Abstract
Preliminary studies of the major pathogen enterovirus 71 (EV71), a member of the Picornaviridae family, have suggested that EV71 may be a major cause of fatal hand, foot and mouth disease cases. Currently, the role of the pathological changes induced by EV71 infection in the immunopathogenic response remains unclear. Our study focused on the interaction between this virus and immunocytes and indicated that this virus has the ability to replicate in CD14+ cells. Furthermore, these EV71-infected CD14+ cells have the capacity to stimulate the proliferation of T cells and to enhance the release of certain functional cytokines. An adaptive immune response induced by the back-transfusion of EV71-infected CD14+ cells was observed in donor neonatal rhesus monkeys. Based on these observations, the proposed hypothesis is that CD14+ cells infected by the EV71 virus might modulate the anti-EV71 adaptive immune response by inducing simultaneous T-cell activation.
Emerging Microbes & Infections (2013) 2, e44; doi:10.1038/emi.2013.44
Introduction
In recent years, frequent and widespread outbreaks of hand, foot and mouth disease in infants and young children have been reported in Asian-Pacific countries.Citation1,Citation2,Citation3,Citation4 Preliminary studies of the major pathogen enterovirus 71 (EV71), a member of the Picornaviridae family,Citation5 have suggested that EV71 may be a major pathological cause of fatal hand, foot and mouth disease casesCitation1,Citation6,Citation7,Citation8 by inducing significant pathological changes in the central nervous system (CNS) that ultimately lead to neurogenic pulmonary failure.Citation9,Citation10 These neurological lesions and the subsequent severe sequelae that result from infection are believed to be a potential growing threat to child health and may be the largest risk to children since polio was ‘eradicated’.Citation8,Citation11 Thus, studies of the pathogenic features of EV71, particularly its mechanism of pathogenesis and the associated immunopathogenesis in EV71-infected human tissues and cells, would contribute to a better understanding of the significance of this virus to public health.Citation12
Despite the lack of understanding of the viral infection processes involved in the migration of the virus from primary infection sites, such as the mucosa in the respiratory or intestinal tracts, to the CNS via the circulation and peripheral nerves, previous studies have described potential pathogenic mechanisms of EV71.Citation13,Citation14,Citation15 Dendritic cell (DC)-specific intercellular adhesion molecule-3-grabbing non-integrin has been reported to be one of the specific EV71 receptors on the surface of immature human DCs.Citation16 Other categories of scavenger receptorsCitation17 as well as P-selectinCitation18 and Annexin IICitation19 are usually expressed on the surface of monocytes, DCs and epithelial cells.Citation20,Citation21 Furthermore, EV71 has been shown to have the capacity to infect immature DCs, in which this virus can proliferate and then presumably migrate to associated organs and tissues such as the CNS.Citation16 Additionally, high viral loads have been detected in the lymphocytes of EV71-infected patients and animal models.Citation22,Citation23 These data suggest that an interaction exists between EV71 and immunocytes during the EV71 infection process. This process likely follows the logical progression of typical pathological changes in CNS tissues and other organs, such as the lungs, and the corresponding inflammatory reactions induced by abnormally functioning immunocytes.Citation1,Citation12,Citation24 In fact, abnormal increases in the level of some inflammatory factors, such as interleukin-6 (IL-6) and interferon-γ (IFN-γ), have been observed in both lethal EV71 clinical cases and in animal model studies of EV71 infection.Citation25,Citation26,Citation27 Thus, further investigation of the interaction between the virus and immunocytes during the EV71 infection process would shed light on the pathogenesis of EV71 infection. In this paper, the impact of EV71 infection on CD14+ cells and the immune activity of T lymphocytes are described. The observations are based on the infection of CD14+ cells by EV71 in a neonatal rhesus monkey model that was previously established in our laboratory. In this model, the pathogenic process of EV71 infection can be monitored based on clinical manifestations, viral load and tissue pathogenic changes.Citation23 The corresponding modulatory and stimulatory functions of this infection on the immune system were investigated with in vivo and in vitro experiments.
Materials and methods
Virus and cells
The FY-23 subgenotype C4 strain of the EV71 virus was isolated from an infected male child with clinical symptoms of severe cardiopulmonary collapse during an epidemic in Fuyang, China, in 2008 (GenBank accession number: EU812515.1).Citation28 The virus was grown in Vero cells (ATCC, Manassas, VA, USA), as previously described.Citation23 The Vero cells were maintained in Dulbecco’s modified Eagle’s medium (HyClone, Logan, UT, USA) with 10% fetal bovine serum (Gibco, Grand Island, NY, USA).
Neonatal rhesus monkeys
All animal work was conducted according to the relevant national and international guidelines. The Office of Laboratory Animal Management of Yunnan Province, China, approved the experimental procedures used with these animals (approval number: SCXK (Dian) 2011-0005). All animals were kept in isolation for 2 weeks before the initiation of the study. Each newborn monkey and its mother were kept in a single cage and were fed according to the guidelines of the Committee on Experimental Animals at the Institute of Medical Biology, Chinese Academy of Medical Sciences.Citation29 A neutralization test was conducted to confirm that the monkeys did not have antibodies against EV71 prior to the experimental infections.Citation23 In accordance with the recommendations of the Weatherall report, the experiments were approved by Experimental Animal Welfare Ethics Committee of the Institute of Medical Biology (approval number: YISHENGLUNZI [2011] 15).
A total of 12 healthy newborn rhesus monkeys with an average weight of 300±350 g and an average age of 1–1.5 months were divided into two groups, with three monkeys in experimental group one and nine monkeys in experimental group 2 (including three monkeys in control group (transfused the normal CD14+ cells from other monkeys), three monkeys in donor group (separated the peripheral blood mononuclear cells (PBMCs), infected CD14+ cells with EV71 in vitro, and then transfused back the EV71-infected CD14+ cells) and three monkeys in non-donor group (transfused the EV71-infected CD14+ cells from other monkeys)).
In experimental group 1, the monkeys were infected with EV71 (104.5 cell culture infective dose 50% (CCID50)/monkey) via the respiratory tract.Citation23 Briefly, the monkeys were anesthetized with ketamine (10 mg/kg of body weight; Phoenix Pharmaceuticals, St Joseph, MO, USA) and then infected using a bronchoscope wedged into the desired trachea. The virus, in 2 mL of sterile saline, was instilled into the trachea via the biopsy channel of the bronchoscope. The three neonates in experimental group 1 were killer by over anesthetization at day 5 post-infection (p.i.) for analysis of EV71-infected CD14+ cells.
The monkeys in experimental group 2 were used to provide PBMCs and were used in the transfusion experiment but were not sacrificed. Briefly, CD4+, CD8+, CD14+ and CD20+ cells were separated from the PBMCs collected from experimental group 2. Cultured CD14+ cells were infected with EV71 for the dynamic analysis of viral proliferation or for transfusion back into three original donors or three non-donor monkeys.
Cell isolation, separation and culture
PBMCs, lymph monocytes and spleen monocytes were isolated from whole blood, the axillary lymph nodes and the spleen, respectively, by density gradient centrifugation over Lymphoprep (Ficoll-Paque PREMIUM; GE Healthcare, Piscataway, NJ, USA). The cells were counted, assayed for viability via trypan blue exclusion, and separated into CD20+, CD14+, CD4+ and CD8+ populations using specific immunoadsorption (monoclonal antibody-coated magnetic beads; Miltenyi Biotec, Bergisch Gladbach, Germany) and flow cytometry. The marker-positive cells and marker-negative cells in the population were cultured at a density of 1.0×107 cells/mL in RPMI 1640 (HyClone, Logan, UT, USA) with 10% fetal bovine serum (Gibco), 100 U/mL penicillin, 100 µg/mL streptomycin, 30 ng/mL granulocyte-macrophage colony-stimulating factor and 10 ng/mL IL-4, at 37 °C in 5% CO2.
Isolation and titration of the virus
The virus samples harvested from cell cultures or isolates of different cell populations (CD4+, CD8+, CD14+ and CD20+ from infected neonates) were analyzed with a microtitration assay. Briefly, the virus sample was diluted sequentially 10-fold and used to inoculate monolayers of Vero cells in a 96-well plate with a volume of 50 µL/well and eight wells for each dilution. After 72 h of incubation at 37 °C post-inoculation, the extent of the cytopathic effect in the inoculated cells was observed to determine the viral titer.Citation30
Extraction of viral RNA and quantitative reverse transcription polymerase chain reaction (qRT-PCR) amplification
Viral RNA was extracted from the cells and tissues of the experimental animals using a Qiagen RNeasy Mini Kit (Qiagen, Hilden, Germany). The viral loads were quantified relative to a standard RNA copy number by real-time real-time RT-PCR analysis, as previously described.Citation30
Immunofluorescence analysis of cells and antigens
The tissue samples from each infected monkey were fixed in formalin, dehydrated, embedded, sectioned, placed on glass slides and fixed in paraformaldehyde as previously described.Citation30 The cell samples, including PBMCs and spleen lymphocytes from infected monkeys in experimental group 1, were washed with phosphate-buffered saline (PBS), resuspended, smeared on a glass plate, fixed with acetone and dried.Citation31 The EV71 antigen was detected using a mouse anti-enterovirus 71 monoclonal antibody (Chemicon, Temecula, CA, USA) and anti-mouse IgG-rhodamine antibodies (Sigma, Deisenhofen, Germany). CD14+ cells were detected using a labeled anti-CD14 antibody (BD Biosciences, San Jose, CA, USA). The staining procedure was performed according to a standard protocol.Citation31 The imaging of labeled samples was performed with a Nikon C1 laser scanning confocal microscope system (Nikon, Tokyo, Japan). The acquisition, storage and analysis of the data were performed using EZ-C1 viewer software from Nikon.
EV71 infects CD14+ cells in vitro
CD14+ cells (1.0×105 cells/well) from the normal neonates of experimental group 2 were cultured for 24 h at 37 °C in 5% CO2 and then infected with EV71 multiplicity of infection (MOI)=1 in vitro. Briefly, the cells were washed twice, infected with EV71, and then washed again prior to culturing at 37 °C. The supernatants and cells were collected at 24 h p.i. 200 µL of supernatant from the infected cells was used for cytokine detection by cytometric bead array (CBA) as described below. The cells were harvested after three freeze–thaw cycles to determine the viral loads by virus titration and qRT-PCR.
T lymphocyte proliferation assay
The supernatants of EV71-infected CD14+ cells were harvested at 24 h p.i. (as described above) and then added to T cells (purified using anti-CD3 monoclonal antibody-coated magnetic beads, 1.0×105 cells/well). The stimulated T cells were divided into two parts for experiments. One part is for cytokines assay. After 24, 48 and 72 h of incubation at 37 °C, the cytokines were detected by CBA as described below. And the other part of cells were incubated for 48, 72 or 96 h at 37 °C in 5% CO2, pulsed with 3H-thymidine (1 µCi/well; NEN/DuPont, Boston, MA, USA) for 8 h, and harvested. The results are expressed as counts per minute of 3H-thymidine incorporation and represent the mean of triplicate cultures.
Flow cytometry-based CBA analysis
Culture supernatants and sera from the transfused monkeys were collected for use in detection assays targeting IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, tumor-necrosis factor-α (TNF-α) and IFN-γ. These assays were conducted simultaneously using a Th1/Th2 cytokine cytometric bead array kit specific for non-human primate (BD Biosciences, San Diego, CA, USA).Citation32 The assay was conducted following the manufacturer’s protocol. Briefly, a mixture of the anti-cytokine beads was added to the supernatant or serum samples, and these samples were incubated with the included phycoerythrin (PE) detection reagent in the dark at room temperature for 3 h, and then washed for twice. The intensity of the fluorescence signal was acquired on a fluorescence-activated cell sorter flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed using CBA software (BD Biosciences, San Jose, CA, USA). The results of the experiments have excluded the levels of previously added IL-4, according to the control.
Transfusion experiment
CD14+ cells (2×105 cells) collected from each of the three normal neonates in experimental group 2 were infected with EV71 (MOI=1) in vitro. At 24 h p.i., the in vitro-infected CD14+ cells (2×105 cells) were collected by centrifugation and washed with PBS three times. Half of these CD14+ cells were transfused back into the three original donor monkeys (auto-transfusion), and the other half of the cells were transfused into three non-donor monkeys (allo-transfusion).Citation33 As a negative control, three additional monkeys in experimental group 2 were treated with PBS. The transfused monkeys were monitored daily for body temperature and general health status. PBMCs were taken from all the monkeys at day 5 post-transfusion, incubated for 24 h and stimulated with EV71 VP1 peptide prior to intracellular cytokine staining and flow cytometry to determine the percentage of cells producing IFN-γ.
Intracellular cytokine staining with flow cytometry
The intracellular cytokine staining assay was performed using freshly isolated PBMCs from transfused or normal monkeys. The cells were stimulated with EV71 antigen (2 ng/mL) for 4 h at 37 °C. The negative controls included cells incubated without peptide. Brefeldin A (10 µg/mL; Sigma, St Louis, MO, USA) was added according to the method described in detail by Karlsson et al.Citation34 All the cells were washed and stained for flow cytometry with fluorescein isothiocyanate-conjugated anti-IFN-γ (BD Biosciences, San Jose, CA, USA) and PE-conjugated anti-CD3 (BD Biosciences, San Jose, CA, USA) for 30 min at 4 °C. After staining, the cells were washed, fixed in 1% paraformaldehyde, and collected with a FACSCalibur instrument using Cell Quest software (BD Biosciences, San Jose, CA, USA). The proportion of cells producing IFN-γ was recorded.
Statistical analysis
The data were expressed as the means and standard deviations. Individual experiments were repeated three times per sample. GraphPad Prism software (San Diego, CA, USA) was used for the statistical analyses. The differences between the two groups were evaluated using one-way analysis of variance. A P value of <0.05 was considered significant.
Results
EV71 has the ability to infect CD14+ cells during the infection of neonatal rhesus monkeys
As demonstrated in our previous studies, an EV71 viremia peak is typically present from days 5 to 8 p.i. in the neonatal monkey model, followed by sustained high viral loads from days 7 to 10 p.i. in the CNS and other organs, including brown adipose tissue and lymph nodes.Citation23 qRT-PCR amplification of viral RNA extracted from the blood samples of monkeys in experimental group 1 collected on day 5 p.i. indicated that EV71 was present in PBMCs (). By contrast, no viruses were observed in the serum, erythrocytes (red blood cells) or platelets (). The sampled PBMCs of these infected monkeys were immobilized on slides for analysis by confocal fluorescence microscopy using an anti-EV71 antibody and anti-CD14, CD4, CD8 and CD20 fluorescent antibodies. EV71 and CD14 fluorescence were observed within the same cells among PBMCs obtained at day 5 p.i. (). However, no EV71 fluorescence was observed in CD4+, CD8+ and CD20+ cells (Supplementary Table S1). Because EV71 is delivered via a respiratory tract spray in our established neonatal monkey model, this study highlights the relationship between CD14+ cells and EV71 antigen expression in the trachea, axillary lymph nodes and spleens of infected animals sacrificed at day 5 p.i. The colocalization of anti-EV71 and anti-CD14 antibodies was observed in the trachea, axillary lymph nodes and spleen tissue (). To confirm these observations, viral microtitration and qRT-PCR were used to detect the virus in Vero cells cocultured with CD14+ cells isolated from either the PBMCs or lymph nodes of infected animals and purified with flow cytometry. The Vero cells cocultured with CD14+ cells were positive for virus by both methods (). Together, these findings are sufficient to suggest that EV71 has the ability to infect host CD14+ cells. In these cells, the virus might be maintained and could subsequently migrate to tissues and/or organs in infected neonatal monkeys.
Dynamic profile of EV71 proliferation in CD14+ cell culture
To explore the potential mechanism of the EV71 infection of CD14+ cells in infected neonatal monkeys, the dynamic proliferation of the virus in infected CD14+ cells and other PBMCs from normal monkeys in experimental group 2 was assessed in vitro. The CD14+ cells and other cells, including CD4+, CD8+ and CD20+ cells, were infected by EV71 at an MOI of 0.5–1, and aliquots were collected every 4 h for viral titration. The results collectively suggest that EV71 has a distinct proliferation profile in CD14+ cells (). This tendency was confirmed by qRT-PCR amplification (). These findings imply that EV71 proliferates in CD14+ cells.
EV71 infection modulates the cytokine profile of CD14+ cells
Based on previously published data, CD14+ cells are a type of active immune cell that are capable of modulating both innate and adaptive immune responses by releasing various cytokines.Citation35,Citation36 Our hypothesis—that EV71 infects and proliferates inside CD14+ cells—suggests that EV71 might induce an abnormal inflammatory response in vivo by interfering with the regulation of the immune response by CD14+ cells. The determination of the cytokine profile of CD14+ cells isolated from infected animals provided evidence that EV71 interferes with the immune response. These CD14+ cells cultured in vitro produced higher levels of several cytokines related to immune regulation, including IL-8, IL-4 and IL-6, compared with the control cells from normal monkeys (). To confirm this observation, the cytokine levels in the supernatants of cultured CD14+ cells isolated from normal monkeys and infected by EV71 in vitro were detected by cytometry. The results indicate that higher levels of IL-8, IL-4 and IL-6 are produced by infected CD14+ cells than by negative control cells (). Higher IL-6 levels in the blood and CSF are usually observed in patients with fatal EV71 infections. The data suggest that IL-8 is in fact involved in inflammatory responses.Citation37 However, whether the upregulation of IL-8 expression in infected CD14+ cells is related to the pathogenesis of EV71 is not completely clear.
Figure 1 CD14+ monocytes are associated with EV71 infection. (A) Viral loads in various cells or components from the blood of infected rhesus monkeys. Viral RNA was extracted and measured using the Taqman-based real-time qPCR assay. P values of PBMC and spleen cells vs. serum, RBC and platelets. (B) Confocal fluorescence microscopy of CD14+ cells and EV71 antigen in PBMCs, trachea, axillary lymph nodes and spleen from EV71-infected neonatal monkeys. (C) Viral load from CD4+, CD8+, CD14+ and CD20+ cells of EV71-infected neonatal monkeys. Viral titer data are shown in lgCCID50 scale. P value of the viral load of CD14+ cells vs. the viral load of CD4+ and CD8+ cells. Samples were collected from EV71-infected neonatal monkeys (experimental group 1, n=3) at day 5 p.i. Three independent experiments were performed. RBC, red blood cell; qPCR, quantitative polymerase chain reaction.

EV71-infected CD14+ cells stimulate the proliferation of T lymphocytes
After the removal of CD20+ cells, the majority of the remaining CD14+ cells are monocytes and partially immature DCs,Citation38 which are believed to be capable of activating lymphocytes upon exposure to viral infection.Citation39,Citation40 Based on this understanding, we cultured T cells with the supernatant of EV71-infected CD14+ cells and subsequently performed T lymphocyte proliferation assays. In parallel, the supernatants of lipopolysaccharide-stimulated CD14+ cells and non-stimulated (negative) cells were used as controls to determine the impact of CD14+ cells on T-cell proliferation. The results indicate that the supernatant of EV71-infected CD14+ cells has a stimulatory effect on T lymphocyte proliferation that is equivalent to the effect of the lipopolysaccharide control relative to the negative control (). The detection of cytokines in the supernatant of T lymphocytes cultured with the supernatant of EV71-infected CD14+ cells indicated a significant increase in TNF-α release after 24 h of culture (). Additionally, the level of IL-6 release also tended to slightly increase after 24 h, whereas IL-4 release tended to significantly increase after 48 h and reached its peak at 72 h (). The levels of other cytokines exhibited no clear differences relative to the levels in the negative control group (with the supernatant of EV71-infected CD14+ cells and without T lymphocytes) (). These findings suggest a potential triggering effect of EV71-infected CD14+ cells on T lymphocyte proliferation.
EV71-infected CD14+ cells activate a specific immune response against EV71 infection in vivo
Our results indicate that EV71-infected CD14+ cells have the ability to stimulate T lymphocyte proliferation, which leads directly to the question as to whether this T lymphocyte proliferation, exhibited by the tendency toward a Th2 response, is significant to the specific anti-EV71 response of the immune system of the monkeys. We decided to study the potential relationship between EV71-infected CD14+ cells and the specific immune response in vivo. When comparing the negative control and the non-donor monkeys in experimental group 2 according to major histocompatibility complex differences, a higher percentage of cells producing IFN-γ was found for the three donor monkeys back-transfused with EV71-infected CD14+ cells (); however, an IFN-γ-specific immune response targeting a specific antigenic peptide in EV71 VP1 was not observed within 5 days after the same quantity of allogeneic EV71-infected CD14+ cells were transfused into non-donor monkeys (). These results imply that the potential function of EV71-infected CD14+ cells in the infection process in monkeys is related not only to viral migration to tissues and organs in the infected monkeys, but also to the activation of the T lymphocyte response and the subsequent development of an adaptive anti-EV71 immune response in the monkeys.
Figure 2 Dynamic profile of EV71 proliferation in CD14+ cells in vitro. The proliferation of viruses in CD14+ cells from normal monkeys (experimental group 2, n=3) measured by virus titration (A) and qRT-PCR assay (B) from 0 to 48 h p.i. in vitro. The values are the average of three independent experiments.

Discussion
EV71, a member of the family Picornaviridae, is known to enter the human body via the mucosa of the respiratory or intestinal tracts, where infection is initiated.Citation15,Citation41 The details of EV71 pathogenesis have remained unclear; however, based on available studies of other enteroviruses, the primary site of EV71 proliferation after entry into the human body is assumed to be the reticuloendothelial system.Citation42,Citation43 Furthermore, evidence suggests that EV71 has the ability to infect cells of the lymphatic system, such as human peripheral blood monocytesCitation44 and immature DCs.Citation16 EV71 receptor studies have demonstrated that DC-specific intercellular adhesion molecule-3-grabbing non-integrin, scavenger-II and P-selectin, among other receptors, expressed on the surfaces of monocytes, lymphocytes, DCs and epithelial cells, are capable of binding specifically to EV71.Citation16,Citation17,Citation18 Taken together, these results suggest that after entry into the respiratory or intestinal tract mucosa, EV71 is taken up into the microcirculation of local tissues and subsequently enters cells via binding to surface-expressed receptors. Although the details of this biological event require further elucidation, our studies provide additional evidence to support this hypothesis. Similar with the reports of Dr Wang and his colleagues,Citation44 we also found that EV71 can infect PBMCs from rhesus monkeys. Furthermore, by separating PBMCs into different cell subsets, we found EV71-infected CD14+ cells in the respiratory tract mucosal tissue of EV71-infected neonatal monkeys (). Analysis of PBMCs and lymph nodes harvested at day 5 p.i. by confocal immunofluorescence microscopy further supports the hypothesis that EV71-infected CD14+ cells enter the cardiovascular and lymphatic systems (). These results suggest that EV71 has the ability to infect CD14+ cells and to subsequently migrate to other target organs or tissues. In our previous studies of EV71 infection in the neonatal monkey model, the peak viral load during the EV71 infection process occurred in the axillary lymph nodes, submaxillary lymph nodes and lung lymph nodes from days 5 to 8 p.i., during which time viral viremia develops.Citation23 The peak viral loads occur in certain tissues and organs, such as the CNS and lungs, on day 7 p.i.Citation23 This linear variation in the viral load, increasing with time, provides further support for the hypothesis that EV71 primarily migrates via the blood. Again, the detection of EV71 genomes in blood samples taken at day 5 p.i. indicated the presence of EV71 in PBMCs (). These observations, taken together, are indicative of EV71 migration via host CD14+ cells throughout the body via circulation in the blood and lymphatic systems. The dynamic profile of EV71 proliferation in infected CD14+ cells cultured in vitro further supports the capacity of EV71 to facilitate proliferation by infecting CD14+ cells (). As a pattern recognition molecule, CD14 has a central role in innate immunity and is a major hallmark of monocytes and immature DCs,Citation45 which are capable of stimulating and regulating innate and adaptive immune responses through various pathways.Citation35,Citation36 Our data suggest that the CD14+ cells modulated the expression profiles of some cytokines after infection by EV71. Thus, EV71-infected CD14+ cells are hypothesized to have the ability to stimulate a T-cell response. Interestingly, our results indicate that when T cells are cultured with the supernatant of EV71-infected CD14+ cells, the T cells proliferate and the production of some functional cytokines increases (). Although further research is needed to determine the detailed mechanism of this process, these results suggest that EV71-infected CD14+ cells are the basis of one potential pathway for the stimulation of T cell activation following viral infection.
Figure 3 EV71-infected CD14+ cells stimulate cytokine production in vitro. (A) Detection of cytokines in the supernatants of CD14+ cells from EV71-infected monkeys (n=3) after 24 h of culturing in vitro. P value vs. control group (the supernatants of CD14+ cells from normal monkeys after 24 h of culturing). (B) Detection of cytokines in the supernatants of EV71-infected CD14+ cells after 24 h post-infection in vitro. P value vs. control group (the supernatants of normal CD14+ cells after 24 h of culturing). The cytokines (IL-4, IL-6, IL-8, IL-10, TNF-α and IFN-γ) were measured using BD System kits in accordance with the section on ‘Materials and methods’. Three independent experiments were performed.
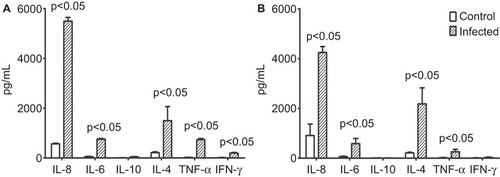
Figure 4 EV71-infected CD14+ cells induce the proliferation of T lymphocytes. (A) The proliferation of T lymphocytes cultured with the supernatants of EV71-infected CD14+ cells was measured by 3H-thymidine. Cultured CD14+ cells were infected by EV71 (MOI=1) for 24 h. The supernatants were collected and added into normal T lymphocytes from the same monkeys. The L, V and N groups were treated with LPS (10 ng/mL, L group), infected with EV71 (MOI=1, V group) or received no treatment (N group), respectively. (B) Detection of cytokines in the supernatants of EV71-infected CD14+ cells cultured with normal donor T lymphocytes (from experimental group 2) in vitro. Cultured CD14+ cells were infected with EV71 (MOI=1) for 24 h. The supernatants were collected and added into normal T lymphocyte cultures from the same monkeys. The cytokines (IL-2, IL-4, IL-5, IL-6, TNF-α and IFN-γ) in the supernatants were measured at different time points using BD System kits. P value vs. control group (the supernatants of EV71-infected CD14+ cells after culturing). Three independent experiments were performed. LPS, lipopolysaccharide.

Figure 5 EV71-infected CD14+ cells induced a specific anti-EV71 immune response in monkeys. The CD14+ cells from three normal monkeys were infected by EV71 (MOI=1) in vitro. After 24 h p.i., EV71-infected CD14+ cells were transfused either back into the same monkeys or into three non-donor monkeys in experimental group 2. PBMCs were collected from the transfused monkeys at 5 days post-transfusion. The percentages of cells producing IFN-γ in the sera of monkeys were determined using CFC as described in the section on ‘Materials and methods’. P value vs. positively and negatively stimulated group. Three independent experiments were performed. CFC, cytokine flow cytometry.

EV71-infected patients and animals provide evidence that EV71 infection may frequently lead to the increased release of particular cytokines, such as IFN-γ and/or IL-6, into the blood or cerebrospinal fluid in infected individuals.Citation25,Citation26,Citation27 Currently, whether the pathological changes induced by EV71 infection are actually involved in the immunopathogenic response is unknown. Because EV71 has the ability to infect CD14+ cells and impart immunological activation and immunoregulation capabilities, studying the impact of EV71-infected CD14+ cells on the immune system and the related immune response is of great importance. The increased proliferation of T cells cultured with the supernatant of EV71-infected CD14+ cells and the significant increases in TNF-α and IL-6 production are sufficient to indicate that the immune responses activated by EV71-infected CD14+ cells are biased toward a Th2 response (). Such model is also suggested by some reports that EV71-infected patients and animals are characterized by an obvious increase in IL-6 release in the peripheral blood.Citation27 However, somewhat differing rates of IFN-γ release have been reported.Citation26 Thus, there may be a currently unknown association between EV71-infected CD14+ cells and the induced inflammatory response that is closely related to pathogenesis. Further exploration of this association will contribute to the overall understanding of EV71 pathogenesis.
Supplementary Figure 1
Download MS Word (38 KB)This work was supported by the National Basic Research Program (2011CB504903 and 2012CB518901), National Natural Sciences Foundation of China (81171573 and 31100127), the Yunnan Natural Science Foundation (2011FB116) and the Peking Union Medical Youth Found (2012J23).
Note: Supplementary information accompanies the paper on Emerging Microbes & Infections's website (http://www.nature.com/EMI/).
- Chan LG, Parashar UD, Lye MS et al.Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. For the Outbreak Study Group. Clin Infect Dis2000;31: 678–683.
- Shimizu H, Utama A, Yoshii K et al.Enterovirus 71 from fatal and nonfatal cases of hand, foot and mouth disease epidemics in Malaysia, Japan and Taiwan in 1997–1998. Jpn J Infect Dis1999;52: 12–15.
- Zhang Y, Tan XJ, Wang HY et al.An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J Clin Virol2009;44: 262–267.
- Ahmad K.Hand, foot, and mouth disease outbreak reported in Singapore. Lancet2000;356: 1338.
- Sim AC, Luhur A, Tan TM, Chow VT, Poh CL.RNA interference against enterovirus 71 infection. Virology2005;341: 72–79.
- Ooi MH, Solomon T, Podin Y et al.Evaluation of different clinical sample types in diagnosis of human enterovirus 71-associated hand-foot-and-mouth disease. J Clin Microbiol2007;45: 1858–1866.
- Lin TY, Chang LY, Hsia SH et al.The 1998 enterovirus 71 outbreak in Taiwan: pathogenesis and management. Clin Infect Dis2002;34( Suppl 2): S52–S57.
- McMinn PC.An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev2002;26: 91–107.
- Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF.Neurologic complications in children with enterovirus 71 infection. N Engl J Med1999;341: 936–942.
- Wang SM, Liu CC, Tseng HW et al.Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis1999;29: 184–190.
- da Silva EE, Winkler MT, Pallansch MA.Role of enterovirus 71 in acute flaccid paralysis after the eradication of poliovirus in Brazil. Emerg Infect Dis1996;2: 231–233.
- Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH.Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis2010;10: 778–790.
- Wong KT, Munisamy B, Ong KC et al.The distribution of inflammation and virus in human enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. J Neuropathol Exp Neurol2008;67: 162–169.
- Chen CS, Yao YC, Lin SC et al.Retrograde axonal transport: a major transmission route of enterovirus 71 in mice. J Virol2007;81: 8996–9003.
- Nagata N, Shimizu H, Ami Y et al.Pyramidal and extrapyramidal involvement in experimental infection of cynomolgus monkeys with enterovirus 71. J Med Virol2002;67: 207–216.
- Lin YW, Wang SW, Tung YY, Chen SH.Enterovirus 71 infection of human dendritic cells. Exp Biol Med (Maywood)2009;234: 1166–1173.
- Yamayoshi S, Yamashita Y, Li J et al.Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat Med2009;15: 798–801.
- Nishimura Y, Shimojima M, Tano Y, Miyamura T, Wakita T, Shimizu H.Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat Med2009;15: 794–797.
- Yang SL, Chou YT, Wu CN, Ho MS.Annexin II binds to capsid protein VP1 of enterovirus 71 and enhances viral infectivity. J Virol2011;85: 11809–11820.
- Laszik Z, Jansen PJ, Cummings RD, Tedder TF, McEver RP, Moore KL.P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood1996;88: 3010–3021.
- Eskelinen EL, Tanaka Y, Saftig P.At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol2003;13: 137–145.
- Chumakov M, Voroshilova M, Shindarov L et al.Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch Virol1979;60: 329–340.
- Liu L, Zhao H, Zhang Y et al.Neonatal rhesus monkey is a potential animal model for studying pathogenesis of EV71 infection. Virology2011;412: 91–100.
- Lin TY, Hsia SH, Huang YC, Wu CT, Chang LY.Proinflammatory cytokine reactions in enterovirus 71 infections of the central nervous system. Clin Infect Dis2003;36: 269–274.
- Wang SM, Lei HY, Su LY et al.Cerebrospinal fluid cytokines in enterovirus 71 brain stem encephalitis and echovirus meningitis infections of varying severity. Clin Microbiol Infect2007;13: 677–682.
- Wang SM, Lei HY, Huang KJ et al.Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis2003;188: 564–570.
- Lin TY, Chang LY, Huang YC, Hsu KH, Chiu CH, Yang KD.Different proinflammatory reactions in fatal and non-fatal enterovirus 71 infections: implications for early recognition and therapy. Acta Paediatr2002;91: 632–635.
- Ma SH, Liu JS, Wang JJ et al.Genetic analysis of the VP1 region of human enterovirus 71 strains isolated in Fuyang, China, during 2008. Virol Sinica2009;24: 162–170.
- Gorska P.Principles in laboratory animal research for experimental purposes. Med Sci Monit2000;6: 171–180.
- Zhang Y, Cui W, Liu L et al.Pathogenesis study of enterovirus 71 infection in rhesus monkeys. Lab Invest2011;91: 1337–1350.
- Hinuma Y, Konn M, Yamaguchi J, Wudarski DJ, Blakeslee JR Jr, Grace JT Jr.Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol1967;1: 1045–1051.
- Chen R, Lowe L, Wilson JD et al.Simultaneous quantification of six human cytokines in a single sample using microparticle-based flow cytometric technology. Clin Chem1999;45: 1693–1694.
- Lopas H, Birndorf NI.Haemolysis and intravascular coagulation due to incompatible red cell transfusion in isoimmunized monkeys. Br J Haematol1971;21: 399–411.
- Karlsson AC, Martin JN, Younger SR et al.Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J Immunol Methods2003;283: 141–153.
- Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA.Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol2000;164: 13–17.
- Hoebe K, Janssen E, Beutler B.The interface between innate and adaptive immunity. Nat Immunol2004;5: 971–974.
- Gomez MI, Lee A, Reddy B et al.Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med2004;10: 842–848.
- Yamaguchi N, Fujimori Y, Fujibayashi Y et al.Interferon-gamma production by human cord blood monocyte-derived dendritic cells. Ann Hematol2005;84: 423–428.
- Bhardwaj N, Bender A, Gonzalez N, Bui LK, Garrett MC, Steinman RM.Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J Clin Invest1994;94: 797–807.
- Kawamura K, Kadowaki N, Kitawaki T, Uchiyama T.Virus-stimulated plasmacytoid dendritic cells induce CD4+ cytotoxic regulatory T cells. Blood2006;107: 1031–1038.
- Ryu WS, Kang B, Hong J, Hwang S, Kim J, Cheon DS.Clinical and etiological characteristics of enterovirus 71-related diseases during a recent 2-year period in Korea. J Clin Microbiol2010;48: 2490–2494.
- Boping Z, Chenrong L. Enterovirus 71 hand mouth foot disease. Beijing: The People’s Medical Publishing House, 2009.
- Wang YF, Chou CT, Lei HY et al.A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection. J Virol2004;78: 7916–7924.
- Wang SM, Chen IC, Su LY, Huang KJ, Lei HY, Liu CC.Enterovirus 71 infection of monocytes with antibody-dependent enhancement. Clin Vaccine Immunol2010;17: 1517–1523.
- Rossi M, Young JW.Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol2005;175: 1373–1381.