Abstract
Clostridium difficile is the leading cause of infectious diarrhea in hospitalized patients. Integrating several infection control and prevention methods is a burgeoning strategy for reducing disease incidence in healthcare settings. We present an up-to-date review of the literature on ‘control bundles’ used to mitigate the transmission of this pathogen. All clinical studies of control bundles reported substantial reductions in disease rates, in the order of 33%–61%. Using a biologically realistic mathematical model we then simulated the efficacy of different combinations of the most prominent control methods: stricter antimicrobial stewardship; the administering of probiotics/intestinal microbiota transplantation; and improved hygiene and sanitation. We also assessed the health gains that can be expected from reducing the average length of stay of inpatients. In terms of reducing the rates of colonization, all combinations had the potential to give rise to marked improvements. For example, halving the number of inpatients on broad-spectrum antimicrobials combined with prescribing probiotics or intestinal microbiota transplantation could cut pathogen carriage by two-thirds. However, in terms of symptomatic disease incidence reduction, antimicrobials, probiotics and intestinal microbiota transplantation proved substantially less effective. Eliminating within-ward transmission by improving sanitation and reducing average length of stay (from six to three days) yielded the most potent symptomatic infection control combination, cutting rates down from three to less than one per 1000 hospital bed days. Both the empirical and theoretical exploration of C. difficile control combinations presented in the current study highlights the potential gains that can be achieved through strategically integrated infection control.
Introduction
Clostridium difficile is the leading cause of infectious diarrhea in hospitalized patients. Although highly variable between countries, the worldwide incidence and severity of C. difficile infection (CDI) have increased in recent years,Citation1,Citation2,Citation3 with a higher proportion of CDI patients undergoing colectomy and dying.Citation4,Citation5 The disease is currently estimated to cost $800 million per year in US acute care facilities.Citation6 Of particular concern are epidemic strains of the pathogen that have emerged in recent years and that incur high mortality rates.Citation7 While the disease has traditionally been associated with healthcare facilities of the industrialized world, it is increasingly recognized as a major contributor to healthcare-acquired infections in developing countries.Citation8 Studies in Argentina, Chile, India and Iran have shown a consistently high prevalence of CDI (6%–17%) in inpatients.Citation9,Citation10,Citation11,Citation12
Until recently, disturbance of the intestinal microbiota resulting from antimicrobials was considered a prerequisite of the disease. However, the epidemiological picture of CDI has been obscured following increased reports of transmission within the community and severe cases occurring in previously low-risk groups, including pregnant women, children and people with no recent exposure to antimicrobials.Citation13,Citation14 An increased frequency of newly emergent epidemic strains has also been described.Citation7 For both endemic and epidemic strains of C. difficile, healthcare facilities act as infection transmission hubs and, therefore, provide obvious targets for intervention.
Although published studies detailing the simulated pathogen transmission dynamics are relatively few in number, almost all have explored the anticipated effects of different interventions.Citation15,Citation16,Citation17,Citation18 These different models with different underlying structures and methods of analysis have yielded a good level of agreement in their projections. In short, their projections agree over the health benefits that can be expected from increased hygiene and sanitation practices within hospitals in order to reduce C. difficile transmission potential.
To date, no study has systematically analyzed the clinical literature for the level of health gains that can be expected from integrating the numerous available control methods. This is surprising given the multicomponent strategies that are routinely employed to combat the spread of disease in hospitals. The Association for Professionals in Infection Control and Epidemiology currently describe a suite of recommendations for preventing CDI.Citation19 Given the recognized major role of the environment in transmission,Citation20 contact precautions are recommended through segregating CDI from non-CDI patients; limiting patient movement through the healthcare facility; vigilant equipment disinfection; and wearing isolation gowns and gloves for each patient encounter. Related to this latter measure, strict adherence to hand hygiene protocols by staff, patients and visitors, and, proper environmental decontamination are further recommended interventions. From a modeling perspective, all of these measures will have the function of reducing within-hospital ward infection transmission potential.
The Association for Professionals in Infection Control and Epidemiology also recommends antimicrobial stewardship as another important component of infection prevention. This is defined as the avoidance of prolonged empiric therapy, targeting therapy by narrowing the spectrum of antimicrobial action, ensuring that the appropriate dosage and duration of therapy are used, and then discontinuing therapy as soon as possible. Because antimicrobial exposure is the primary risk factor associated with CDI development,Citation21 stewardship is expected to attenuate infection rates by reducing the overall susceptibility of hospital patients. These guidelines essentially reiterate the general recommendations of multifactorial infection control measures as described in preceding guidelines.Citation22,Citation23,Citation24
Our aim is to explore effective strategies for combining C. difficile control measures in order to develop an infection control framework that capitalizes upon a multipronged interruption of the pathogen’s transmission. First, we review the clinical literature for evidence to support (or refute) the additional efficacy in reducing C. difficile burden by combining different controls. Then we describe a stochastic, event-driven mathematical model of C. difficile transmission (adapted and updated from reference Citation25) and use it to simulate several control combinations—including both standard and novel control measures. The model is used to inform improved efficacy in infection control practices within healthcare facilities.
MATERIALS AND METHODS
Literature search strategy and study selection
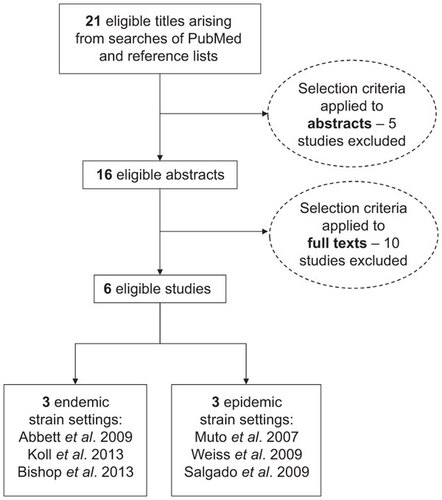
A search was conducted of all relevant articles published up until March 2014, identified from the PubMed database. Key terms used in the search strategy included: ‘Clostridium difficile or C. difficile’ and ‘bundle or multiple control or control package or integrated control or multipronged or multi-pronged’. Review of bibliographies of papers was also carried out to ensure completeness of inclusion of all relevant clinical studies. Studies eligible for inclusion were those describing patient levels of symptomatic C. difficile infection before and after the implementation of multiple, overlapping infection transmission interventions. Articles that involved formalized strategies for enhancing the rates of multiple, pre-existing controls were included along with reports describing the introduction of control methods that were previously absent from the study setting (). We discuss the outcome of this literature search in conjunction with results from our stochastic simulations of bundle approaches to controlling C. difficile.
C. difficile and its transmission
C. difficile is a gram-positive toxin-producing anaerobic bacterium transmitted via the fecal-oral route. While disturbed gut microbiota resulting from exposure to broad-spectrum antimicrobials is the prevailing predisposing factor,Citation26 this is no longer believed to be a prerequisite for the successful colonization of the gut.Citation27 Hence, there are two alternative routes of infection: one in an antimicrobial-treated, predisposed subpopulation and the other in a subpopulation of individuals that have not recently received treatment with antimicrobials. The inclusion of these parallel routes of bacterial colonization is key to understanding the modern epidemiology of C. difficile. The following section describes the compartmental framework that maps out the connections between the different epidemiological groups of patients in an acute healthcare facility. This mathematical model is then used to assess different integrated control strategies (or, ‘control bundles’) for reducing the transmission of C. difficile and ameliorating the burden of associated disease.
The mathematical model
We adapted our recently published model of Clostridium difficile transmission dynamicsCitation25 to account for an increased level of biological realism before simulating different control combinations (the new model structure is shown in and the further improvements made to this model are detailed throughout the model description). The ordinary differential equations describing the instantaneous rates of change between the seven possible epidemiological states are as follows:
Figure 2 Compartmental design of the stochastic, event-driven mathematical model of C. difficile transmission within a simulated 1000-bed acute care hospital.
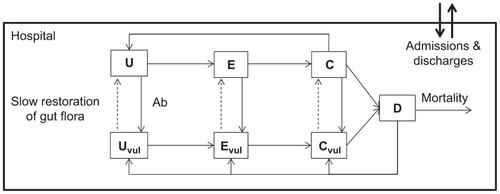
Here, the total hospital inpatient population, N=U+Uv+E+Ev+C+Cv+D, was maintained at 1000 (assuming that a hospital bed is filled more or less as soon as it is emptied). Roman letters denote the number of individuals in the given state and Greek letters denote rates (and proportions) of change. ‘U’nexposed individuals become ‘E’xposed to C. difficile before they are asymptomatically ‘C’olonized, and, subsequently, symptomatically ‘D’iseased. There are two subpopulations described by the equations, differentiating individuals who have, and who have not, recently taken broad-spectrum antimicrobials. Infection in individuals who have not recently taken antimicrobials is a key feature of the modern epidemiology of C. difficile and is believed to have come about through the successful spread of hypervirulent strains.Citation7,Citation28 The subscript ‘v’ denotes the groups that are currently taking, or have recently taken antimicrobials, and are more vulnerable to CDI progression than those who are not exposed to antimicrobials. The pathogen transmission coefficient is denoted β. Following exposure to C. difficile spores, it takes an average of five days (η−1=5) before patients become asymptomatically colonized and infectious.Citation29 Following recent evidence, antimicrobial use does not increase the likelihood of colonization.Citation30 Predisposed patients consist of those that are currently on antimicrobials, or whom have taken antimicrobials in the preceding three months. This predisposed group is assumed to make up 50% of all inpatients.Citation31,Citation32 Progression to symptomatic disease (CDI) takes five days (θ−1=5) following colonization and is five times more likely for predisposed patients (ε−1=5).Citation30 In other words, the key mechanisms by which vulnerable and normal inpatients differ are the proportion of colonized individuals who become symptomatic and the rate at which they become symptomatic (which is higher for those who have been recently exposed to antimicrobials). This enhanced biological realism is a key distinguishing feature between this current model and previously published models including our own previous simulation model.Citation25
Patient admissions, ϕ, were assumed to perfectly balance discharges summed with CDI deaths (ϕ=κ(N−D)+μ(1−ζ)D, assuming a constant hospitalized population) and were split proportionally across the different epidemiological categories according to ξ (with corresponding subscripts). Discharge rates were calculated simply as the inverse of the average length of stay, assumed to be 6 days.Citation33 Patients can be newly admitted in any epidemiological state but can only be discharged if they are not symptomatically infected. Patients can switch from non-predisposed to predisposed at rate α (accounting for the rate of antimicrobial prescription) and λ denotes the reverse process whereby a patient’s gut microbiota recovers following discontinued antimicrobial use—assumed to take approximately three months.Citation34 It is assumed that the administering of probiotics or intestinal microbiota transplantation acts by expediting this recovery rate.Citation35,Citation36 The symptoms of 33% of patients with CDI are assumed to self-resolveCitation37 within 2 days,Citation38 reflecting the high percentage of mild symptoms reported for this infection.Citation39 This rapid self-resolution of the significant percentage of CDI sufferers with milder symptoms is another element of enhanced biological realism that distinguishes this model from all previous simulation analyses.
CDI treatment (ρ−1) takes 10 daysCitation40 with an unsuccessful clearance rate, σ, of 20% per treated patient.Citation41 6.8% of CDI sufferers die within 60 days of symptoms onset (the daily mortality rate, μ, is therefore calculated as [1−(1−0.068)(1/60)]=0.0012).Citation30 This mortality rate is only experienced by the patients who suffer more severe symptoms—a logical and novel inclusion to this model. Symptomatic infection is itself treated with antimicrobials and, because of the damaged gut microbiota associated with symptoms, patients remain in vulnerable categories post-treatment. CDI sufferers are immediately quarantined from other inpatients and so do not contribute to transmission. While this does represent an optimistic simplification of the epidemiological system, our previous analyses have shown that within-hospital transmission is insensitive to a wide range of simulated screening/isolation levels.Citation25 The model parameters and associated studies are described in
Table 1 Epidemiological model symbology and parameterization
Using the methods outlined by Keeling and RohaniCitation42 this deterministic set of equations was converted into an event-driven Direct Gillespie simulation system.Citation43 Stochasticity incorporation is justified by the low prevalence of symptomatic infection harbored by the small simulated population.Citation42 This stochastic simulation model was then run until steady state (1000 days) and used to explore the effects of different integrated control scenarios.
Simulated colonization and disease interventions
Four control methods were explored in this analysis: (i) improved hand hygiene and sanitation; (ii) stricter antimicrobial stewardship; (iii) reduced length of stay (LoS) for inpatients; and (iv) expedited gut microbiota recovery which can be achieved either through administering probiotics or through intestinal microbiota transplantation. Antimicrobial stewardship can be interpreted as a reduction in rates of prescribed broad-spectrum antimicrobials that are known to be risk factors of C. difficile infection.Citation44 While the first two control methods represent quite typical control methods for attenuating the spread of nosocomial infections, LoS reduction, probiotics and intestinal microbiota transplantation are not typically included in intervention strategies. We included LoS reduction because of the strong impetus of clinicians and hospital managers to limit inpatient duration following evidence of LoS as a key risk factor for healthcare acquired infection.Citation45,Citation46 We included probiotics and intestinal microbiota transplantation (also referred to as ‘fecal bacteriotherapy’) following the strong evidence in recent systematic reviews supporting the protective effect that they can have against C. difficile.Citation36,Citation47
Previous studies demonstrated the utility of improved sanitation and reduced average length of stay in reducing the transmission potential of C. difficile. Therefore, we began by exploring the effects of coupling these control tools. Most theoretical studies published to date have downplayed the efficacy of antimicrobial stewardship in reducing C. difficile transmission, but none has ascertained whether there are any additional benefits of complementing this strategy with the prescription of probiotics (both strategies might be expected to operate in the same epidemiological direction by reducing the proportion of inpatients that have heightened predisposition to CDI). All other combinations of the four control tools were simulated to ensure that no unexpected synergistic interactions were missed.
RESULTS
Clinical studies of the efficacy of bundles in controlling C. difficile
In 2000, an outbreak investigation recommended the sequential introduction of control measures and the development of a comprehensive C. difficile infection control ‘bundle’. The successful implementation of this bundle consisting of antimicrobial stewardship and improved hospital-wide sanitation was subsequently reported by Muto and colleagues.Citation48 The authors describe a 58% reduction in the annual rate of C. difficile through the use of combined controls. Despite recommendations for integrated control existing in the literature for nearly two decades, studies pertaining to the benefits of a combination approach to control have been scant since the study of Muto et al.Citation48
Following the NAP1/027 epidemic in Quebec in 2002, Weiss and colleagues conducted a five-year ‘multipronged’ C. difficile control strategy in an acute care tertiary hospital (the largest medical centre) in Quebec.Citation49 The strategy included rapid C. difficile testing of patients with unformed stools (with subsequent isolation of test-positives), a global hand hygiene program and the hiring of a team of infection control practitioners. They observed a 61% reduction in CDI rates over the study period.Citation49 Abbett et al.Citation50 and Salgado et al.Citation51 describe the use of a C. difficile prevention bundle in their university-affiliated tertiary care facilities. They also report encouraging reductions (of 40% and 45% respectively) in CDI rates over the study period through the use of rapid isolation of test-positives and enhanced infection control practices including escalated environmental cleaning. A collaborative effort of 35 New York metropolitan area healthcare facilities showed a statistically significant combined reduction in CDI rates (approximately 30%) following the implementation of an infection control bundle comprising of segregation of CDI patients, improved hygiene practice and enhanced environmental cleaning.Citation52 Bishop et al.Citation53 recently documented a similar reduction level (36%) in CDI case numbers following implementation of a bundle approach to controlling infection in surgical inpatients. Hence, the relatively few studies detailing a bundle approach to C. difficile control indicate substantial reductions in disease incidence in healthcare settings ( summarizes the findings of all relevant studies).
However, these combination control assessments share an obvious and important disadvantage: they cannot partition the level of infection reduction to the individual control methods. Disentangling the efficacies of the different controls when they are used in conjunction is impossible, as is the precise estimation of any synergistic effect between controls. This presents strong motivation for capitalizing upon biologically realistic simulation modeling to inform optimal C. difficile control combinations.
Table 2 Summary of the clinical studies examining the efficacy of control bundles in mitigating Clostridium difficile infection
Combinations of control for reducing pathogen colonization
shows the combined effect of the four simulated control methods in reducing the ratio of C. difficile colonized patients discharged relative to those admitted. All control methods generated marked improvements in reducing the colonized ratio. However, probiotics/bacteriotherapy were less effective than antimicrobial stewardship, reductions in transmission and LoS. Antimicrobial stewardship levels resulting in a halved proportion in the vulnerable epidemiological categories reduced the colonized ratio by a half and it improved the reduction achieved by all other control methods. For example, the maximum reduction in the colonized ratio achieved in combination with probiotics/bacteriotherapy (i.e., through halving the proportion on broad-spectrum antimicrobials from 50% to 25%, while expediting gut flora recovery from 90 days to 10 days) was two-thirds compared to the reduction by a factor of one-third achievable with probiotics/bacteriotherapy alone.
Figure 3 The effect of control combinations on the ratio of patients discharged relative to those admitted with asymptomatic C. difficile colonization. Controls include: λ, rate of gut microbiota recovery which is expedited by probiotics or intestinal microbiota transplantation; α, rate of antimicrobial prescription which is reduced through stricter stewardship; β, the rate of transmission which is reduced through improvements to hygiene and sanitation; κ, the rate of patient discharge (inverse of average length of stay), which is increased to minimize patient exposure window.
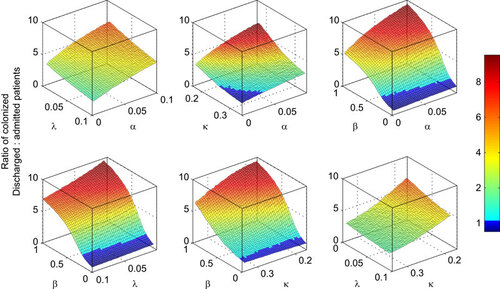
All combinations of other methods with reduced transmission coefficient yielded parameter spaces in which the numbers of colonized patients admitted to hospital exceeded those discharged (a colonized ratio of less than 1). Interestingly, in the (highly idealized) absence of within-hospital transmission, simulations showed that extended length of stay was actually beneficial in reducing the colonized ratio. This is because no patients are newly exposed to the pathogen in this idealized (theoretical) setting, combined with the fact that some colonized patients lose carriage of C. difficile during their stay in hospital.
Combinations of control for reducing disease incidence
In the absence of additional infection control (‘additional’ because hospitals are never in a state of no-control) the incidence of disease is 2.8 per 1000 hospital bed days (SD: 4.2). This lies towards the top of the range described in the most comprehensive survey which was carried out in Europe,Citation54 accounting for the high rates of underreporting associated with milder, symptomatic infection.Citation39 shows the simulated reduction in CDI incidence in hospital inpatients (per 1000 hospital bed days) as a result of the different combinations of control methods. The surfaces are more jagged because of the increased influence of stochastic effects in the smaller sub-population in diseased (versus colonized) categories.
Figure 4 The effect of control combinations on C. difficile symptomatic disease incidence per 1000 hospital bed days. Controls include: λ, rate of gut microbiota recovery which is expedited by probiotics or intestinal microbiota transplantation; α, rate of antimicrobial prescription which is reduced through stricter stewardship; β, the rate of transmission which is reduced through improvements to hygiene and sanitation; κ, the rate of patient discharge (inverse of average length of stay) which is increased to minimize patient exposure window.
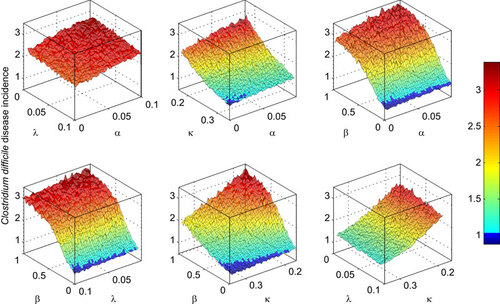
Antimicrobial stewardship yielded meager benefits in terms of reducing the incidence of CDI, regardless of combination with other methods. Likewise, prescribing probiotics/bacteriotherapy in order to expedite gut microbiota recovery were ineffective control tools and combining them with other transmission reduction methods failed to yield any synergistic effect.
Reducing the transmission coefficient (β) through improvements to hygiene and sanitation had a comparatively large effect in decreasing the incidence of disease. However, even complete elimination of within-hospital transmission fails to completely eliminate the incidence of CDI because patients who are already exposed or colonized will still import the infection when admitted. Combining this method with either antimicrobial stewardship or prescription of probiotics/bacteriotherapy yielded little additional benefit compared with transmission reduction alone (with marginal improvement attained by combination with antimicrobial stewardship). Reducing the average length of stay (κ−1) was also effective in decreasing disease incidence. Although probiotics/bacteriotherapy did not improve upon control based on LoS reduction, simulations indicated a small benefit in combining LoS reduction with antimicrobial stewardship. The only combination of methods that provided significant gains in ameliorating CDI incidence was the simultaneous reduction in LoS and the transmission coefficient. When both of these parameters were set to the minimum values (maximum control level includes eliminating within-ward transmission, β=0, by improving sanitation and reducing average length of stay from 6 days to 3 days), the resulting incidence in CDI for hospital inpatients was reduced by two-thirds: from 2.8 (SD: 4.2) to 0.9 per 1000 hospital bed days (SD: 1.5).
DISCUSSION
Mathematical model development offers a framework for safely assessing the efficacies of available infection control methods through simulation and scenario analysis. To date, models of C. difficile transmission are sparse and most are very simplistic, omitting factors that are known to be crucial to the epidemiology of this globally relevant disease. Such factors include the possibility of colonization and disease in individuals who have not recently taken antimicrobials—an alarming characteristic that has recently received a great deal of attention.Citation13,Citation28 Here, we have presented a biologically realistic model of C. difficile; used it to simulate the modern epidemiology of the pathogen; and, analyzed control combinations in order to strategize a more integrated approach to control.
We have shown that more stringent antimicrobial stewardship and the prescription of probiotics/bacteriotherapy are both ineffective at reducing symptomatic disease incidence, either in isolation or combination with each other or the other simulated control methods. Although evidence for the benefits reported from administering probiotics/bacteriotherapy is variable,Citation47,Citation55,Citation56 recent studies have unanimously suggested antimicrobial stewardship to be an effective method of reducing the rate of CDI in hospitals.Citation57,Citation58,Citation59,Citation60 However, attributing the level of infection reduction from this particular control method alone is not yet possible because these studies describe stewardship in conjunction with (often unspecified) additional infection control procedures.Citation59,Citation60
A recent hospital-based study from the UK surveyed the bacterial isolates from 1223 cases of symptomatic C. difficile infection.Citation61 From analyzing whole-genome sequence similarity (two or fewer single nucleotide variants), these researchers inferred that 35% of patients with C. difficile infection had been infected by other patients (the remaining 65% having been infected outside of the Oxford-based hospital). Our simulation output agrees in that it also demonstrates an inability to eliminate C. difficile from the hospital simply through cessation of within-hospital transmission. However, simulations indicate that under this highly idealized scenario of no within-hospital transmission, closer to 60% of infections can be controlled (). This qualitatively similar but quantitatively distinct result requires further investigation. One plausible explanation could be that new infections originating from patients with milder symptoms may have been missed in the Oxford study due to the under-reporting of disease that is known to occur for milder C. difficile infection.Citation37,Citation62
In addition to the benefits in transmission reduction achieved with improvements to sanitation and hygiene, simulations demonstrate the very substantial infection control achieved with reducing the average LoS. Moreover, the combined benefit of reducing LoS and improving sanitation and hygiene significantly exceeds that achieved with either method alone. In other words, adopting a strategy combining both tools will reduce the extent to which either would otherwise be required in isolation to achieve the same gains in CDI reduction.
In terms of the ratio of colonized patients discharged relative to those admitted, all control methods performed well. Antimicrobial stewardship showed greater efficacy in colonization control than it did for disease control, resulting in a maximum reduction of around 50%. Additionally, combining antimicrobial stewardship (halving the proportion of inpatients in the vulnerable epidemiological categories) with probiotic/bacteriotherapy prescription (expediting gut recovery from 90 to 10 days) reduced the colonized ratio by up to two-thirds. Improved sanitation and hygiene and reduced LoS provided notable reductions in the colonized ratio and each was complemented with the addition of any of the other control tools.
As with other infection models, the transmission coefficient is critical to the disease’s epidemiology. The transmission coefficient in this healthcare setting, as is the case for all infectious disease models, is difficult to define according to the numerous behavioral elements entailed. An important limitation in the current study is that infection was only simulated to pass between inpatients (or, at least, infection occurred at a level that was proportional to the prevalence of infectious patients). In reality, hospital staff and patient visitors will also act as infection sources and reservoirs. Partitioning the relative contribution of these (and other) separate sources of infection can easily be achieved in a modeling framework, but parameterization will be impossible until the molecular epidemiology of this disease is better described. Rubin et al.Citation18 recently made some progress to this end by using an agent-based modeling approach for simulating combinations of controls (isolation, hand hygiene, environmental cleaning) across a complex contact network of individuals within a hospital. Despite a very simplified epidemiological description of C. difficile (individuals were either susceptible, asymptomatically infected or symptomatically infected), simulation output qualitatively matched our own: environmental cleaning/hand hygiene was very effective at reducing within-hospital transmission.
A further limitation of our study is our inability to simulate a given strain in a given setting. Instead, we have had to source the parameterization of our model across multiple settings (and multiple strains). No single study presents all the required parameter values for our model. Understandably, this is a common issue among biologically realistic simulation models.Citation42 Importantly, in the event of a thorough epidemiological analysis of a particular strain of C. difficile whereby complete (or, at least, near-complete) model parameterization will be made possible, we have a functional and biologically realistic model that will provide a valuable contribution to future outbreak analysis. The next phase of development for this research is the conversion of the general, strategic framework presented here into a more tactical (idiosyncratic) tool for exploring control options for CDI in a specified healthcare setting. This requires location-specific data collection to inform model parameterization (e.g., pre-intervention rates of infection and colonization; local antimicrobial prescribing behaviors; the average length of stay for a particular hospital and the feasible level to which this can be reduced, etc.).
Despite advances in other infectious disease epidemiology settings,Citation63,Citation64,Citation65,Citation66 research into strategic infection control combinations for healthcare-acquired pathogens is underdeveloped. By reviewing the literature on control bundles for reducing C. difficile transmission and presenting simulation results for what we consider to be the most biologically realistic model of C. difficile reported to date, we hope to have provided important contributions to this burgeoning field. Whether our conclusions translate to other relevant epidemiological settings, such as long-term care facilities,Citation67 requires further investigation. Given the similarities between C. difficile and other important healthcare acquired infections (e.g., methicillin-resistant Staphylococcus aureus), the framework that we present here should be easily adaptable to other pathogens in future studies.
Acknowledgments
The study was supported by funding from the National Health and Medical Research Council of Australia (grant number APP1006243).
- McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis2006; 12: 409–415.
- Wilcox MH, Smyth ET. Incidence and impact of Clostridium difficile infection in the UK. J Hosp Infect1998; 39: 181–187.
- Lo Vecchio A, Zacur G. Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol2012; 28: 1–9.
- Muto CA, Pokrywka M, Shutt K et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol2005; 26: 273–280.
- Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg2007; 142: 624–631.
- McGlone SM, Bailey RR, Zimmer SM et al. The economic burden of Clostridium difficile. Clin Microbiol Infect2012; 18: 282–289.
- He M, Miyajima F, Roberts P et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet2012; 45: 109–113.
- Garcia C, Samalvides F, Vidal M, Gotuzzo E, Dupont HL. Epidemiology of Clostridium difficile–associated diarrhea in a peruvian tertiary care hospital. Am J Trop Med Hyg2007; 77: 802–805.
- Dhawan B, Chaudhry R, Sharma N. Incidence of Clostridium difficile infection: a prospective study in an Indian hospital. J Hosp Infect1999; 43: 275–280.
- Fernandez-Canigia L, Nazar J, Arce M et al. [Clostridium difficile diarrhea: frequency of detection in a medical center in Buenos Aires, Argentina.] Rev Argent Microbiol2001; 33: 101–107. Spanish.
- Herrera P, Cotera A, Fica A, Galdo T, Alvo M. [High incidence and complications of Clostridium difficile diarrhea among patients with renal diseases.] Rev Med Chil2003; 131: 397–403. Spanish.
- Sadeghifard N, Salari MH, Grassemi MR et al. Prevalence of Clostridium difficile-associated diarrhea in hospitalized patients with nosocomial diarrhea. Iran J Public Health2005; 34: 67–72.
- Benson L, Song X, Campos J, Singh N. Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol2007; 28: 1233–1235.
- Centers for Disease Control and Prevention (CDC). Severe Clostridium difficile-associated disease in populations previously at low risk—four states, 2005. MMWR Morb Mortal Wkly Rep2005; 54: 1201–1205.
- Lanzas C, Dubberke ER, Lu Z, Reske KA, Gröhn YT. Epidemiological model for Clostridium difficile transmission in healthcare settings. Infect Control Hosp Epidemiol2011; 32: 553–561.
- Starr JM, Campbell A. Mathematical modelling of Clostridium difficile infection. Clin Microbiol Infect2001; 7: 432–437.
- Starr JM, Campbell A, Renshaw E, Poxton IR, Gibson GJ. Spatio-temporal stochastic modelling of Clostridium difficile. J Hosp Infect2009; 71: 49–56.
- Rubin MA, Jones M, Leecaster M et al. A Simulation-based assessment of strategies to control Clostridium difficile transmission and infection. PLoS ONE2013; 8: e80671.
- Rebmann T, Carrico RM. Preventing Clostridium difficile infections: an executive summary of the Association for Professionals in Infection Control and Epidemiology’s elimination guide. Am J Infect Control2011; 39: 239–242.
- Weber DJ, Anderson DJ, Sexton DJ, Rutala WA. Role of the environment in the transmission of Clostridium difficile in health care facilities. Am J Infect Control2013; 41: S105–S110.
- Owens RC, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis 2008, 2008; 46: S19–S31.
- Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol1995; 16: 459–477.
- Health Protection Agency. Clostridium difficile: findings and recommendations from a review of the epidemiology and a survey of directors of infection prevention and control in England. London: HPA, 2006. Available at http://www.hpa.org.uk/webc/hpawebfile/hpaweb_c/1194947403482 (accessed 29 April 2014).
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; the Healthcare Infection Control Practices Advisory Committee. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. Atlanta, GA: CDC, 2007. Available at http://www.cdc.gov/hicpac/pdf/isolation/isolation2007.pdf (accessed 29 April 2014).
- Yakob L, Riley T, Paterson D, Clements A. Clostridium difficile exposure as an insidious source of infection in healthcare settings: an epidemiological model. BMC Infect Dis2013; 13: 376.
- Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect1998; 40: 1–15.
- Rouphael NG, O’Donnell JA, Bhatnagar J et al. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obst Gynecol2008; 198: 635.e1–6.
- Clements ACA, Magalhães RJ, Tatem AJ, Paterson DL, Riley TV. Clostridium difficile PCR ribotype 027: assessing the risks of further worldwide spread. Lancet Infect Dis2010; 10: 395–404.
- Johnson S, Clabots CR, Linn FV et al. Nosocomial Clostridium difficile colonisation and disease. Lancet1990; 336: 97–100.
- Loo VG, Bourgault AM, Poirier L et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med2011; 365: 1693–1703.
- MacDougall C, Polk RE. Variability in rates of use of antibacterials among 130 US hospitals and risk—adjustment models for interhospital comparison. Infect Control Hosp Epidemiol2008; 29: 203–211.
- Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis2007; 44: 664–670.
- The Organisation for Economic Co-operation and Development. Health at a glance2011. Paris: OECD, 2011. Available at http://dx.doi.org/10.1787/health_glance-2011-en (accessed 29 April 2014).
- Rafii F, Sutherland JB, Cerniglia CE. Effects of treatment with antimicrobial agents on the human colonic microflora. Ther Clin Risk Manag2008; 4: 1343–1358.
- McFarland LV. Evidence-based review of probiotics for antibiotic-associated diarrhea and Clostridium difficile infections. Anaerobe2009; 15: 274–280.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis2011; 53: 994–1002.
- Bartlett JG. Treatment of antibiotic-associated pseudomembranous colitis. Rev Infect Dis1984; 6 (S1): S235–S241.
- Centers for Disease Control and Prevention. Frequently asked questions about Clostridium difficile for healthcare providers 2010. Atlanta, GA: CDC, 2010. Available at: http://www.cdc.gov/hai/organisms/cdiff/cdiff_faqs_hcp.html (accessed 29 April 2014).
- Khanna S, Pardi D, Aronson S et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol2012; 107: 89–95.
- McFarland LV. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol2008; 5: 40–48.
- Leffler DA, Lamont JT. Treatment of Clostridium difficile-associated disease. Gastroenterol2009; 136: 1899–1912.
- Keeling M, Rohani P. Modeling infectious diseases in humans and animals. Princeton, NJ: Princeton University Press, 2008.
- Gillespie DT. Exact stochastic simulation of coupled chemical reactions. J Phys Chem1977; 81: 2340–2361.
- Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect2009; 58: 403–410.
- Delgado-Rodríguez M, Bueno-Cavanillas A, López-Gigosos R et al. Hospital stay length as an effect modifier of other risk factors for nosocomial infection. Eur J Epidemiol1990; 6: 34–39.
- Freeman J, McGowan JE. Risk factors for nosocomial infection. J Infect Dis1978; 138: 811–819.
- Goldenberg JZ, Ma SS, Saxton JD et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev2013; 5: CD006095.
- Muto CA, Blank MK, Marsh JW et al. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach. Clin Infect Dis2007; 45: 1266–1273.
- Weiss K, Boisvert A, Chagnon M et al. Multipronged intervention strategy to control an outbreak of Clostridium difficile infection (CDI) and its impact on the rates of CDI from 2002 to 2007. Infect Control Hosp Epidemiol2009; 30: 156–162.
- Abbett SK, Yokoe DS, Lipsitz SR et al. Original article: proposed checklist of hospital interventions to decrease the incidence of healthcare-associated Clostridium difficile infection. Infect Control Hosp Epidemiol2009; 30: 1062–1069.
- Salgado CD, Mauldin PD, Fogle PJ, Bosso JA. Analysis of an outbreak of Clostridium difficile infection controlled with enhanced infection control measures. Am J Infect Control2009; 37: 458–464.
- Koll BS, Ruiz RE, Calfee DP et al. Prevention of hospital-onset Clostridium difficile infection in the New York Metropolitan Region using a collaborative intervention model. J Health Qual2014; 36: 35–45.
- Bishop J, Parry MF, Hall T. Decreasing Clostridium difficile infections in surgery: impact of a practice bundle incorporating a resident rounding protocol. Conn Med2013; 77: 69–75.
- Bauer MP, Notermans DW, van Benthem BH et al. First results of the European Clostridium difficile Infection Survey (ECDIS). 19th European Congress of Clinical Microbiology and Infectious Diseases; 16–19 May 2009; Helsinki, Finland.
- Pillai A, Nelson R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev2008; 1: CD004611.
- Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet1989; 1: 1156–1160.
- Aldeyab MA, Kearney MP, Scott MG et al. An evaluation of the impact of antibiotic stewardship on reducing the use of high-risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J Antimicrob Chemother2012; 67: 2988–2996.
- Jump RL, Olds DM, Seifi N et al. Effective antimicrobial stewardship in a long-term care facility through an infectious disease consultation service: keeping a LID on antibiotic use. Infect Control Hosp Epidemiol2012; 33: 1185–1192.
- Nathwani D, Sneddon J, Malcolm W et al. Scottish Antimicrobial Prescribing Group (SAPG): development and impact of the Scottish National Antimicrobial Stewardship Programme. Int J Antimicrob Agents2011; 38: 16–26.
- Valiquette L, Cossette B, Garant MP, Diab H, Pépin J. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis2007; 45 (S2): S112–S121.
- Eyre DW, Cule ML, Wilson DJ et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med2013; 369: 1195–1205.
- Khanna S, Pardi DS, Aronson SL et al. The epidemiology of community acquired Clostridium difficile infection: a population based study. Am J Gastroenterol2012; 13: 89–95.
- Yakob L, Clements AC. A mathematical model of chikungunya dynamics and control: the major epidemic on Réunion Island. PLoS ONE2013; 8: e57448.
- Yakob L, Dunning R, Yan G. Indoor residual spray and insecticide-treated bednets for malaria control: theoretical synergisms and antagonisms. J Roy Soc Interface2011; 8: 799–806.
- Ferguson N, Keeling MJ, Edmunds WJ et al. Planning for smallpox outbreaks. Nature1991; 425: 681–685.
- Meyers LA. Contact network epidemiology: bond percolation applied to infectious disease prediction and control. Bull Am Math Soc2007; 44: 63–86.
- Simor AE, Bradley SF, Strausbaugh LJ et al. Clostridium difficile in long-term-care facilities for the elderly. Infect Control Hosp Epidemiol2002; 23: 696–703.