Abstract
Co-infection of visceral leishmaniasis (VL) and human immunodeficiency virus type 1 (HIV-1) is known to have higher rates of initial treatment failure, relapse and mortality than in those without HIV-1 infection. Co-infection of VL and HIV-1 usually results in death by the end of treatment in previously reported cases in China. Here we report on a patient with VL and HIV-1 co-infection who received a high dose and an extended course of sodium stibogluconate treatment in addition to antiretroviral therapy (ART). This treatment regimen resulted in good control of VL and HIV-1 infection, while the conventional protocol of sodium stibogluconate treatment was not able to prevent multiple VL relapses. To the best of our knowledge, this is the first surviving case of VL and HIV-1 co-infection with this particular treatment regimen in China.
Introduction
Visceral Leishmaniasis (VL), also called ‘kala-azar’ in China and South Asian countries, is an epidemic infectious disease caused by infection with the protozoan parasites, Leishmania donovani or L. infantum.Citation1 VL has become a significant opportunistic infection in human immunodeficiency virus type 1 (HIV-1)-infected patients in the epidemic region over the past 30 years. Patients with leishmania and HIV-1 co-infection have emerged in Europe, Africa, Central and South America and Asia (India, Nepal, Oman and other countries). HIV-infected patients with VL have higher rates of initial treatment failure, relapse and mortality than HIV-negative patients with VL. Six patients with Leishmania and HIV-1 co-infection have been reported in China, all of whom died within a few weeks after diagnosis except one who was lost to follow up. The patient with VL/HIV-1 co-infection reported here received both anti-Leishmania and antiretroviral therapy (ART) and is still alive after one and a half years of treatment; the patient's VL and HIV-1 infections are under control.
CASE REPORT
A 46-year-old male from Sichuan Province who presented with progressive abdominal distension for two years was admitted to our hospital on 25 July 2012. In 1990, he was bitten by a sandfly at his place of employment in a VL epidemic region. At the end of 1992, he presented with fever, hepatomegaly and splenomegaly, was diagnosed with liver cirrhosis by local hospitals and treated without major improvement. His liver and spleen continued to enlarge, accompanied by severe anemia and weight loss. He was hospitalized at Sichuan Provincial Hospital with a diagnosis of VL via bone marrow examination. Treatment included sodium stibogluconate (pentavalent antimony), 6 mL/day, containing antimony 0.6 g, by intravenous infusion. After six days of treatment, his condition improved and the patient was discharged. He moved to work in a non VL-endemic area and denied a history of drug abuse, blood transfusion and paid blood donation, but did acknowledge to have multiple sexual partners. In August 2010, he found an abdominal mass in the left upper quadrant, accompanied by abdominal distension. Leishmania amastin (Leishmania amastigote form) was found in his bone marrow smear. He was again diagnosed with VL in October of the same year. He received the conventional treatment of pentavalent antimony for six days for a second time and his condition improved. During hospitalization, the patient was screened for HIV-1 antibody and the result of the screen was positive. The local disease prevention and control center confirmed HIV-1 infection by Western blot analysis, with low CD4+ T lymphocytes of 42/µL. He was diagnosed with acquired immunodeficiency syndrome (AIDS) based on very low CD4+ T lymphocyte counts. Since July 2011, he has received ART including lamivudine+zidovudine+efavirenz. After two months of treatment, he presented with severe anemia. Zidovudine-induced bone marrow suppression was considered. Zidovudine was replaced by tenofovir. After one year of ART, his HIV-1 plasma RNA level was undetectable, but his CD4+ T lymphocytes were still less than 50/µL (CD4+ T lymphocyte counts were tested every three months). In 2011, the patient was diagnosed with liver cirrhosis by a local hospital because of splenomegaly and portal hypertension, and treated with traditional Chinese medicine. He was referred to our hospital for splenectomy.
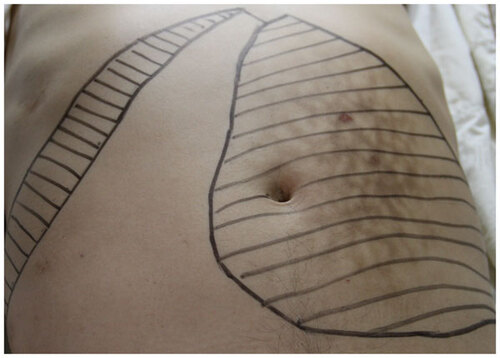
Physical examination showed a temperature of 36.9 °C, heart rate 113 bpm, respiratory rate 20 bpm, blood pressure 112/78 mmHg, severe pallor and hepatosplenomegaly (the liver was 2 cm below the costal edge and the spleen was palpable 7 cm below the umbilical level) (Figure ). Leishmania amastin (Leishmania amastigote form) was found in the bone marrow aspirate (Figure ). The blood chemistry panel including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), blood urea nitrogen (BUN), creatinine (Cr) and glucose was normal; albumin, globulin and β2 microglobulin were remarkable abnormal (); serum sodium 131.5 mmol/L, potassium 3.32 mmol/L, calcium 1.66 mmol/L, magnesium 0.66 mmol/L. Routine urine and stool tests found no abnormalities. His CD4+ T lymphocyte counts were 56/µL. Serum tests showed HBsAg, negative; HBsAb, positive; hepatitis C antibody, negative; HIV-1 antibody, positive; Treponema pallidum-specific antibodies, positive; rapid plasma reagin, negative. Abdominal ultrasonography showed hepatosplenomegaly and gallstones. Electrocardiography was normal. Co-infection of VL with AIDS was diagnosed.
Figure 2 Leishmania amastin (Leishmania amastigote form) was observed in a bone marrow aspirate of the patient. Both nucleus and kinetoplast can be seen within some amastigotes.
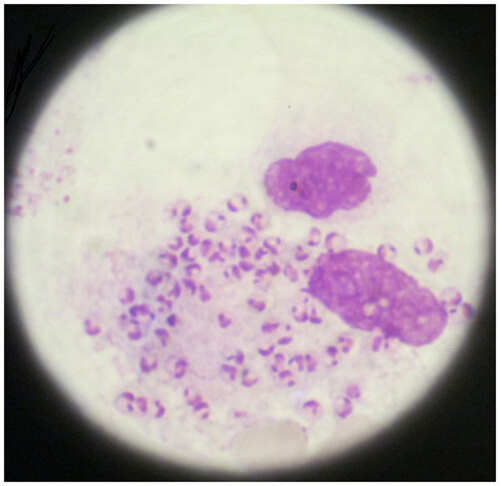
Table 1 The changes of the patient with VL/HIV co-infection in key indicators during treatment
After admission, the patient still presented with abdominal distension, normal body temperature, acceptable spirit, appetite and sleep, continuing to take lamivudine+tenofovir+efavirenz. Anti-leishmania therapy was given on 28 July 2012, using sodium stibogluconate (pentavalent antimony, 6 mL/day, containing antimony 0.6 g) by intravenous infusion. After four days of treatment, no adverse reactions were found. Pentavalent antimony was increased to 12 mL/day (including antimony 1.2 g) for an additional 4 days. The liver and spleen began to shrink, but the patient presented with a significant loss of appetite, fatigue and a worse pancytopenia. Drug-induced bone marrow suppression was suspected. Whole blood cells increased after stopping pentavalent antimony therapy. The usage of pentavalent antimony therapy, 6 mL (including antimony 0.6 g) was renewed every other day. The total dose of the first phase treatment was 204 mL of pentavalent antimony (containing antimony 20.4 g) from 28 July 2012 to 22 September 2012.
In October 2012, the degree of abdominal distension was mitigated. The spirit, appetite and energy levels of the patient were better than before. Upon physical examination, anemia was milder than before, the liver was not palpable below the right costal edge and the spleen was palpable 2 cm below the umbilical level. Leishmania amastin (Leishmania amastigote form) was not found in the bone marrow aspirate. Blood cell counts increased (). Blood chemistry panel of ALT, AST, TBIL, BUN, Cr and glucose was normal, and albumin was nearly normal; globulin and β2 microglobulin declined; serum sodium, potassium, calcium and magnesium returned to normal. CD4+ T lymphocyte counts were 225/µL, HIV-1 plasma RNA was below detection. Abdominal ultrasonography showed splenomegaly and gallstones. The patient was given 12 mL pentavalent antimony every other day. The total dose of the second phase treatment was 108 mL of pentavalent antimony (containing antimony 10.8 g) from 13 November 2012 to 22 November 2012.
In December 2012, the degree of abdominal distension was obviously mitigated. His general condition was much better than before. Upon physical examination, the patient had rosy lips, the liver could not be felt below the costal edge and the spleen was 1 cm over the umbilical level. Leishmania amastin (Leishmania amastigote form) was not found in his bone marrow aspirate. Blood cell counts are shown in . Blood ALT, AST, TBIL, BUN, Cr and glucose were normal, albumin and globulin returned to normal, and β2 microglobulin further declined; serum sodium, potassium, calcium and magnesium were maintained at a normal range. CD4+ T lymphocyte counts were 214/µL; HIV-1 plasma RNA was still not detected. Abdominal ultrasonography showed splenomegaly and gallstones. Electrocardiography was normal. He was given 12 mL pentavalent antimony every other day. The total dose of the third phase treatment was 108 mL of pentavalent antimony (containing antimony 10.8 g) from 8 January 2013 to 24 January 2013.
In July 2013, after 1 year treatment of sodium stibogluconate, it was determined that the patient was in good mental health, had a good appetite, was sleeping well and had a good level of physical strength via telephone follow-up. Abdominal distension disappeared. At his local hospital, it was determined that normal whole blood cells counts were restored (); the abdominal ultrasound showed the spleen was 3 cm below the costal edge and the number of CD4+ T lymphocytes were up to 330/µL. In January 2014 after one and half years of treatment with anti-Leishmania and ART, the abdominal ultrasound showed that the spleen of the patient was not palpable below the left costal edge, and the number of CD4+ T lymphocytes were up to 360/µL. His condition was considered stable after 12 months of pentavalent antimony withdrawal. The patient was still taking ART at the time this report is prepared.
DISCUSSION
More than 30 countries around the world have reported cases of VL/HIV-1 co-infection. Following the introduction of ART, one-third of patients with VL in Spain are co-infected with HIV-1.Citation2 HIV-1 infection rates among patients with VL range from 2% to 5% in India Citation3 and up to 34% in parts of Ethiopia.Citation4 VL can be spread through a sandfly bite or through exposure to contaminated blood. Intravenous drug users sharing syringes spread both VL and HIV-1, which is a main reason for the high rate of VL/HIV-1 co-infection in the aforementioned countries. VL is epidemic in multiple Chinese regions including Xinjiang, Gansu, Inner Mongolia, Sichuan, Shanxi and Shaanxi provinces. AIDS was found in China in 1985, while HIV-1-infected individuals were found all over China in 1998. There were tens of thousands of HIV-1 infected individuals in Xinjiang and Sichuan and intravenous drug use was an important reason for HIV-1 prevalence in these regions. There were four reports about VL/HIV-1 co-infection in China, which include a total of six patients.Citation5,Citation6,Citation7,Citation8 Given the large number of undetected HIV-1 infected individuals and the atypical clinical manifestations of VL/HIV-1 co-infection, it is easy to be misdiagnosed. Therefore, it is estimated that the actual number of patients with VL/HIV-1 co-infection far exceeds the number of reported cases.
There are limited data to guide recommendations for optimal therapy of VL in HIV-1 infected patients. Chemotherapeutic agents with efficacy against VL include pentavalent antimonial drugs, amphotericin B, pentamidine isethionate, paramomycin (a parenteral aminoglycoside) and miltefosine (the first oral drug for treatment of VL).
Pentavalent antimonial drugs, sodium stibogluconate (SSG) and meglumine antimoniate have been used for past decades as first line drugs for treatment because of their low cost and availability in most countries. Currently, this option has been sidelined due to their unacceptable toxicity and high rate of treatment failure and mortality. However, because of the high cost and lack of access to alternative agents, SSG at a dose of 20 mg/kg for 28–30 days is still used in low-resource settings.Citation9 Mortality rates with SSG treatment in patients with VL/HIV-1 co-infection in Ethiopia range from 16% to 25%, compared with 3%–4% in HIV-1 negative patients. A recent small study from Ethiopia reported cure rates of only 58% in VL/HIV-1 patients treated with meglumine antimoniate, while rates of side effects including pancreatitis was high.Citation10,Citation11,Citation12 In India, there is widespread parasite drug resistance to SSG, with efficacy rates as low as 35% in Bihar.Citation13,Citation14
Amphotericin B deoxycholate has high anti-leishmanial efficacy but it is associated with a high risk of renal toxicity and other side effects and has been replaced in recent years in countries with sufficient financial resources by lipidic formulations (L-AmB) of the drug. In HIV-1-negative patients with VL, L-AmB at a total dose of 20 mg/kg is extremely effective treatment, and in Indian HIV-1-negative VL patients, even a single 10 mg/kg dose has a treatment efficacy of 96%.Citation15,Citation16,Citation17 But outcomes following L-AmB treatment in HIV-1 infected VL patients have been disappointing. In India, a retrospective cohort of 55 ART-naive HIV-1/VL co-infected patients treated with L-AmB 20 mg/kg between 2007 and 2010 had both high relapse rates (eight patients, 14.5%) and high mortality (seven patients, 12.7%). Although the treatment was well tolerated, the overall death/treatment failure rate of 26.5% after 2 years compares poorly to the less than 1% mortality in HIV-1-uninfected VL patients in this study.Citation3 In a retrospective report on treating 116 HIV-1-infected patients with a first episode of VL and 79 relapse cases with L-AmB 30 mg/kg, parasitological failure rates were 32% (versus 0% of HIV-1 -negative patients treated with the same regimen). HIV-1-infected visceral leishmaniasis patients presenting with relapses had a particularly high parasitological failure rate of 56%, a concerning finding in a setting in which parasitological failure appears to be invariably followed by relapses and high mortality.Citation18
Pentamidine isethionate, a second-line alternative treatment, is rarely used due to suboptimal efficacy and toxicity. Although VL is treated similarly in patients with and without HIV-1 infection, co-infected patients generally have low cure and high mortality rates. Furthermore, HIV-1-infected patients are more likely to suffer treatment-related adverse events than the HIV-1-negative population.
The patient presented in the current report is a confirmed case of VL/HIV-1 co-infection. This patient had a long history (since the mid-1990s) of VL infection and was treated twice with pentavalent antimony treatment (0.6 g/day, 6 days); his condition improved, but the disease relapsed after several years. He was also diagnosed with HIV-1 infection in 2010 and then received ART. The recurrence of VL may be related to a shorter period of treatment with pentavalent antimony treatment in addition to a lower dose of the treatment. HIV-1-induced immunodeficiency may also play an important role in the recurrence. In recent years, the patient presented with splenomegaly and pancytopenia, but without fever. He had long been misdiagnosed as having liver cirrhosis. It should be noted that the clinical manifestations of patients co-infected with VL and HIV-1 may be atypical.
After one year of ART, his HIV-1 plasma RNA level was undetectable, but no significant increase in the number of CD4+ T lymphocytes was observed. Two months after treatment with the retraction of his liver and spleen, the number of CD4+ T lymphocytes rapidly increased to 200/µL, which suggested that the low CD4+ T lymphocytes may not only be related to HIV-1 infection, but that VL may play an important role on the patient's suspected immunodeficiency status. After the patient received pentavalent antimony and ART, his symptoms disappeared. The patient survived and lived normally.
Previously, there have been six reported cases of VL/HIV-1 co-infection in China.Citation5,Citation6,Citation7,Citation8 None had received VL treatment; five of these patients died within a few weeks and one was lost to follow up, which may be associated with the patients’ inadequate treatment.
Use of the pentavalent antimonials in VL/HIV-1 co-infected patients is no longer recommended by most experts in the field, owing to their toxicity and high rate of treatment failure and mortality.Citation9 Amphotericin B (AmB) or its lipid-based formulation (L-AmB) is currently recommended by the WHO as a first-line treatment for HIV-1-associated VL;Citation9 however, the failure and relapse rates of treatment are still high. Given the poor outcomes with the above drugs used as single agents in VL, interest has recently focused on drug combinations and development of new drugs. AmB has many side effects and L-AmB is very expensive; therefore, there are some difficulties with use of L-AmB in poor-resourcing countries. In the current case study, whole blood cells of the patient decreased during the period of pentavalent antimony treatment (daily dose of 1.2 g). His blood cells recovered after 1 week of withdrawal; therefore, we posit that the side effects of pentavalent antimony are likely reversible. When the dose of pentavalent antimony was reduced to 0.6 g per day or 1.2 g every other day, there were no obvious side effects. Since the side effects of pentavalent antimony are related to dose administered, appropriate dosage can avoid adverse reactions.
At present, trial data to guide the use of secondary prophylaxis for VL/HIV-1 co-infection are lacking. This patient had been given pentavalent antimony therapy with a total dose of up to 42 g, while also receiving ART. Currently, his overall condition is good and the number of CD4+ T lymphocytes is above 200/µL (up to 360/µL). After 12 months of pentavalent antimony withdrawal, his condition is stable.
Miltefosine is a new oral drug with an efficacy of 90%–94% in HIV-1-uninfected patients with VL.Citation19,Citation20 In patients with HIV-1-associated VL, miltefosine treatment appears to be well tolerated, but outcomes are suboptimal. In a study of 107 HIV-1-infected VL patients in Ethiopia, mortality outcomes were similar in miltefosine- and SSG-treated patients (5% versus 7%), but rates of initial treatment failure (17.5% versus 2.3%) and subsequent relapse (25% versus 11%) were higher in those receiving miltefosine.Citation11 However, whether miltefosine can prevent secondary infection is worth exploring. Proper dosage and course of pentavalent antimony treatment can prolong the life of patients with VL/HIV-1 co-infection and improve their quality of life. Furthermore, VL-disseminated histoplasmosis and penicillium marneffei are important opportunistic infections associated with HIV-1 infection; they have a similar clinical manifestation, are easily confused among these pathogens, and should be carefully discriminated from each other.Citation21 Due to the presence of a large number of migrating populations in China in recent years, medical staff needs to be vigilant of unique infectious diseases. In VL and HIV-1 endemic areas, the medical staff should pay more attention to the early diagnosis of VL, HIV-1 and VL/HIV-1 co-infection and design effective treatment strategies to control the diseases and prevent their further spread.
- Desjeux P.Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis2004;27: 305–318.
- Gil-Prieto R, Walter S, Alvar J, de Miguel AG.Epidemiology of leishmaniasis in Spain based on hospitalization records (1997–2008). Am J Trop Med Hyg2011;85: 820–825.
- Sinha PK, van GJ, Pandey K et al.Liposomal amphotericin B for visceral leishmaniasis in human immunodeficiency virus-coinfected patients: 2-year treatment outcomes in Bihar, India. Clin Infect Dis2011;53: e91–e98.
- ter Horst R1, Tefera T, Assefa G, Ebrahim AZ, Davidson RN, Ritmeijer K.Field evaluation of rK39 test and direct agglutination test for diagnosis of visceral leishmaniasis in a population with high prevalence of human immunodeficiency virus in Ethiopia. Am J Trop Med Hyg2009;80: 929–934.
- Gu Y, Ma CH, Zhou LB. [A HIV antibody-positive patient with kala azar.] Shi Yong Yi Ji Za Zhi2008;16: 2156–2157.Chinese.
- Bao YX, Pan KJ, Mai MT et al. [One case of AIDS with kala-azar.] Zhong Guo Bing Du Bing Za Zhi2012;6: 75–76.Chinese.
- Xia HJ, Yi YJ, Liao YQ. [The analysis of misdiagnosis about three cases of kala-azar with HIV infection.] Zhong Guo Pi Fu Xing Bing Xue Za Zhi2011;7: 567–568.Chinese.
- Zhang GJ, Cao BL, Xing JY. [One case with HIV infection and kala-azar.] Pi Fu Xing Bing Xue Za Zhi2010;11: 1072–1074.Chinese.
- Jarvis JN, Lockwood DN.Clinical aspects of visceral leishmaniasis in HIV infection. Curr Opin Infect Dis2013;26: 1–9.
- Hurissa Z, Gebre-Silassie S, Hailu W et al.Clinical characteristics and treatment outcome of patients with visceral leishmaniasis and HIV co-infection in northwest Ethiopia. Trop Med Int Health2010;15: 848–855.
- Ritmeijer K, Dejenie A, Assefa Y et al.A comparison of miltefosine and sodium stibogluconate for treatment of visceral leishmaniasis in an Ethiopian population with high prevalence of HIV infection. Clin Infect Dis2006;43: 357–364.
- Hailu W, Weldegebreal T, Hurissa Z et al.Safety and effectiveness of meglumine antimoniate in the treatment of Ethiopian visceral leishmaniasis patients with and without HIV co-infection. Trans R Soc Trop Med Hyg2010;104: 706–712.
- Chakravarty J, Sundar S.Drug resistance in leishmaniasis. J Glob Infect Dis2010;2: 167–176.
- Sundar S, More DK, Singh MK et al.Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis2000;31: 1104–1107.
- Sundar S, Jha TK, Thakur CP, Mishra M, Singh VR, Buffels R.Low-dose liposomal amphotericin B in refractory Indian visceral leishmaniasis: a multicenter study. Am J Trop Med Hyg2002;66: 143–146.
- Sundar S, Mehta H, Suresh AV, Singh SP, Rai M, Murray HW.Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulations. Clin Infect Dis2004;38: 377–383.
- Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW.Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med2010;362: 504–512.
- Mueller M, Ritmeijer K, Balasegaram M, Koummuki Y, Santana MR, Davidson R.Unresponsiveness to AmBisome in some Sudanese patients with kala-azar. Trans R Soc Trop Med Hyg2007;101: 19–24.
- Sundar S, Singh A, Rai M et al.Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis2012;55: 543–550.
- Sundar S, Rosenkaimer F, Makharia MK et al.Trial of oral miltefosine for visceral leishmaniasis. Lancet1998;352: 1821–1823.
- Gui XE, Guan LR. [Differential diagnosis of visceral leishmaniasis, progressive disseminated histoplasmosis and penicilliosis marneffei.] Zhong Guo Ji Sheng Chong Xue He Ji Sheng Chong Bing Za Zhi2007;25: 69–72.Chinese.