Abstract
Hand, foot and mouth disease (HFMD) is an important public health problem that has emerged over the past several years. HFMD predominantly infects children under seven years old and occasionally causes severe disease in adults. Among the enteroviruses, enterovirus 71 (EV71) and coxsackievirus 16 (CA16) are the major causative agents of HFMD. In addition, adenovirus cocirculates with enterovirus and has become a possible additional pathogenic factor for HFMD in some cases. Here, we have investigated the neutralizing antibody responses to both enterovirus and adenovirus in adults, with the aim of exploring the prevalence trends of these viruses and the nature of protective immunity in humans to these viral infections. Sera from 391 healthy adults from 21 provinces and cities in China were tested for the presence of antibodies against EV71, CA16, adenovirus human serotype 5 (AdHu5) and chimpanzee adenovirus pan7 (AdC7) using neutralization tests. High seroprevalence rates of EV71, CA16 and AdHu5 were found in the population (85.7%, 58.8% and 74.2%, respectively). The coseropositivity rate of these three viruses was 39.4% (154 of 391), with median neutralizing antibody titers of 80, 40 and 640, respectively, and the neutralizing antibody titer for EV71 was found to be correlated with those of CA16 and AdHu5. AdC7 was found to be a rare adenovirus serotype in the human population, with a seropositivity rate of 11.8%, suggesting that it could be a good choice for a vaccine carrier that could be used in vaccine development.
Introduction
The enterovirus genus of single-stranded RNA viruses includes poliovirus, coxsackie A virus, coxsackie B virus, echoviruses and other viruses that affects millions of people worldwide each year. Over 100 human enterovirus serotypes have been identified based on antibody neutralization tests, including enterovirus 71 (EV71) and coxsackievirus 16 (CA16), the main causative agents of hand, foot and mouth disease (HFMD), which has emerged as an important public health problem in recent years, especially in Asia and Pacific regions. EV71 and CA16 usually infect infants and children under seven years old and present with a papulovesicular rash.Citation1 A few children suffer complications such as myocarditis, pulmonary edema and aseptic meningoencephalitis, which can be fatal. Occasionally, HFMD is observed in adults.Citation2,Citation3
The major pathogens responsible for HFMD differ across countries. In China, HFMD is primarily associated with EV71, which caused the outbreaks of HFMD in Anhui province in 2008, in Guangdong province in 2009 and in Jiangsu province in 2012.Citation4,Citation5,Citation6 However, in other countries, HFMD is mostly associated with CA16.Citation7 The seroprevalence trends of these two viruses are changing over time. There are no vaccines or antiviral drugs available for HFMD. Although immunoglobulins and the antiviral agent ribavirin are commonly used, their efficacy remains uncertain.Citation8 Thus, the development of effective vaccines to prevent HFMD is critical. Knowing the seroprevalence of EV71 and CA16 is necessary to generate a suitable and effective vaccine. The seroprevalence of EV71 and CA16 has been investigated in children and pregnant women,Citation6,Citation9,Citation10,Citation11 but little is known about the seroprevalence of these viruses in healthy adults, despite the fact that adults do suffer from HFMD and can present with serious symptoms.Citation2,Citation3 However, efforts to investigate the seroprevalence of these viruses in adults are increasing and may reflect the continued spread of these viruses.
Co-infection with enterovirus and a second virus, such as adenovirus, has been suggested as another possible pathogenic factor for HFMD.Citation12 Adenoviruses are a family of double-stranded DNA viruses with more than 100 identified serotypes; adenoviruses cause a wide range of illnesses, from mild respiratory infections to multiorgan disease. Both enterovirus and adenovirus are transmitted via the respiratory or fecal–oral routes, and both can be neurotoxic. These two viruses can be shed for a prolonged period after infection in humans, so they can reach similar co-infection rates.Citation13 During an HFMD outbreak in Sarawak in 1997, a subgroup B adenovirus and an enterovirus were isolated from three fatal cases, supporting the concept of co-infection with these viruses in humans.Citation14
Pre-existing neutralizing antibodies in humans not only tell us the history and prevalence trends of certain pathogens but also indicate the nature of protective immunity in humans to the corresponding infections.Citation15,Citation16 In this study, we have investigated the prevalence of neutralizing antibodies to four viruses, EV71, CA16, adenovirus human serotype 5 (AdHu5) and chimpanzee adenovirus pan7 (AdC7), in the serum of healthy adults. AdHu5 is a subgroup C adenovirus and is one of the most common adenoviruses circulating in both children and adults. AdC7 originated in chimpanzees and is thought to be a rare serotype in humans, although little is known about its prevalence in the human population. Chimpanzee adenoviruses have been considered ideal carriers for the development of vaccines against a broad range of pathogens because their neutralizing antibodies are rare in humans, and this low antibody prevalence would circumvent the negative effects of pre-existing immunity to common human serotypes of adenovirus.Citation17,Citation18 Evaluating the prevalence of neutralizing antibodies to AdC7, compared with AdHu5, may provide evidence supporting the potential use of AdC7 as an additional or alternative vaccine carrier.
MATERIALS AND METHODS
Human serum samples
Serum samples from 391 healthy adult humans were collected at random times throughout 2012 by the Suzhou Industrial Park Centers for Disease Control and Prevention. Two hundred and seven study participants were from Jiangsu province, while the remaining participants were from the other 20 provinces and cities in China. All serum samples were heat inactivated at 56 °C for 30 min prior to testing. Informed consent was obtained from each study participant, and the collection and use of serum samples for this study were approved by the Human Ethics Committee of the Suzhou Industrial Park Centers for Disease Control and Prevention.
Cells and viruses
Human rhabdomyosarcoma (RD) and human embryonic kidney (HEK293) cells were maintained in complete Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco BRL, Grand Island, NY, USA), 2% penicillin and streptomycin (HyClone, Logan, UT, USA). EV71 strain G082 (genogroup C4) and CA16 strain SZ05 (GenBank accession NO EU262658), kindly provided by Dr Zhong Huang (Institute Pasteur of Shanghai, Shanghai, China), were amplified in RD cells and purified by sucrose density gradient centrifugation. Recombinant replication-defective adenoviruses AdHu5 and AdC7 expressing green fluorescent protein were constructed in our laboratory using published protocols.Citation17 The recombinant adenoviruses were amplified in HEK293 cells to generate high-titer viral stocks and then purified by cesium chloride density gradient centrifugation. All four viruses were dissolved in phosphate buffered saline with 10% glycerol and stored at −80 °C for use in all tests in this study.
EV71 and CA16 neutralization assays
The titers of the EV71 and CA16 viruses were determined using the Reed and Muench method to analyze the cytopathic effects observed in infected RD cells and were expressed as 50% tissue culture infectious doses (TCID50). The neutralization antibody tests for EV71 and CA16 were performed as previously described.Citation19,Citation20 Viruses were diluted to 2 TCID50/µL. Fifty microliters of diluted viruses was mixed with 50 µL of two-fold serially diluted serum, at dilutions of 1∶10–1∶1280, in 96-well plates and incubated at 37 °C for 1 h. RD cells (1×104) were added to each well after incubation. Seventy-two hours later, cytotoxicity was observed under a microscope. The neutralizing titer was defined as the highest serum dilution factor that resulted in a 50% inhibition of the cytopathic effect. A neutralizing titer ≥10 was considered positive.
Adenovirus neutralization assays
An adenovirus neutralization assay was performed based on previously described methods.Citation21 Before testing, 10-fold serial dilutions of the recombinant adenoviruses were prepared, and 50 µL of each dilution was added to 96-well plates, followed by 50 µL of Dulbecco’s modified Eagle’s medium with 5% fetal bovine serum. One hundred microliters of HEK293 cell suspension (2.5×105 cells/mL) was added to wells in the same 96-well plate. Twenty-four hours later, green fluorescent protein levels were examined by fluorescence microscopy to determine a suitable virus concentration to use in the neutralization test. Viruses were then diluted to this concentration, mixed with two-fold serially diluted (1∶10–1∶1280) human serum in 96-well plates and incubated at 37 °C for 1 h. After incubation, 100 µL of an HEK293 cell suspension (2.5×105 cells/mL) was added to each well and the plates were then incubated at 37 °C in a 5% CO2 atmosphere. Twenty-four hours later, the 96-well plates were examined by fluorescence microscopy. Dulbecco’s modified Eagle’s medium without serum was used as the negative control. The neutralizing antibody titer was expressed as the reciprocal of dilutions for which the proportion of green fluorescent protein-expressing cells was reduced to approximately 50% of that for the negative control. A titer ≥20 was regarded as positive for the serotype-specific neutralizing antibodies.Citation22
Data analysis
Seroprevalence rates were compared between viruses using the Chi-square test. Analysis of varianceCitation23 was performed to compare the means of neutralizing antibody titers across different regions, genders and ages. The correlations between neutralizing antibody titers of the viruses studied were calculated using Spearman’s method.Citation24 All statistical analysis was performed using SPSS (Statistical Package for the Social Sciences, Chicago, IL, USA) software (version 16). A P value of less than 0.05 was considered to be statistically significant.
RESULTS
Study participants and characteristics
This study enrolled 391 healthy adults from 21 provinces and cities (). Participants were aged 18–71 (mean: 30.7±8.9) years and included 226 males and 165 females. Participants were divided into five age groups. The study sample was representative of the geographical distribution of the population of China, with 239 of participants from coastal (Jiangsu, Zhejiang, Shanghai and Shandong) regions and 152 from inland areas (Henan, Shanxi, Hunan, Hubei and other provinces).
Table 1 Demographics of all serum sample donors (n=391)
Seroprevalence and distribution of neutralizing antibodies to four viruses
The seroprevalence rates were as follows: EV71, 85.7% (335/391, 95% confidence interval (CI): 82.2%–89.2%); CA16, 58.8% (230/391, 95% CI: 53.9%–63.7%); AdHu5, 74.2% (290/391, 95% CI: 69.8%–78.5%); AdC7, 11.8% (46/391, 95% CI: 8.6%–15.0%). Seropositivity for anti-EV71 neutralizing antibody was greater than that for anti-CA16 (P<0.0001), and the seroprevalence of anti-AdHu5 neutralizing antibodies was much higher than that of anti-AdC7 neutralizing antibodies (P<0.0001). Because EV71, CA16 and AdHu5 have high seroprevalences, the prevalence of neutralizing antibodies to all three of these viruses was calculated and found to be 39.4% (154/391, male/female ratio: 69∶85), with median neutralizing antibody titers of 80, 40 and 640 for EV71, CA16 and AdHu5, respectively.
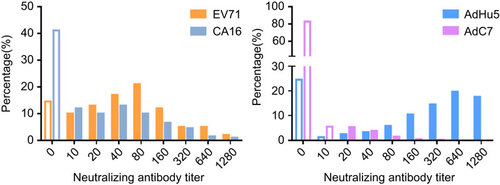
The distribution of titers for the four viruses is shown in . Positive titers were primarily concentrated in the ranges of 10–160 for EV71 and CA16, 160–1280 for AdHu5 and 20–40 for AdC7. Neutralizing antibody titers for AdHu5 in these participants were very high, while the titers for AdC7 were very low, with only one titer reaching 320.
Figure 1 Distribution of EV71, CA16, AdHu5 and AdC7 neutralizing antibody titers. The blank bars represent the negative ratios, while colored bars represent the corresponding positive ratios in each titer group. The ratio of the number of participants in each titer group to the total number of subjects is shown as a percentage.
Virus seroprevalence in different geographical regions
Of the 391 healthy adults whose serum samples were collected, 61.1% were from coastal regions and 38.9% were from inland regions. Viral seroprevalence rates in adults from coastal regions were higher than those from inland regions, except for AdC7. However, only the difference in AdHu5 seroprevalence rates between coastal and inland areas reached statistical significance (P=0.001; ). The median neutralizing antibody titers to EV71, CA16, AdHu5 and AdC7 in seropositive individuals were 80, 40, 640 and 40, respectively, in coastal regions, and 60, 40, 320 and 40, respectively, inland. However, there were no statistically significant differences in the neutralizing antibody titers for any of the four viruses between coastal and inland regions ().
Figure 2 EV71, CA16, AdHu5 and AdC7 neutralizing antibody titers of seropositive individuals from different areas. No significant differences in neutralizing antibody titers of all viruses between coastal and inland areas were found. The box plot shows the minimum, first quartile, median, third quartile and maximum titer levels.
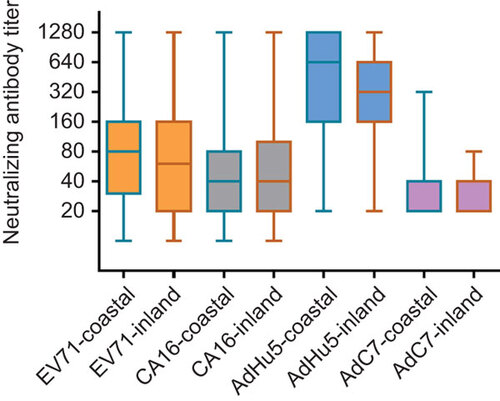
Table 2 Seroprevalence of EV71, CA16, AdHu5 and AdC7 in different regions
Virus seroprevalence by gender and age group
The seroprevalence rates for all four viruses were higher in females () but only reached statistical significance for CA16 and AdHu5. A significant difference in neutralizing antibody titer was only observed between males and females for CA16 (P=0.006) ().
Comparing the seroprevalence rates among different age groups, no significant difference was found for EV71, CA16 or AdC7. The seropositivity rate for AdHu5 in the 21–30 years old age group was significantly lower than that in the 41–50 years old age group (P=0.011), but there was no other significant difference between other age groups for AdHu5 (). There were no significant differences in titer values of neutralizing antibodies between age groups for all four viruses ().
Figure 3 Neutralizing antibody titers against EV71, CA16, AdHu5 and AdC7 of seropositive individuals, male and female. Significant difference in neutralizing antibody titers was only observed between males and females for CA16 (P=0.006). The box plot shows the minimum, first quartile, median, third quartile and maximum titer levels.
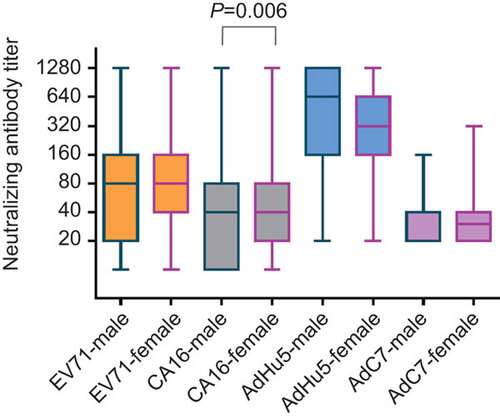
Figure 4 Neutralizing antibody titers against EV71, CA16, AdHu5 and AdC7 of seropositive individuals among different age groups. No significant differences were found in titer values of neutralizing antibodies between age groups for all four viruses. The box plot shows the minimum, first quartile, median, third quartile and maximum titer levels.
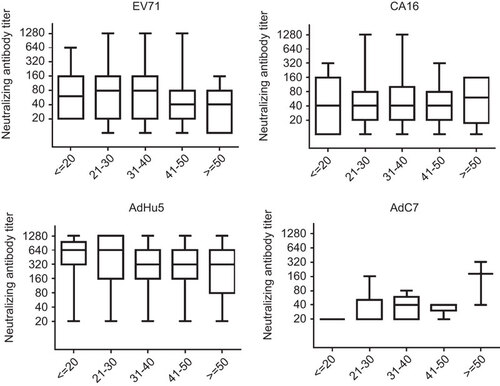
Table 3 Seroprevalence of EV71, CA16, AdHu5 and AdC7 by gender
Table 4 Seroprevalence of EV71, CA16, AdHu5 and AdC7 among different age groups
Correlation between neutralizing antibody titers for different viruses
To analyze the relations between infections with different viruses, a correlation analysis was performed and showed that neutralizing antibody titers for EV71 were associated with those for CA16 (r=0.145, P=0.004) and AdHu5 (r=0.126, P=0.013). A significant correlation between neutralizing antibody titers for other viruses was not observed.
DISCUSSION
HFMD predominantly occurs in children and can manifest severe symptoms, especially in those younger than three years old.Citation10 For this reason, most seroprevalence studies of EV71 and CA16 have focused on the pediatric population.Citation6,Citation9,Citation11 Adults can also develop HFMD and neutralizing antibodies when exposed to EV71 and CA16. Previous reports showed that humoral immunity mediated by neutralizing antibodies plays a critical role in protection against EV71 and CA16,Citation7,Citation25,Citation26 although cell-mediated immunity also contributes to protection against EV71 and CA16.Citation27,Citation28 Adult infection with these two viruses is mostly latent and asymptomatic, but the mechanisms underlying this response in adults have not been clarified.
A study in Shanghai demonstrated that subclinically EV71-infected children served as a source of continued spread of EV71 in the population.Citation9 Thus, adults who carry EV71 and CA16 asymptomatically may pose a significant threat to children. Children are likely to become infected with EV71 and CA16 from adults, although further research is needed in this area. Protection from HFMD should address both children and adults. A seroprevalence study of pre-existing immunity in adults is indispensable, as this immunity may be associated with less clinical severity in adults, and such a study can shed light on the spread of HFMD in children and the general population.
A survey conducted using serum samples from patients tested for other viruses in Germany revealed that CA16 seroprevalence rates were 70%–85% in adults aged 20–59 years, whereas EV71 seroprevalence rates were 40%–48% in adults in the same age range.Citation7 However, another study in Germany found that 75% of healthy adults aged 20–40 years old had neutralizing antibodies to EV71.Citation25 In our study, the EV71 seroprevalence rate was 85.7% in healthy adults, higher than that in Germany, while the seroprevalence rate for CA16 (58.8%) was much lower, which may be because HFMD in Germany tends to be associated with CA16 infections and is less commonly due to EV71.Citation7 Yang et al.Citation28 reported that 83.3% (10 of 12 samples) of healthy adults were antibody-positive to EV71 in 2008 in Fuyang City, China, where the national HFMD outbreak originated in that year. Another study on neutralizing antibodies in pregnant women in Jiangsu, China found the seropositivity rate for EV71 to be 85.3%, which was similar to our result, while the CA16 seropositivity rate was 89.1%, which was much higher than we found.Citation10 We showed that seroprevalence rates for EV71 and CA16 in adults did not vary significantly across age groups and were comparable (EV71: >80%, CA16: 54%) to those in children between 5 and 15 years of age, as reported previously,Citation6 suggesting that infection rates for these viruses may be saturated in children older than 5 years.
Enterovirus outbreaks primarily occur during summer and fall in tropical areas, so infection rates may vary across geographic regions. However, no significant differences in seroprevalence between coastal and inland areas were observed in our study. Higher EV71 and CA16 seroprevalence and neutralizing antibody titer values were found in females, but only those for CA16 were statistically significantly different. We speculate that Chinese women are involved in more household cleaning and care of the sick, so their likelihood of getting infected is higher.
Children are susceptible to adenovirus infection, which accounts for 10% of febrile illnesses in children.Citation29 A previous study indicated that neutralizing antibody levels for adenovirus, together with those for EV71 and CA16, approached adult levels after seven years of age.Citation1,Citation30 Thus, adenovirus may cocirculate with enterovirus in humans. AdHu5 is one of the most common serotypes to infect both children and adults. AdHu5 seroprevalence rates in adults vary from 60% to 70% in EuropeCitation31,Citation32 and the USACitation33,Citation34 and up to 98% in warmer African and Asian tropical countries.Citation19,Citation35,Citation36,Citation37 Several studies have shown that AdHu5 seropositivity rates in some areas of China ranged from 72% to 77.34% in adults,Citation22,Citation38,Citation39 consistent with our results (74.2%). We found AdHu5 seroprevalence to be significantly higher in coastal regions than in inland regions, which could be due to the mild and humid climate in coastal areas; it is possible that the coastal climate contributes to the spread of adenovirus infections. We also found a gender difference in AdHu5 seroprevalence, which was not consistent with previous reports.Citation35 However, AdHu5 and CA16 have similar gender distributions of seroprevalence. In our study, AdHu5 seroprevalence increased with age (21–50 years), with an older population (41–50 years old) having the highest seropositivity (87.5%), in agreement with a previous report.Citation22
Because the prevalence of AdHu5 is so high in humans and because pre-existing immunity to AdHu5 dampens the immune response induced by AdHu5-based vaccines, rare adenovirus serotypes from other species, such as chimpanzees, have been developed and tested as vectors for vaccine development. AdC7 represents a distinct serotype related to human adenovirus and has already been used as a vaccine carrier in many studies, with promising results.Citation40,Citation41,Citation42 However, the seroprevalence status of AdC7 in humans has not been investigated extensively. In our study, we showed that the AdC7 seropositivity rate in adults is low (11.8%) and similar to that for AdC68 (12.7%) in Chinese adults,Citation22 and that the AdC7 seroprevalence rate does not vary significantly among regions, genders or age groups. Like AdC7, AdC68 is another rare serotype, related to human adenovirus, from chimpanzees.Citation17 The neutralizing antibody titer for EV71 correlated with those of CA16 and AdHu5, which implies these three viruses cocirculate in adults and that adenovirus infection also should be taken into consideration in the prevention and treatment of HFMD.
Here, we have demonstrated that EV71, CA16 and AdHu5 were highly epidemic among healthy adults in China. The correlation between neutralization titers for EV71, CA16 and AdHu5 further confirms that enterovirus cocirculates with human adenovirus in humans. AdC7 rarely circulates in adults, suggesting that it could be a good choice as a vaccine carrier for vaccine development.
This work was supported by grants from the Knowledge Innovation Program (NO 54PCL2010-0030101) and the 100 Talents Program (NO 2A201331121100301) of the Chinese Academy of Sciences to Dongming Zhou. We gratefully acknowledge Dr Zhong Huang, Institute Pasteur of Shanghai, Chinese Academy of Sciences, China, for his generous assistance and reagents. We thank the participating nurses for their assistance with data and sample collection, and we thank all the study participants for taking part in the study.
- Yu H, Wang M, Chang H et al.Prevalence of antibodies against enterovirus 71 in children from Lu’an City in Central China. Jpn J Infect Dis2011;64: 528–532.
- Toya M, Endo Y, Tanizaki H, Fujisawa A, Tanioka M, Miyachi Y.An adult case of severe hand-foot-mouth disease accompanying persistent fever and systemic arthritis. Dermatol Online J2012;18: 14.
- Hamaguchi T, Fujisawa H, Sakai K et al.Acute encephalitis caused by intrafamilial transmission of enterovirus 71 in adult. Emerg Infect Dis2008;14: 828–830.
- Yang F, Ren L, Xiong Z et al.Enterovirus 71 outbreak in the People’s Republic of China in 2008. J Clin Microbiol2009;47: 2351–2352.
- De W, Changwen K, Wei L et al.A large outbreak of hand, foot, and mouth disease caused by EV71 and CAV16 in Guangdong, China, 2009. Arch Virol2011;156: 945–953.
- Ji H, Li L, Liu Y et al.Seroepidemiology of human enterovirus 71 and coxsackievirusA16 in Jiangsu province, China. Virol J2012;9: 248.
- Rabenau HF, Richter M, Doerr HW.Hand, foot and mouth disease: seroprevalence of Coxsackie A16 and Enterovirus 71 in Germany. Med Microbiol Immunol2010;199: 45–51.
- Chen X, Wang C, Xu L et al.A laboratory evaluation of medicinal herbs used in china for the treatment of hand, foot, and mouth disease. Evid Based Complement Alternat Med: eCAM2013;2013: 504563.
- Zeng M, El Khatib NF, Tu S et al.Seroepidemiology of Enterovirus 71 infection prior to the 2011 season in children in Shanghai. J Clin Virol2012;53: 285–289.
- Zhu FC, Liang ZL, Meng FY et al.Retrospective study of the incidence of HFMD and seroepidemiology of antibodies against EV71 and CoxA16 in prenatal women and their infants. PloS ONE2012;7: e37206.
- Luo ST, Chiang PS, Chao AS et al.Enterovirus 71 maternal antibodies in infants, Taiwan. Emerg Infect Dis2009;15: 581–584.
- Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE.Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis2003;9: 78–85.
- Ruohola A, Waris M, Allander T, Ziegler T, Heikkinen T, Ruuskanen O.Viral etiology of common cold in children, Finland. Emerg Infect Dis2009;15: 344–346.
- Cardosa MJ, Krishnan S, Tio PH, Perera D, Wong SC.Isolation of subgenus B adenovirus during a fatal outbreak of enterovirus 71-associated hand, foot, and mouth disease in Sibu, Sarawak. Lancet1999;354: 987–991.
- Catanzaro AT, Koup RA, Roederer M et al.Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis2006;194: 1638–1649.
- McElrath MJ, de Rosa SC, Moodie Z et al.HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet2008;372: 1894–1905.
- Xiang Z, Gao G, Reyes-Sandoval A et al.Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol2002;76: 2667–2675.
- Xiang Z, Li Y, Cun A et al.Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg Infect Dis2006;12: 1596–1599.
- Shi J, Huang X, Liu Q, Huang Z.Identification of conserved neutralizing linear epitopes within the VP1 protein of coxsackievirus A16. Vaccine2013;31: 2130–2136.
- Ku Z, Ye X, Huang X et al.Neutralizing antibodies induced by recombinant virus-like particles of enterovirus 71 genotype C4 inhibit infection at pre- and post-attachment steps. PloS ONE2013;8: e57601.
- Sprangers MC, Lakhai W, Koudstaal W et al.Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol2003;41: 5046–5052.
- Zhang S, Huang W, Zhou X, Zhao Q, Wang Q, Jia B.Seroprevalence of neutralizing antibodies to human adenoviruses type-5 and type-26 and chimpanzee adenovirus type-68 in healthy Chinese adults. J Med Virol2013;85: 1077–1084.
- Davenport CB.Analysis of variance applied to human genetics. Proc Natl Acad Sci USA1940;1: 1–3.
- Spearman C.The proof and measurement of association between two things. Int J Epidemiol2010;5: 1137–1150.
- Diedrich S, Weinbrecht A, Schreier E.Seroprevalence and molecular epidemiology of enterovirus 71 in Germany. Arch Virol2009;154: 1139–1142.
- Zhu Z, Zhu S, Guo X et al.Retrospective seroepidemiology indicated that human enterovirus 71 and coxsackievirus A16 circulated wildly in central and southern China before large-scale outbreaks from 2008. Virol J2010;7: 300.
- Chang LY, Hsiung CA, Lu CY et al.Status of cellular rather than humoral immunity is correlated with clinical outcome of enterovirus 71. Pediatr Res2006;60: 466–471.
- Yang C, Deng C, Wan J, Zhu L, Leng Q.Neutralizing antibody response in the patients with hand, foot and mouth disease to enterovirus 71 and its clinical implications. Virol J2011;8: 306.
- Fox JP, Hall CE, Cooney MK.The Seattle Virus Watch. VII. Observations of adenovirus infections. Am J Epidemiol1977;105: 362–386.
- Thorner AR, Vogels R, Kaspers J et al.Age dependence of adenovirus-specific neutralizing antibody titers in individuals from sub-Saharan Africa. J Clin Microbiol2006;44: 3781–3783.
- Kostense S, Koudstaal W, Sprangers M et al.Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS2004;18: 1213–1216.
- Mast TC, Kierstead L, Gupta SB et al.International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine2010;28: 950–957.
- Nwanegbo E, Vardas E, Gao W et al.Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin Diagn Lab Immunol2004;11: 351–357.
- Sumida SM, Truitt DM, Lemckert AA et al.Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol2005;174: 7179–7185.
- Pilankatta R, Chawla T, Khanna N, Swaminathan S.The prevalence of antibodies to adenovirus serotype 5 in an adult Indian population and implications for adenovirus vector vaccines. J Med Virol2010;82: 407–414.
- Abbink P, Lemckert AA, Ewald BA et al.Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol2007;81: 4654–4663.
- Dudareva M, Andrews L, Gilbert SC et al.Prevalence of serum neutralizing antibodies against chimpanzee adenovirus 63 and human adenovirus 5 in Kenyan children, in the context of vaccine vector efficacy. Vaccine2009;27: 3501–3504.
- Yu B, Zhou Y, Wu H et al.Seroprevalence of neutralizing antibodies to human adenovirus type 5 in healthy adults in China. J Med Virol2012;84: 1408–1414.
- Sun C, Zhang Y, Feng L et al.Epidemiology of adenovirus type 5 neutralizing antibodies in healthy people and AIDS patients in Guangzhou, southern China. Vaccine2011;29: 3837–3841.
- Roy S, Kobinger GP, Lin J et al.Partial protection against H5N1 influenza in mice with a single dose of a chimpanzee adenovirus vector expressing nucleoprotein. Vaccine2007;25: 6845–6851.
- Kobinger GP, Figueredo JM, Rowe T et al.Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine2007;25: 5220–5231.
- Kobinger GP, Feldmann H, Zhi Y et al.Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology2006;346: 394–401.
