaged epithelial layers. This enables such soluble adhesion molecules to be good biomarkers that reflect the mucosal leukocyte reaction to pro-inflammatory conditions.Citation7,Citation17 However, when being treated with other non-surfactants toxic compounds that do not cause epithelial disruption, the CVL levels of the adhesion molecules did not display significant increases, as there was a relatively intact epithelium and less inflammation at the topical site. In the present study, we directly investigated the recruitment of immune cells, including HIV-targeted cells, in vaginal mucosal tissues instead of soluble adhesion molecules in vaginal fluids and developed in vitro cells models. Our data demonstrated that the recruitment of immune cells at the topical mucosal sites and the upregulation of HIV receptor/coreceptors in vitro are highly sensitive biomarkers for the influence of microbicide candidates as compared with assays involving evaluation of histopathology and local secretion of pro-inflammatory cytokines.
Though there are many safety evaluation approaches, including topical immune cell recruitment assays, which have been explored and added into the existing body of use in preclinical settings, it remains unknown how to mutually interpret the data generated from different approaches, the true assay sensitivities and how to effectively combine these evaluations. Our data suggests that safety evaluation assays might be categorized into three levels according to their sensitivities, provisionally named as low sensitive, sensitive and highly sensitive assays. The low sensitive assay is represented by histopathological examination and most likely could be extrapolated to the rabbit vaginal irritation approach. These approaches can identify visible damage to the mucosal barrier or significant inflammation induced by N9, though they hardly detect the changes resulting from high-dose CS. The sensitive assays are represented by the quantification of inflammatory cytokine production in mucosal wash in vivo or supernatants of stimulated cell lines in vitro, the examination of the integrity of the cell layer by occludin staining or transepithelial electrical resistance assays in vitro and apoptosis assays in vitro. These assays are capable of identifying the changes caused by high-dose CS but not low-dose CS, which is 100-fold lower than high-dose CS. Finally, the highly sensitive assays include the recruitment of immune cells at the topical mucosal sites and the upregulation of surface markers, including HIV receptor/coreceptors and activation markers. These approaches are able to detect the minor changes (invisible damage) resulting from the administration of low-dose CS at very early stages. Because the assays at the varied sensitivity levels provide different perspective information, we recommended that at least one assay from each sensitivity level should be included in the safety evaluation algorithm and preferentially two assays from the same sensitive level with one being in vivo and the other in vitro for comparison purposes and for mutual corroboration. Interestingly, no significant change was observed for 10 mg/mL (1%) TFV, even with the most sensitive assays, which is in accordance with previous clinical and preclinical observations.Citation3 Although the result of Vaginal and Oral Interventions to Control the Epidemic (VOICE) trial is opposite to Centre for the AIDS Program of Research in South Africa (CAPRISA) 004, which indicated that a vaginal gel containing 1% TFV could reduce risk 39% overall in women using the active product and 54% in highly adherent users,Citation3 it still confirmed the safety of 1% TFV gel.Citation18 In addition, there are many differences between these two trials. For example, CAPRISA 004 used ‘before and after’ sex doses, whereas VOICE tested a daily dosing regimen. Therefore, at least for now, people cannot ascribe the ineffectiveness of TFV gel in the VOICE trial to safety factors such as inflammation, recruitment or aberrant activation of immune cells before all of the clinical data are analysed in full and further investigations are performed.
The application of highly sensitive assays in the safety evaluation of microbicide candidates is emphasized by its capacity to detect the recruitment of HIV-1 target cells and the upregulation of activation markers and HIV-1 receptor/coreceptors at very early stages. In our in vitro experiments, we observed that low-dose CS upregulated the expression of HIV receptor/coreceptors on macrophage or lymphocyte cells in the absence of triggering the production of inflammatory cytokines. The dramatic upregulation of α4β7, a newly discovered HIV receptor that serves both as the receptor for HIV mucosal infectionCitation19,Citation20 and as mucosal homing receptor,Citation21,Citation22 can partially explain why the recruitment of immune cells at the mucosal sites occurred after topical administration of low-dose CS. Taken together, our results indicate that low-dose CS may enhance the acquisition of HIV via the recruitment of HIV target cells and the upregulation of the HIV-1 receptor/coreceptors in the absence of causing apoptosis and inflammatory cytokine production, which cannot be identified by traditional models. Our observations may explain the observation in previous studies in which CS was more prone to enhance HIV infection when a low dose rather than a high dose was used.Citation15
Taken together, the data presented here not only broaden the understanding of the potential mechanisms of microbicide candidates that result in the enhancement of HIV infection, but also provide a more stringent preclinical assessment tool for the safety evaluation of microbicide candidates, and therefore, have important implications for the development of microbicides.
This work was supported by the National Basic Research Program of China (2012CB519001), Natural Science Foundation of China (81102282, 812111038 and 81171553) and National Grand Program on Key Infectious Disease Control (2012ZX10001-007-008 and 2013ZX10001-006).
- Hillier SL, Moench T, Shattock R, Black R, Reichelderfer P, Veronese F.In vitro and in vivo: the story of nonoxynol 9. J Acquir Immune Defic Syndr2005;39: 1–8.
- McGowan I.Microbicides for HIV prevention: reality or hope? Curr Opin Infect Dis2010;23: 26–31.
- Abdool Karim Q, Abdool Karim SS, Frohlich JA et al.Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science2010;329: 1168–1174.
- Fichorova RN, Bajpai M, Chandra N et al.Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of vaginal microbicidal contraceptives. Biol Reprod2004;71: 761–769.
- Cummins JE Jr, Doncel GF.Biomarkers of cervicovaginal inflammation for the assessment of microbicide safety. Sex Transm Dis2009;36: S84–S91.
- Fichorova RN.Guiding the vaginal microbicide trials with biomarkers of inflammation. J Acquir Immune Defic Syndr2004;37( Suppl 3): S184–S193.
- Zhong M, He B, Yang J et al.L-selectin and P-selectin are novel biomarkers of cervicovaginal inflammation for preclinical mucosal safety assessment of anti-HIV-1 microbicide. Antimicrob Agents Chemother2012;56: 3121–3132.
- Li LZ, Yang Y, Yuan SH et al.Establishing a Th17 based mouse model for preclinical assessment of the toxicity of candidate microbicides. Chin Med J2010;123: 3381–3388.
- Mesquita PM, Cheshenko N, Wilson SS et al.Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J Infect Dis2009;200: 599–608.
- Abner SR, Guenthner PC, Guarner J et al.A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J Infect Dis2005;192: 1545–1556.
- Cummins JE Jr, Guarner J, Flowers L et al.Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother2007;51: 1770–1779.
- Patton DL, Cosgrove Sweeney YT, Paul KJ.A summary of preclinical topical microbicide vaginal safety and chlamydial efficacy evaluations in a pigtailed macaque model. Sex Transm Dis2008;35: 889–897.
- Tien D, Schnaare RL, Kang F et al.In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses2005;21: 845–853.
- Galen BT, Martin AP, Hazrati E et al.A comprehensive murine model to evaluate topical vaginal microbicides: mucosal inflammation and susceptibility to genital herpes as surrogate markers of safety. J Infect Dis2007;195: 1332–1339.
- Tao W, Richards C, Hamer D.Enhancement of HIV infection by cellulose sulfate. AIDS Res Hum Retroviruses2008;24: 925–929.
- Li L, Ben Y, Zhu Z et al.Minocycline down-regulates topical mucosal inflammation during the application of microbicide candidates. PLoS ONE2012;7: e43211.
- Trifonova RT, Bajpai M, Pasicznyk JM, Chandra N, Doncel GF, Fichorova RN.Biomarkers of leukocyte traffic and activation in the vaginal mucosa. Biomarkers2007;12: 608–622.
- Microbicide Trials Network. MTN statement on decision to discontinue use of tenofovir gel in VOICE, a major HIV prevention study in women. Pittsburgh, PA: MTN, 2011.Available at http://www.mtnstopshiv.org/node/3909 (accessed 30 January 2013).
- Buffa V, Stieh D, Mamhood N, Hu Q, Fletcher P, Shattock RJ.Cyanovirin-N potently inhibits human immunodeficiency virus type 1 infection in cellular and cervical explant models. J Gen Virol2009;90: 234–243.
- Sexton A, Harman S, Shattock RJ, Ma JK.Design, expression, and characterization of a multivalent, combination HIV microbicide. FASEB J2009;23: 3590–3600.
- Wang X, Xu H, Gill AF et al.Monitoring alpha4beta7 integrin expression on circulating CD4+ T cells as a surrogate marker for tracking intestinal CD4+ T-cell loss in SIV infection. Mucosal Immunol2009;2: 518–526.
- Hawkins RA, Rank RG, Kelly KA.Expression of mucosal homing receptor alpha4beta7 is associated with enhanced migration to the Chlamydia-infected murine genital mucosa in vivo. Infect Immun2000;68: 5587–5594.
