Abstract
Streptococcus suis is an important pathogen causing economic problems in the pig industry. Moreover, it is a zoonotic agent causing severe infections to people in close contact with infected pigs or pork-derived products. Although considered sporadic in the past, human S. suis infections have been reported during the last 45 years, with two large outbreaks recorded in China. In fact, the number of reported human cases has significantly increased in recent years. In this review, we present the worldwide distribution of serotypes and sequence types (STs), as determined by multilocus sequence typing, for pigs (between 2002 and 2013) and humans (between 1968 and 2013). The methods employed for S. suis identification and typing, the current epidemiological knowledge regarding serotypes and STs and the zoonotic potential of S. suis are discussed. Increased awareness of S. suis in both human and veterinary diagnostic laboratories and further establishment of typing methods will contribute to our knowledge of this pathogen, especially in regions where complete and/or recent data is lacking. More research is required to understand differences in virulence that occur among S. suis strains and if these differences can be associated with specific serotypes or STs.
Introduction
Streptococcus suis is one of the most important pathogens in the porcine industry causing septicemia, meningitis and many other infections.Citation1 In addition, it is an emerging zoonotic agent responsible for septicemia with or without septic shock, meningitis and other less common infections in humans.Citation1 During the last decade, the number of reported human cases due to S. suis has dramatically increased, and while most sporadic human cases of infection appear to be due to close occupational contact with pigs/pork products, particularly in Western countries (farmers, veterinarians, butchers, food processing workers, etc.), two epidemics were recorded in China in 1998 and 2005.Citation1 As of 2006, the number of human cases reported in Asia has increased.Citation2,Citation3 In fact, in some Asian countries, the general population is at risk.Citation2 However, an update on the distribution of the different serotypes and sequence types (STs), as determined by multilocus sequence typing (MLST), of strains responsible for infections in both pigs and humans from around the world have not been recently compiled. Yet, knowledge of this distribution is necessary in order to not only understand the current situation regarding S. suis, but also to evaluate areas where knowledge is lacking. This review covers the global distribution of the S. suis serotypes and STs responsible for infections reported in pigs from 1 January 2002 to 31 December 2013. A complete review on serotypes from humans since the first description in 1968 was also carried out. However, data of the STs from human cases are available only since 2002. Complete reviews have already covered the different virulence factors implicated in the pathogenesis of the infection caused by this important pathogen,Citation4,Citation5 and this will not be further addressed in this review.
Brief description of the general aspects of infection in pigs and human
The natural habitat of S. suis is the upper respiratory tract of pigs, more particularly the tonsils and nasal cavities, but also the genital and digestive tracts.Citation6 With almost 100% of pig farms worldwide having carrier animals, S. suis is one of the most important bacterial pig pathogens. Transmission of S. suis among animals is considered to be mainly through the respiratory route.Citation6 Of the various manifestations of the disease, septicemia and meningitis are by far the most striking features, but endocarditis, pneumonia and arthritis can also be observed.Citation7 Nevertheless, in peracute cases of infection, pigs are often found dead with no premonitory signs of disease.Citation7 Presumptive diagnosis of infection in pigs is usually based on clinical signs and macroscopic lesions.Citation8 Confirmation of the infection is mandatory and must be achieved by isolation and characterization of the pathogen.Citation8
Since first described in Denmark in 1968,Citation9 over 1600 human cases of S. suis infection have been reported with many more probably never diagnosed or misdiagnosed. S. suis is the most common cause of adult meningitis in Vietnam, the second most common in Thailand and the third most frequent cause of community-acquired bacterial meningitis in Hong Kong.Citation10,Citation11,Citation12 Different from pigs, the main route of entry of S. suis in humans is thought to be through contact of cutaneous lesions, most usually on the hands and arms, with contaminated animals, carcasses or meat.Citation3 However, this situation seems to be different in some Asian countries where the oral route is taken into consideration since many cases of infection have been reported after ingestion of contaminated raw pork products.Citation13 In Western countries, infections in humans most usually occur sporadically.Citation1 After an incubation period that ranges from a few hours to days,Citation13 S. suis usually produces meningitis in humans, but is also responsible for cases of endocarditis, pneumonia, peritonitis, arthritis and other less common diseases usually related to generalized septicemia.Citation14,Citation15 In addition, peracute infections with shock and a high mortality rate have been described, particularly in the case of the streptococcal toxic shock-like syndrome (STSLS) closely associated with the 2005 Chinese epidemic, but also observed independently of this outbreak.Citation16
IDENTIFICATION OF S. suis
S. suis is an encapsulated gram-positive bacterial coccus that occurs singly, frequently in pairs, or occasionally in short chains. The organism grows well in aerobiosis, but growth is enhanced by microaerophilic conditions. The majority of strains are alpha-hemolytic on bovine and sheep blood agar plates after 24 h of incubation at 37°C.
Pig and human strains isolated from clinical cases of infection
For clinical cases in pigs, isolation and identification of strains is relatively easy since successful identification can be achieved by a minimum of biochemical tests and confirmed by serotyping (see below).Citation17 However, the use of rapid multi-test biochemical kits may be misleading as some strains of S. suis can be misidentified.Citation18 Although S. suis field isolates readily grow on media used for culturing meningitis-causing bacteria and veterinary diagnostic laboratories easily identify this pathogen, many human diagnostic laboratories are less aware of this pathogen and may misidentify it as enterococci, Streptococcus pneumoniae, Streptococcus bovis, viridans group streptococci (e.g., Streptococcus anginosus and Streptococcus vestibularis) or even Listeria monocytogenes.Citation14,Citation18,Citation19,Citation20,Citation21,Citation22 In many cases, the initial gram stain presumptive diagnosis of the cerebrospinal fluid specimen is pneumococcal meningitis. This confusion may have led to the misdiagnosis of S. suis infections in the past. Many cases were diagnosed retrospectively after the isolates were initially misidentified.Citation23 More recently, polymerase chain reaction (PCR) tests have been used to directly detect S. suis DNA from cerebrospinal fluid samples with a sensitivity considerably higher than direct culture, especially if antibiotics have been used.Citation2 However, most PCR tests used for humans detect serotype 2 strains only and will not detect infections caused by other S. suis serotypes (see below). Instead of the serotype 2 PCR test, it is advisable to use other validated PCR tests that allow the detection of the S. suis species (see below).
Strains isolated from clinically healthy pigs
S. suis is a normal inhabitant of the oral cavity of pigs. The biochemical identification of isolates recovered from clinically healthy pigs (mainly from tonsil samples) is difficult to achieve due to the presence of other streptococci that are part of the normal oral microflora and that are phenotypically similar to S. suis. For this reason, molecular biology techniques have been developed during the last decade to allow the detection and identification of S. suis strains, such as a PCR assay targeting the specific house-keeping gene encoding for the glutamate dehydrogenase (gdh).Citation24 Although widely used, the gdh PCR test can sometimes fail to correctly identify certain S. suis isolates.Citation25 Using this test, it has also been reported that there was a risk of misidentifying Streptococcus gallolyticus as S. suis.Citation26
SEROTYPING
Definition of serotypes
Serotyping, which should be a part of the routine identification of S. suis strains recovered from diseased pigs and humans, will provide further confirmation regarding the pathogen's identity. A total of 35 serotypes of S. suis have been described and are defined based on the antigenicity of their capsular polysaccharide (CPS).Citation6 Evidence accumulated throughout the years has demonstrated a high level of genetic diversity in the S. suis species, as shown by sequence analysis of the 16S rRNA and cpn60 genes: these two methods suggested that serotypes 32 and 34 were divergent, being clustered together at a significant distance from the other serotypes. This led to the suggestion that these two serotypes might be reclassified as a different species (Streptococcus orisratti).Citation27,Citation28 However, field strains belonging to these serotypes have been isolated from diseased pigs in recent years in Canada and China.Citation25,Citation29,Citation30 More recently, further studies have suggested that the reference strains of additional serotypes (20, 22, 26, and 33) do not belong to the S. suis species.Citation31 As such, there is an urgent need for a consensus in defining whether a serotype belongs to S. suis or not.Citation32
In order to understand the important roles of the CPS in serotyping and in the interactions of S. suis with the host, knowledge regarding the specific structure that confers capsular properties to each individual serotype is necessary. Van Calsteren et al.Citation33,Citation34 have described the composition and structures of the S. suis serotypes 2 and 14 CPS. Still, the chemical composition and structure of the CPS of the serotypes other than 2 and 14 are presently unknown.
Serological methods
Proper serological typing, which is one of the most important features of the S. suis infection diagnosis, must be performed using either a co-agglutination test, capillary precipitation test or with Neufeld's capsular reaction using reference antisera.Citation35,Citation36 Details on how to perform the co-agglutination test have already been published.Citation37 This test is preferred by many laboratories, especially in North America.Citation17 Some serotypes cross-react, indicating the presence of common antigenic determinants. This cross-reaction is probably due to similar or closely related structural features of the CPS. To date, the following cross-reactions have been described: serotype 1/2 with serotypes 1 and 2, serotypes 6 and 16, serotypes 2 and 22, and serotypes 1 and 14.Citation35,Citation38 In some cases, absorption is recommended in order to obtain monospecific antisera.Citation17 However, levels of cross-reactions for some serotypes vary with field strains (Gottschalk M, unpublished data, 2014).
Serotyping by multiplex PCR
The idea of molecular serotyping by PCR amplification of serotype specific cps genes using either a simplex or multiplex PCR is attractive due to the fact that animals are not used for serum production, ease of development and effectiveness. Early studies reported the use of assays targeting some serotypes.Citation39,Citation40 Liu et al.Citation41 reported a new protocol using four multiplex PCR assays allowing the detection of the 33 serotypes of S. suis (serotypes 1 to 31, 33 and 1/2), but not those related to S. orisratti (serotypes 32 and 34). Strains reacting in serology with more than one serotype could be confirmed using this technique. More recently, Okura et al. developed a PCR for all 35 described serotypes of S. suis.Citation42 Regardless of the PCR test used, a major disadvantage is the fact that the serotypes 2 and 14 cannot be distinguished from serotypes 1/2 and 1, respectively, since both of these serotype pairs do not possess unique cps genes.Citation43 This represents a major problem for pig isolates since serotypes 1, 1/2, 2 and 14 are commonly isolated in swine.Citation3,Citation10 The use of specific antisera is mandatory for such strains. For this reason, only serologically confirmed isolates of serotypes 2 and 14 recovered from pigs were considered in the present review. Although it is also necessary to confirm isolates from humans, it may represent a less important drawback since serotype 1/2 has never been isolated from humans and serotype 1 has been reported in only three non-serologically confirmed cases (at least with both serotypes 1 and 14 antisera), which is important considering these serotypes cross-react (see above).Citation44,Citation45 For this reason, isolates from humans reported in the literature as being serotype 2 or 14 based only on PCR reactions (although not serologically confirmed) will be considered as such in the present review.
‘Serotyping’ by biochemical identification
In the past, some reported cases of S. suis infection in humans have been attributed to serotype 2 based on the biochemical analyses obtained using the rapid multi-test commercial kits. While many of these kits claim to differentiate between serotype 1 and 2 strains based on sugar fermentation, there is still no evidence of a correlation between a specific serotype and its biochemical properties.Citation17,Citation20,Citation46 As a result, some human cases have been reported as serotype 2, while others were reported as serotype 1, but since the serotypes of these strains have not been confirmed using antisera (and not even by PCR), their serotypes are herein reported as ‘unconfirmed by reference antisera or PCR tests'.
Non-typable strains
Some S. suis isolates do not agglutinate with any of the typing antisera directed against the 35 serotypes and are identified as non-typable isolates.Citation29 Non-typable S. suis strains may correspond to either truly encapsulated strains that belong to novel, not yet described, encapsulated serotypes, or to non-encapsulated strains, which are impossible to serotype using the serological methods based on CPS antigens. The proportion of non-typable isolates varies between studies depending on the number of serotypes detected using antisera.
Using antisera against all 35 serotypes, Gottschalk et al.Citation25,Citation47 demonstrated that 89% of non-typable S. suis strains presented high surface hydrophobicity, suggesting that they were poorly or non-encapsulated. This was confirmed using transmission electron microscopy, demonstrating that highly hydrophobic strains (74%–93%) were non-encapsulated.Citation25 More than 40% of these non-encapsulated strains have been recently shown to belong to known serotypes using a multiplex PCR for all 35 serotypes.Citation42 It is also difficult to be certain if these strains were already non-encapsulated when causing disease, or if they lost their CPS during isolation and culture. It has been previously reported that 34% of isolates belonging to serotype 1/2 or 2 recovered from cases of endocarditis in Japan were non-encapsulated due to deletions and insertions in the genes of the CPS locus.Citation48 It was concluded that although the CPS is considered an important virulence factor for S. suis, loss of capsular production might be beneficial to S. suis in the course of infective endocarditis. In fact, non-encapsulated strains were shown to possess not only high adhesion properties to mammalian cells, but also a capacity to form biofilms.Citation47 Since the sites of isolation of these non-typable strains were similar to those of the most important serotypes (meninges/brain, joints, heart and lungs), their potential virulence capacity should not be disregarded.
MULTILOCUS SEQUENCE TYPING
Being a pathogen capable of causing sporadic cases of infection and epidemics in both pigs and humans, the global surveillance of S. suis is very important in order to better understand the epidemiology of this bacterial species.Citation49 Though different methods based on DNA have been used for the surveillance of S. suis, these methods are effective for short-term epidemiology only as they are based on non-characterized genomic differences between isolates.Citation49 In contrast, MLST distinguishes a large number of genotypes while using genetic variations that accumulate very slowly, in housekeeping genes, and has allowed global and long-term epidemiology for many important meningitis-causing bacteria by determining the STs present within a population.Citation49,Citation50
In 2002, King et al.Citation51 established a model of MLST for S. suis using seven different house-keeping genes (cpn60, dpr, recA, aroA, thrA, gki and mutS). Since being established, this MLST model has been used by multiple laboratories throughout the world to determine the STs of S. suis strains isolated from pig and human cases of infection. When used alongside serotyping, MLST allows gathering further information about the genetic diversity of the S. suis strains within the different serotypes. The popularity of this typing method for S. suis continues to increase due to the ease in carrying out the technique (PCR and sequencing) and due to the fact that the data are transferrable and comparable from one laboratory to another. Thanks to the S. suis MLST Database, the global distribution of the different S. suis STs can be shared and compared. When the MLST data take into consideration the serotypes, it is important that a trustable and complete serotyping system has been applied to the field strains; otherwise, the final data are difficult to interpret. More recently, studies have begun combining data obtained from MLST with the presence or absence of different S. suis virulence-associated markers at the gene and protein levels including the suilysin (SLY, encoded by the sly gene), muramidase-released protein (MRP, encoded by the mrp gene), extracellular factor (EF, encoded by the epf gene) and different pili in order to compare STs data with phenotypic characteristics.Citation52,Citation53
METHODOLOGY USED IN THE PRESENT REVIEW
In the present review, literature searches were completed using the following databases: Medline (PubMed; http://www.ncbi.nlm.nih.gov/pubmed/), Web of Science (http://thomsonreuters.com/web-of-science-core-collection/) and Science Direct (http://www.sciencedirect.com/). Relevant articles in English were used, while references in other languages were considered when available. MLST results were obtained from the S. suis MLST Database (http://ssuis.mlst.net/). Regarding the distribution of serotypes, the results reported for clinical cases in pigs correspond only to those where serotyping was carried out using antisera and not PCR, while for human cases, reports where serotyping was performed by either antisera or PCR (for serotypes 2 and 14) are both reviewed. This consideration is primarily based on the fact that serotype 1 and serotype 1/2 isolates have not yet been recovered (or confirmed) from human cases of infection. It is important to note that when the correlation between STs and serotypes are reported in this review, it is impossible to confirm the methodology used for the serotyping for many cases.Citation51 We decided to take the data into consideration, especially for those included in the S. suis MLST Database, since the number of cases used for this review would otherwise have been highly limited. For the distribution of STs, the ST complexes represent hyperinvasive lineages to which two or more STs belong as a result of genetic proximity and are based on the MLST phylogeny diagram presented by Lachance et al.Citation54 STs considered to be unrelated to another ST according to this phylogeny diagram are identified as being ‘unrelated’ to any known ST complex. With the exception of the serotype distribution of S. suis human cases, which comprises all cases reported since first described in 1968, distribution of serotypes for clinical cases in pigs and distribution of all STs are based on data published between 1 January 2002 to 31 December 2013.
WORLDWIDE DISTRIBUTION OF SEROTYPES
Diseased pigs
In order to fully appreciate the prevalence of human infections and the zoonotic potential of S. suis, one must understand the situation at the farm level since transmission from diseased pigs or pork-derived products is a prerequisite for infection of humans. The data compiled from studies since 1 January 2002 are shown in .
Table 1 Worldwide distribution of serotypes for reported clinical S. suis cases of infection in pigs by country from 1 January 2002 to 31 December 2013
While S. suis is part of the normal microflora of pigs and many studies have focused on carriage in healthy pigs, these studies were not included in this review since the serotype distribution in healthy carriers greatly differs from that of clinical strains, with serotype 2 being much less frequently isolated.Citation65,Citation66,Citation67,Citation68,Citation69,Citation70,Citation71 In addition, since S. suis is a normal inhabitant of the upper respiratory tract,Citation6 most of these studies identified only a small part of the real population of each carrier. Finally, in the case where species-specific PCR tests were not used, many of the ‘untypable’ strains detected probably did not belong to the S. suis species. The contribution of these commensal strains to the risk posed to humans in close-contact with pigs or with pork-derived products remains to be further studiedCitation14,Citation72,Citation73 and seems to occur particularly in immunocompromised patients. The situation may be different when animals are not really ‘healthy carriers’ but rather convalescent animals, carrying in their tonsils virulent strains. However, this situation is almost impossible to define in field studies.
During the last 12 years, more than 4500 serologically confirmed strains recovered from diseased pigs have been reported (). Globally, the predominant S. suis serotypes isolated from clinical cases in pigs are, in decreasing order, serotypes 2, 9, 3, 1/2 and 7, along with 15.5% of the strains being non-typable by serotyping. However, there is a clear geographical effect on the distribution of serotypes (see below) and these figures are influenced by the number of published studies.
Almost 70% of studies on worldwide isolates recovered from diseased pigs are from North America (). Nevertheless, this is not an indication of a higher number of cases but simply of a higher number of published reports and studies. In fact, 97% of North American data are from Canada and the rest from the United States, with no data from Mexico. In North America, serotype 2 is the most prevalent in Canada, while in the United States, it is serotype 3. Meanwhile, the second most prevalent serotype in Canada is serotype 3 and is serotype 2 in the United States. However, only slight differences in percentages have been observed in both countries, demonstrating a similar distribution when the data are combined: both serotypes 2 and 3 are the two most prevalent serotypes isolated from clinical pig cases in North America with 24.3% and 21.0% of prevalence, respectively, followed by serotypes 1/2, 8 and 7. Similar distributions of serotypes in Canada and the United States may be explained by a fluid movement of animals between the two countries.
In South America, only two studies have been published, both from Brazil, which report serotype 2 as being the most prevalent with a mean of 57.6% of cases, followed by, in decreasing order of prevalence, serotypes 1/2, 14, 7 and 9 (). Interestingly, although no clinical cases in pigs have been published, human cases have been reported in other South American countries (see below).Citation74,Citation75,Citation76,Citation77 On the other hand, no human cases have yet been reported in Brazil.
In Asia, where the vast majority of human cases have been reported (see below), studies on clinical cases in pigs account for only 14.0% of the data available and are provided from China and South Korea only. The most prevalent serotypes in infected pigs are, in decreasing order of prevalence, serotypes 2, 3, 4, 7 and 8 (). The South Korean study surprisingly reported serotypes 3 and 4 as being predominant, followed by serotype 2 at a low prevalence of only 8.3%. This anomaly may be due to the fact that the paper reported a relatively low number of cases (only 20 isolates) from animals with acute polyserositis only.Citation61 Even more disturbing is the fact that the information available regarding the status of S. suis infections in pigs in Asia is the most scarce of the three continents, while this is the continent where cases of zoonosis occur the most frequently in the general population. Although many hospitals and microbiologists in both Thailand and Vietnam are aware of S. suis infections when it comes to diagnosis,Citation2 the four veterinary studies published from these countries investigated healthy or slaughterhouse pigs only.Citation69,Citation70,Citation78,Citation79 Similarly, there have been 10 human S. suis cases reported in Japan between 1994 and 2009, but no complete study on the distribution of isolates from clinically ill pigs including all serotypes has been recently published. In fact, data from the last epidemiological studies in Japan date back to between 1987 and 1991, more than 20 years ago, which predate the human cases reported in this country and illustrate the necessity to gather data in order to increase our knowledge of the current epidemiological situation.Citation80 In Hong Kong, where S. suis has been reported as the third most common culture-confirmed cause of community-acquired bacterial meningitis, there have been 47 human cases from 1983 to 2001 and 21 from 2003 to 2005,Citation12 yet there is a complete absence of epidemiological data regarding S. suis in pigs, with the exception of two studies investigating the prevalence of S. suis in pork meat sold in wet markets.Citation81,Citation82 In Singapore, the Philippines, Laos and Cambodia, where human cases have occurred during the last decade (), there are no data available on the epidemiology of S. suis infections in pigs.
Table 2 Worldwide distribution of reported clinical S. suis cases of infection in humans by country until 31 December 2013
Important pig producing European countries, such as Denmark, Belgium, France, Germany, Italy and the United Kingdom, have not recently reported the distribution of serotypes recovered from clinical cases in pigs: the last reports from these countries regarded strains isolated between 1990 and 2000.Citation66,Citation83 Before the year 2000, serotype 2 was the most common serotype recovered in Italy, France and Spain, whereas serotype 9 was more frequently found in the Netherlands, Germany and Belgium.Citation76 This lack of information is of high importance. The only two countries with more recent data are Spain and the Netherlands. In fact, in Spain, serotype 2 is no longer the most prevalent serotype, but the second behind serotype 9, followed by serotypes 7, 8 and 3.Citation63,Citation64,Citation65 In the Netherlands, between 2002 and 2007, serotype 9 was still the most prevalent, followed by serotypes 2, 7, 1 and 4.Citation62 Although serotype 9 is the most prevalent serotype in Spain and in the Netherlands and was also prevalent in many European countries before the year 2000, no human cases associated with this serotype have yet been reported. Taken together, these two European countries provide a relatively similar serotype distribution with serotypes 9, 2, 7, 8 and 3 in order of importance (). In studies previous to 2002, serotype 1 also appeared to be prevalent in countries such as Belgium and the United Kingdom;Citation76 however, it is not clear if this is the current situation. The situation is similar in Denmark where only serotype 7 isolates were characterized during the last twelve years and where the last serological study regarded isolates recovered in 1995–1996.Citation84,Citation85 For the time period covered in this review, two reports, from France and Italy, have been published, although the data from none of these was considered since one of them used serotyping by PCR, detecting serotypes 1 (and 14), 2 (and 1/2), 7 and 9 only and, thus, did not meet the inclusion criterion (see above), while the other studied the distribution of serotypes in healthy animals.Citation67,Citation86 Interestingly, human cases have emerged in other European countries in many of which few or no studies on S. suis isolation from diseased pigs have been conducted. In Serbia, a recent prevalence study was published using isolates from swab samples taken from dead animals, clinically healthy pigs, slaughterhouse pig carcasses and butchers' knives, but it was impossible to associate corresponding serotypes to the diseased animals only.Citation87 In Croatia, only one study pertaining to the antimicrobial susceptibility of S. suis type 2 isolates was published,Citation88 but no epidemiological studies on the distribution of serotypes is available. Moreover, in Greece and Poland, where human cases have been reported, there are still no pig data available. As such, there is an urgent need to evaluate fresh data on the prevalence of S. suis in clinical isolates from pigs in Europe where many countries are among the more important pig producers in the world.
Finally, there is also a lack of relatively recent serotyping data from Australia and New Zealand. In Australia, where three human cases have been recently reported, the studies concerning serotype distribution in pigs predate these human cases, thus showing that there were once active surveillance studies in this country.Citation89,Citation90 One human case of S. suis was also reported in New Zealand,Citation91 but no recent studies regarding isolates recovered from diseased pigs have been published in this country.
In summary, there are countries where the serotype distribution of field strains recovered from diseased pigs has been systematically undertaken during the last 12 years, generating data that may influence any analysis of the worldwide distribution of serotypes. There is an urgent need for new data from European countries on the serotype distributions of S. suis strains isolated from diseased pigs, especially considering the fact that currently available vaccines are bacterins, which are supposed to confer serotype-specific protection. Some countries with an important pig production, such as Brazil, have few studies on pig disease and no human cases declared. Other countries commonly report human cases, but data from diseased animals are almost absent.
Human cases
One of the goals of this review was to compile the serotype distribution of S. suis infections in humans, which has not been completed since 1989,Citation92 and to update the total number of cases from recent reviews on human cases, all of which are excellent comprehensive reviews regarding the clinical features of S. suis infections in humans.Citation3,Citation18,Citation93 In the present study, and differently from clinical pig isolates, serotype 2 and 14 isolates were considered to be as such even if identified by PCR, which is unable to differentiate them from serotypes 1/2 and 1, respectively. This consideration is primarily based on the fact that serotype 1 and serotype 1/2 isolates have not yet been recovered (or confirmed) from human cases. A total of 1642 cases have been reported worldwide as of 31 December 2013 ().
Of these cases, serotype 2 was the most frequently reported with 74.7%, followed by serotype 14 with 2.0%. It is important to note that 262 (21.5%) serotype 2 strains and two (6.0%) serotype 14 strains were identified by PCR only. The most striking aspect regarding human cases of infection is that 377 cases (23.0%) of all reported cases either do not specify the serotype in the case report or employed a method for serotype identification that was judged inadequate (‘biochemical serotyping’). Even though many of these reports claim to be caused by serotype 2, which is probably true, serotyping, or at the very least PCR, should be performed if the strains are still available in order to increase our knowledge of S. suis infections in humans. Meanwhile, the remaining five human cases of infection were caused by the following serotypes: 4,Citation14 5,Citation133 16,Citation146 21Citation77 and 24.Citation133 Since 2011, human S. suis cases of infection have been reported for the first time in Cambodia, Chile, French Guiana, Poland and South Korea.
The vast majority of human cases have occurred in Asia, which account for more than 90% of all reported cases, particularly in Vietnam, Thailand and China. These three countries alone account for 83.6% of all cases worldwide. However, in China, almost all cases were described during the 1998 and 2005 outbreaks.Citation16,Citation105 In East and Southeast Asia, S. suis zoonosis should be considered endemic due in part to the high density of pigs, relatively high number of backyard-type production farms, slaughtering practices with the use of few preventive measures, presence of wet markets and consumption of ill pigs and/or consumption of uncooked or undercooked pork products.Citation3 In Thailand, most of the reported cases were caused by serotype 2, while serotype 14 is the second highest, the latter accounting for 21 cases of meningitis and sepsis out of a total of 530 cases. However, infections by other serotypes, such as a serotype 5 peritonitis and a serotype 24 sepsis, have also been reported. In Vietnam, where S. suis is now the most frequent cause of adult bacterial meningitis,Citation10 most cases are also due to serotype 2, six cases of meningitis were caused by serotype 14, and one case of peritonitis by serotype 16. The diversity of infections caused by serotypes other than serotype 2 could be explained by the awareness of diagnosticians to S. suis infections. Consequently, this could also explain why Vietnam and Thailand have the largest number of reported cases and a higher probability of encountering atypical cases on a more regular basis. It is also interesting that the patients who suffered infections by serotypes 5, 16 and 24 were also suffering from cirrhosis: a likely explanation is that these infections were the result of immunocompromisation. The compilation of human cases in China includes sporadic cases and the two S. suis serotype 2 epidemics, the first in Jiangsu province in 1998, where 14 deaths out of 25 cases occurred,Citation16 and the second in Sichuan province in 2005, where 38 deaths out of 215 cases were reported. This second epidemic remains the largest outbreak of S. suis in humans.Citation105 These epidemics were unprecedented for S. suis infections in humans considering the high incidence of systemic disease, the low number of cases of meningitis, and the high rate of mortality observed.Citation1 The patients presented cases consisting of either sepsis, meningitis, or STSLS, based on the presence of the following symptoms: sudden onset of high fever, diarrhea, hypotension, blood spots and petechial, clear erythematous blanching rash and dysfunction of multiple organs, such as acute respiratory distress syndrome, liver and heart failure, disseminated intravascular co-agulation, and acute renal failure.Citation1 Even though China has the largest pig production in the world and is the site of the 1998 and 2005 outbreaks that attracted much attention from the scientific community, the number of reported cases is considerably lower than in Thailand and Vietnam. One possible explanation is that dishes containing raw pork and blood, very popular in Thailand and Vietnam, are uncommon in China. In fact, since the last outbreak in 2005, only four other sporadic human cases were reported in mainland China.Citation107,Citation113
Human cases were also reported in Cambodia, Hong Kong, Japan, Laos, Singapore, South Korea and Taiwan. It is of interest to note that in Hong Kong, S. suis serotype 2 was reported as the third most frequent cause of adult bacterial meningitis.Citation12,Citation108 There is also a human case of infection from the Philippines, although it was not officially reported in this country. In fact, even though the infection was diagnosed as S. suis serotype 2 meningitis and treated in the United States, the patient had just returned from a seven-month vacation in the Philippines where he had consumed raw pork, making this an Asian case and considered as such in this study.Citation119
The second continent where most human infections have been described is Europe, accounting for 8.5% of all reported human cases (), with approximately 71.4% of all European human cases occurring in countries with a highly developed pig industry: the Netherlands, United Kingdom, France and Spain. Cases were also reported in Austria, Belgium, Croatia, Denmark, Germany, Greece, Ireland, Italy, Poland, Portugal, Serbia and Sweden. Surprisingly, no cases have yet been reported in Russia, a country with an increased developing pig production. Human cases of S. suis infection were reported for the first time in Europe, with the first ever case in Denmark, in 1968. It is interesting to note that all cases reported before 1983 were from Western Europe.Citation9,Citation108 Only two countries consider S. suis infections in humans as an industrial disease: the United Kingdom and France since 1983 and 1995, respectively.Citation18 This recognition led to legislation and regulations which may have contributed to reducing the number of cases in both of these countries, with the last human case in the United Kingdom being reported in 2001. In France, although few cases have been reported since 1995, most were related to wild boar hunters. It is known that wild boars also carry S. suis.Citation163 Consequently, hunters should be aware of the necessary precautions when preparing the carcasses, which present the greatest risk.Citation176,Citation177 Most human cases of infection contracted from wild boars were serotype 2, with two cases of meningitis caused by serotypes 4 and 14 in the Netherlands, one case of meningitis by serotype 14 in Denmark, and one case of septicemia by serotype 14 in the United Kingdom.Citation14,Citation158,Citation226
Although being the continent with the highest number of S. suis infection reports from diseased pigs (), only a few sporadic cases of S. suis infection in humans have been reported in North America. As previously mentioned, the isolation rate of S. suis serotype 2 from diseased pigs is considerable lower than that of Europe or Asia. The lower number of pig cases of disease due to this serotype may be a reason for the lower number of human cases of infection. It has been suggested that S. suis strains of serotype 2 from Canada and the United States present lower virulence properties than those from Europe and Asia.Citation54 For these two countries, confirmed human cases were all serotype 2, with the exception of one serotype 14 case of meningitis in Canada.Citation95 In the United States, two of the cases were from the continent while the third was from Hawaii, an island in the Pacific Ocean importing pigs from Asia. While the case in Hawaii is considered American (unlike the case from the Philippines), it would perhaps be more appropriate to compare the features of this infection with those from Asia. In fact, the strain recovered from Hawaii presents phenotypic characteristics typical of Asian strains (Gottschalk M, unpublished data, 2014).
Sporadic cases were also reported elsewhere throughout the world, such as those in South America (Argentina, Chile and French Guiana), and Oceania (Australia and New Zealand), but when combined, they only account for 0.8% of all reported cases. As was shown by Wertheim et al.,Citation3 sporadic cases of S. suis infections in humans are encountered in most regions where pig rearing is important, with notable exceptions being Mexico and Brazil. In the case of Mexico, even though one study evaluated upper respiratory tract colonization of slaughterhouse workersCitation233 (see below), no official reports on clinical pig cases or human infections are available, which is alarming, knowing full well that in previous studies, clinical strains of S. suis isolated from pig and human cases were included.Citation83,Citation234 Despite the lack of available reports, this infection is very common in Mexican farms (Gottschalk M, unpublished data, 2014). As such, action is required in order to remedy the troubling situation in this country which has well-developed veterinary diagnostic laboratories. It remains to be seen if the absence of human cases is due to a lack of awareness of S. suis as a zoonotic pathogen in these regions when diagnosing bacterial infections, or if these strains are of lower virulence, as with some strains from Canada and the United States.
shows the frequency of the clinical manifestations of infection by confirmed serotype, illustrating that the serotypes 2 and 14 are involved in similar proportions in meningitis (50%–70%) and septicemia (20%–25%). Septic shock was defined as a category comprising sepsis, septicemia, bacteremia and/or STSLS, while meningitis comprises all meningeal-related symptoms. Serotype 2 infections also represented 2.9% of the cases where other afflictions where diagnosed such as endocarditis, septic arthritis, pneumonia, peritonitis, pulmonary edema, myocarditis and other infections. Serotypes 4 and 21 were isolated from cases of meningitis, serotypes 5 and 16 from cases of peritonitis, and serotype 24 from a case of sepsis.
Table 3 Clinical manifestations of reported clinical S. suis cases of infection in humans by serotypes confirmed by co-agglutination
Why reported strains isolated from human cases are almost exclusively serotypes 2 and 14 is puzzling. While the serotypes 3, 9 and 1/2 are among the most highly prevalent in diseased pigs after serotype 2, at least in North America, no human infections due to these serotypes have yet been reported in any country. These differences in virulence between pig and human isolates may be partially due to serotype-specific CPS structural features, for which the CPS structures are still unknown, except for serotypes 2 and 14, and/or due to other serotype-specific bacterial factors involved in the pathogenesis. We could speculate that the serotypes 2 and 14 are more virulent than the other serotypes based on the observation that three out of the five cases caused by other serotypes (5, 16 and 24) occurred in patients suffering from cirrhosis, which in turn may have immunocompromised them and made them susceptible to serotypes uncommon in human infections.Citation133,Citation146 These differences could also be explained by varying serotype-specific colonization abilities. However, many cases caused by serotypes 2 and 14 have also been described in individuals with predisposing conditions. More research is required in order to understand how differences in virulence of S. suis strains occurs and if these differences can be associated with specific serotypes.
Subclinically infected humans: zoonotic potential to workers?
Subclinically infected pigs are carriers of S. suis mainly in the tonsils.Citation6 However, this information is not clearly available for humans. Only a handful of studies have investigated S. suis subclinical infections in humans as displayed in , either by serology (antibodies) or by bacterial detection.
Table 4 Studies exploring human carriage and exposure to S. suis in risk groups
Three of these were serological studies, with two using an indirect enzyme-linked immunosorbent assay (ELISA) with S. suis serotype 2 whole bacteria (formalin-inactivated or not) as antigen (). In New Zealand, the authors examined sera from 70 pig farmers, 96 dairy farmers, 107 meat inspectors and 16 veterinary students.Citation235 Twenty-one percent of pig farmers, 10% of meat inspectors and 9% of dairy farmers (most of them also raising pigs on their farms) were positive for ‘anti-S. suis serotype 2 antibodies’. It is impossible to be certain if these titers were specific for serotype 2 since cross-reacting antibodies with other S. suis serotypes and/or with other bacterial species were probably detected. Antibody titers were associated with longer occupational exposure for both pig farming and meat inspection. In the United States, using a similar antigen, sera from 73 pig-exposed and 67 non-pig-exposed adults from Iowa were titrated. Ten percent of pig-exposed workers were found to be positive compared to only one positive individual (1.5%) from the non-pig-exposed group.Citation237 Once again, the authors concluded that positive titers were associated with either working with pigs or living on a pig farm for more than 10 years, and also found that study participants who worked with both finishing and nursery pigs had 8.8 times the odds of having a positive titer when compared to non-exposed participants. The third serological study, from the Netherlands, is interesting since the authors chose to perform Western blot analysis, with higher specificity, targeting two virulence-related markers of S. suis serotype 2 that are widely present among virulent strains in that country, the MRP and EF proteins, in sera from 102 veterinarians and 191 pig farmers.Citation236 Results showed that 6% of veterinarians were positive for anti-MRP and 2% were positive for anti-EF, while 1% of pig farmers were positive for anti-MRP and 0.5% were positive for anti-EF. It is important to note that these antigens have not been reported to be present in bacterial species other than S. suis. These three serological studies, while the titers obtained cannot be directly compared, illustrate the zoonotic potential of S. suis based on the presence of antibodies possibly generated through long-term exposure and/or exposure-related subclinical infections. It should be noted that the presence of antibodies does not guarantee a carriage status for positive individuals.
In addition to these serological studies, three studies have directly focused on carriage in the upper respiratory tract of humans by trying to isolate S. suis from pharyngeal or tonsil swabs of pig-exposed workers (). In Italy, 10 volunteer slaughterhouse workers were swabbed and two were found to be positive for S. suis serotype 2.Citation238 In Mexico, the tonsils from 69 slaughterhouse workers were swabbed and four were found to be positive.Citation233 Of the four strains isolated, one was serotype 2, another was serotype 27 and two were non-typable by serotyping. The virulence of these four strains was also evaluated in mice and it was determined that three of them were mildly virulent (30%–80% mortality) and one of the non-typable strains was highly virulent (70%–90% mortality). Finally, in Germany, the tonsils from 132 meat workers (from either a slaughterhouse or meat processing factory) and 140 controls were swabbed and seven workers were found to be positive carriers of S. suis serotype 2, while none of the controls showed positive results. For the seven workers found to be positive carriers, four were retested 3 weeks later and were confirmed to still be positive carriers, indicating that the bacteria probably remained in the tonsils of healthy individuals for a relatively long period of time, which was also shown to be the case in pigs.Citation6 However, since these individuals were continuously exposed to pork products, repeated recolonization could not be ruled out.Citation2 All of the positive workers were employed for an average of 9.9 years ranging from three to 21 years. These three studies regarding the nasopharyngeal carriage all report positive samples in pig-related meat workers and none in the population without occupational exposure to pig. It is also very important to note that of the 13 strains isolated from healthy workers, 84.6% were serotype 2.
Taken together, these studies illustrate the zoonotic potential through long-term exposure, either by demonstrating the presence of antibodies towards S. suis or by isolating S. suis from tonsils or pharynx in pig-exposed workers. It must be noted that the positive rates in the studies of carriers of the upper respiratory tract are probably underestimated since detection of the pathogen was achieved using isolation methods, which are considered as having low sensitivity. Studies using molecular techniques (species-specific PCR) would probably present a significantly higher sensitivity. Exposure to S. suis may lead to subclinical infections, induction of antibodies, serious disease, as was the case in the human cases discussed previously, or just an insignificant and transitory atypical colonization of the mucosal membranes within the respiratory route.Citation110,Citation181 The annual risk of developing meningitis due to S. suis in the Netherlands was estimated to be 3.5/100 000 for slaughterhouse workers, 2.7/100 000 for pig breeders and 1.2/100 000 for butchers.Citation14 Compared to the non-exposed general population (0.002/100 000), the risk for slaughterhouse workers is 1500 times higher. In the United Kingdom, the risk for butchers is even higher.Citation239 The annual incidence for the occupational group (direct contact with pigs) in Hong Kong was 32/100 000, 350 times higher than that of the general population (0.09/100 000) and 30 times higher than the homologous group in the Netherlands.Citation82 In addition, a less dramatic difference between the occupational group and the general population was observed in Hong Kong (350 times higher in Hong Kong versus 1500 times in the Netherlands). All these differences may be due, at least in part, to a closer contact between people and pork carcasses in Asia.Citation2 Also, a case–control study conducted in Vietnam from 2006 to 2009 showed that eating high risk dishes common in Vietnam, such as fresh (Tiẽt Canh) or undercooked blood, tonsils, tongue, stomach, intestines and uterus from pigs within 2 weeks prior to admission was the most significant risk factor for S. suis infection, followed by exposure to pigs, pork products and preparation of pork in the presence of skin lesions within two weeks prior to admission.Citation72 The authors also investigated the presence of S. suis in pork by-products collected from slaughterhouses and wet/retail markets in Vietnam, and found an 11% contamination rate with S. suis serotype 2 in internal organ samples. Unfortunately, they were unable to demonstrate S. suis carriage rates in 1022 healthy individuals or patients without S. suis infection using a PCR targeting S. suis serotype 2 and 1/2. A PCR for the detection of S. suis may have been more sensitive and could possibly have identified potential carriers of other serotypes.Citation233 The authors suggested that their results, which include six positive rectal swabs from patients, strengthen the hypothesis that the gastrointestinal tract may be a route of entry for S. suis, which is in agreement with cases following the ingestion of raw pork meat. Similarly, data from Thailand suggest a high incidence rate (6.2/100 000) of S. suis infection in the general population in 2010, mostly related to consumption of raw pork products.Citation130
WORLDWIDE DISTRIBUTION OF SEQUENCE TYPES
Global distribution of sequence types
As presented in , different serotype 2 STs predominate in different regions of the world. The ST1 is mostly associated with disease in both pigs and humans in Europe (though the ST20 is important in the Netherlands), Asia (Cambodia, mainland China, Hong Kong, Japan, Thailand and Vietnam) and Argentina ( and ). Meanwhile, the ST7, responsible for the 1998 and 2005 epidemics, is mostly endemic to mainland China ( and ). North American cases greatly vary from those in Eurasia, with most strains being either ST25 or ST28, two STs also recovered in Thailand and Japan, respectively ( and ). Finally, the ST101 to 104 are endemic to Thailand and appear to be more and more commonly isolated from human cases, especially the ST104 (). As such, it can be easily observed that the current distribution of the S. suis serotype 2 STs greatly varies throughout the world, though data have only been available for a little over a decade, from only a few countries and mostly only for the serotype 2.
Figure 1 Worldwide distribution of the most important S. suis serotype 2 sequence types isolated from both clinical pig and human cases of infection.
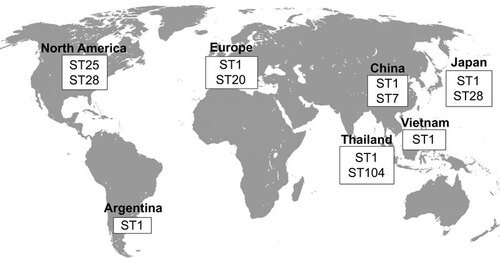
Nevertheless, there is an important issue regarding the serotypes attributed to the different strains typed by MLST. Many of the studies, including most of the strains from the S. suis MLST Database, do not specify the method used for serotyping. In other cases, such as the study of King et al.,Citation51 the authors did not necessarily confirm the serotypes of the strains used. As explained above, the use of PCR for serotyping of serotypes 2 and 14 strains is an inappropriate method, especially for clinical pig cases, even though cases serotyped using this method were still considered for this part of the review. As such, in the cases where more than one serotype was identified for a single ST, it is possible that the serotypes were misidentified and remain to be confirmed using reference antisera. Hence, it is important to keep in mind these discrepancies when analyzing the distribution of STs, particularly for the clinical pig cases. On the other hand, if confirmed, it would be extremely interesting to study strains of different serotypes sharing the same ST, since capsular switching has not been clearly demonstrated for this pathogen.
Diseased pigs
In contrast to the serotype distribution of diseased pigs (the section on ‘Diseased pigs’ under ‘WORLDWIDE DISTRIBUTION OF SEROTYPES’), results where the serotype was identified by either serological methods, PCR or unidentified methods and where the ST was determined using the MLST method described by King et al.,Citation51 were taken into consideration for the worldwide distribution of the STs of clinical cases in pigs.
In North America, the majority of MLST studies conducted on S. suis strains isolated from diseased pigs have been serotype 2 (). It was determined that 44% of North American strains are ST25, 51% ST28 and only 5% are ST1.Citation52 In Canada, the proportions of ST25 and ST28 are similar to 54% and 46%, respectively, but in the United States, 75% of strains were shown to be ST28 and only 10% ST25, while the remaining 15% are ST1.Citation52 As with North American S. suis strains, the majority of European studies have been conducted using serotype 2 strains (). Most of these studies have demonstrated that ST1 strains are predominately isolated from diseased pigs in the Netherlands, Spain and the United Kingdom.Citation51,Citation62,Citation244 King et al. had already associated the serotype 2 ST1 strains with invasive infections.Citation51 Nevertheless, many strains of serotype 9 have also been recovered from diseased pigs and typed.Citation62,Citation244 In both the Netherlands and Spain, serotype 9 isolates were identified as belonging to the ST16, where they represent 43% of strains in the Netherlands.Citation62 Unlike some countries in Europe, where the serotype 9 is as important as the serotype 2, most S. suis strains isolated from diseased pigs in Asia are serotype 2, representing 90% of cases in mainland China.Citation59 However, as mentioned above, there are relatively few reports of isolation from diseased pigs in Asia. Of these serotype 2 cases, the predominant STs are ST1, ST7 and ST28 (). In mainland China, Chen et al.Citation59 demonstrated that 22% of serotype 2 strains are ST1 and 77% are ST7. Meanwhile, the few ST28 strains recovered in that country were mostly associated with cases of pneumonia.Citation246 Regarding Japan, ST1 and ST28 strains isolated from cases of endocarditis in diseased pigs account for 8% and 76% of serotype 2 strains, respectively.Citation248
Table 5 Determined sequence types of reported clinical S. suis cases of infection in pigs from 1 January 2002 to 31 December 2013
Human cases
With 97% of all serotype confirmed human cases of S. suis infection due to serotype 2, the determination of the STs responsible for these cases becomes highly important (). Globally, ST1 strains have been described as mostly responsible for S. suis serotype 2 human cases, particularly in South America, Europe and Asia, but also one case in North America.Citation10,Citation62,Citation86,Citation129,Citation242,Citation249 Nevertheless, multiple other STs have been described worldwide, though these appear to be endemic to certain geographical regions. For example, the ST20 is important in the Netherlands and France but not in the rest of Europe.Citation62 ST7 strains, to which belong the strains responsible for the 1998 and 2005 Chinese epidemics, were isolated from human patients only in mainland China and Hong Kong.Citation242,Citation249 Meanwhile, ST25 and ST28 strains have been particularly associated with human cases in North America and Japan, respectively.Citation53,Citation116,Citation242 The situation is particular in Thailand, where ST1 and ST104 strains are predominant, causing mainly meningitis and non-meningitis cases, respectively.Citation53,Citation128,Citation129 A few cases of ST25, ST28, ST101, ST102 and ST103 have also been described.Citation129 Interestingly, ST101–104 are so far endemic to Thailand only.
Table 6 Determined sequence types of reported clinical S. suis cases of infection in humans from 1 January 2002 to 31 December 2013
Though S. suis serotype 14 infections are less frequent in humans than serotype 2 cases, representing 2% of all the serotype-confirmed cases, the number of human infections caused by this serotype appears to be increasing. The ST105 is prevalent in Southeast Asia, particularly in Vietnam and Thailand. In the latter country, 92% of human serotype 14 cases are caused by this ST ().Citation10,Citation132
Only three human S. suis cases, other than those caused by serotypes 2 and 14, have been typed by MLST (), and all three were described as newly identified STs. Serotype 5, 16 and 24 human cases of infection were identified as ST181, ST106 and ST221, respectively.Citation133,Citation146 Interestingly, no data have yet been published on the possible presence of these three newly identified STs for human isolates in diseased pigs.
Association between pig and human sequence types
Albeit no study has yet associated STs of strains isolated from human cases with those from diseased pigs in the same geographical region, it would seem reasonable to suggest that this association exists, particularly for the serotype 2, as most human strains appear to originate from contact with either pigs or pork by-products. In North America, where serotype 2 ST25 and ST28 strains are predominately isolated from diseased pigs, it is of no surprise that human ST25 cases were identified.Citation52,Citation98,Citation242 Interestingly, no cases due to ST28 have been diagnosed in humans. It has been suggested that ST25 strains from pigs are more virulent than their ST28 counterparts,Citation52 which may explain this situation. Moreover, it is interesting to note that even though ST1 strains account for only 5% of isolates from diseased pigs, a human case caused by an ST1 strain was reported in the United States.Citation96 It is possible that ST1 strains were imported from pigs from Europe and Asia. Being more virulent than their ST25 and ST28 counterparts, it may be hypothesized that a higher number of cases in pigs due to ST1 strains will appear in the near future in North America. Furthermore, similar STs have been described in some Asian countries for both diseased pigs and humans. For example, in Japan, ST1 and ST28 strains have been isolated from both species, while ST1 and ST7 strains have been identified for human and pig isolates in mainland China.Citation53,Citation59,Citation116,Citation128,Citation241,Citation242,Citation248 The situation is similar in Europe where serotype 2 ST1 clonal complex strains are predominately isolated from diseased pigs in Spain, Italy, the Netherlands and the United Kingdom and where most human cases have also been typed as belonging to this complex.Citation51,Citation62,Citation86,Citation204,Citation240,Citation244
This association between pig and human strains within a geographical region, though not definitive and currently only reflecting the situation for the serotype 2, confirms the results obtained by Chatellier et al.Citation250 whereby using randomly amplified polymorphic DNA, they concluded that strains isolated from pigs and humans could not be genotypically distinguished and were similar.
Association between sequence types and virulence markers
Presently, the most popular virulence markers used in association with STs are the SLY (sly), MRP (mrp) and EF (epf). It is important to note that although the genotypes of strains belonging to other serotypes have been reported, these factors are mainly associated with serotype 2 strains.
Serotype 2 ST1 strains recovered from both clinical pig and human cases have for the most part been genotyped/phenotyped as sly+mrp+epf+/SLY+MRP+EF+, regardless of the geographic origin (whether it be mainland China, Japan, North America or Spain for both diseased pigs and human cases), which is identical to the serotype 2 ST7 strains isolated from diseased pigs and humans in mainland China.Citation52,Citation242,Citation244,Citation245,Citation246,Citation248 Nevertheless, other important genetic differences vary between ST1 and ST7 strains including the presence of a 89K pathogenicity island in ST7 strains.Citation251 These ST1 complex strains interestingly differ from not only the human serotype 2 ST104 strains of Thailand which are sly+mrp‐epf‐ but also from the human serotype 2 ST20 strains recovered in the Netherlands that were epf‐.Citation62,Citation129,Citation242 Also of interest is a human case from Spain where the serotype 2 strain isolated was typed as being a ST3, and presented a large variant of the mrp, identified as mrp* (which has a higher molecular weight), though being sly+epf+.Citation204,Citation252 In Europe, it was determined that strains isolated from diseased pigs belonging to the ST16 complex differ from the ST1 complex strains in being mrp* rather than mrp, while in Spain, the endemic ST123 and ST125 are both mrp‐ and epf‐.Citation244 As for ST25 strains isolated from diseased pigs in North America, these were identified as being SLY‐MRP‐EF‐, while the ST28 isolated from North America, mainland China and Japan were sly‐mrp+epf‐ or SLY‐MRP+EF‐.Citation52,Citation246,Citation248 Though currently not as widely used as the above mentioned virulence markers, different pili (srtB, srtC, srtD, srtF and srtG) have also been associated with different STs. It was identified that ST1 strains isolated from both diseased pigs and human cases of serotype 2 infections in Japan and Thailand were srtBCD+ and srtF+ but srtG‐.Citation53 Meanwhile North American ST25 strains isolated from diseased pigs and human cases were srtF- and srtG+ and ST28 strains isolated from diseased pigs and human cases from North America and Japan were srtF+ and srtG+.Citation52,Citation248
It remains difficult to be certain of the association between STs and virulence markers as being universal, but it appears to be representative of populations within a region and may be a useful diagnostic tool with methods identifying the genotypic or phenotypic presence or absence of the different virulence markers.
CONCLUDING REMARKS
S. suis serotype 2 remains the most isolated serotype and the one most associated with disease in both pigs and humans in most parts of the world. Due to its endemic status in Southeast Asia and the two epidemics it caused in Jiangsu (1998) and Sichuan (2005), China, characterized by STSLS and high mortality rates, this bacterium should no longer be considered merely an important pig pathogen, but also an important and emerging zoonotic agent. Many avenues of research remain regarding the definition of a ‘true’ S. suis serotype and the adoption and use of a complete serotyping system. In this regard, the recent availability of new complete PCR serotyping systems will allow any laboratory in the world to serotype S. suis isolates without the need of specific antibodies. The identification of this pathogen by medical and veterinary laboratories significantly varies among countries, and in only a few regions both are well developed. In some important pig producing countries in the Americas, such as Canada, the United States, Mexico and Brazil, diagnostic laboratories in human medicine should be more aware of the zoonotic potential of S. suis due to occupational exposure to pigs and/or pork-derived products. The scientific community should request that the specific serotype of each reported case in humans be identified using acceptable techniques to keep useful epidemiological data. On the other hand, in some countries in Asia where human cases are routinely reported, the number of studies provided by diagnostic laboratories working in veterinary medicine should be significantly increased, since there are almost no data regarding strains recovered from diseased animals. The same applies to European countries which are important pig producers and from which no data regarding the distribution of serotypes from diseased animals were reported in the last 12 years, hampering the use of species-specific protective bacterins.
Though the MLST data currently available for S. suis strains isolated from both diseased pigs and human cases of infection come from different countries throughout the world, there remains a long way to go before a complete picture of the current situation of S. suis can be obtained. Yet, this picture is necessary as it could hint at both dominating and emerging STs and could possibly help identify epidemic strains quickly and categorize the zoonotic potential of specific STs. With the dissemination of information about STs across the internet, particularly thanks to the S. suis MLST Database, it would be important to set up a complete and reliable serotype identification of the different strains added. Although this database is a great tool that facilitates the sharing of knowledge, the lack of many details pertaining to each strain such as the serotype, method used for serotyping, health status of the host, and host itself, limit the full potential of the STs as was demonstrated in this review where many strains could not be included. Furthermore, in the absence of identification of the serotyping method, it is impossible to confirm if the serotype indicated is correct. Nevertheless, the global distribution of S. suis, particularly serotype 2 strains, greatly varies, an aspect that could be useful in developing diagnostic and preventive tools specific for a particular geographical distribution. Despite the fact that studies have recently associated virulence markers and profiles with given STs, the biological significance of these associations still needs to be studied, as only a handful of studies have compared STs from different geographical origins. In fact, some STs found in North America and described as low virulent are frequently isolated from patients in Asia. There is an urgent need to compare virulence properties of strains of similar STs from different geographical origins. Finally, though still tentative, the possibility of associating strains from diseased pigs and human cases in a given region, as determined based on serotypes and STs, would provide further epidemiological support for the zoonotic potential of this pig pathogen, demonstrating the need for proper hygiene practises in order to reduce the risk of zoonotic infections. With more and more laboratories using a complete serotyping system and MLST as a tool for S. suis classification, we will one day have the complete picture regarding the S. suis distribution.
The research carried out on Streptococcus suis in our laboratories is funded by the Natural Sciences and Engineering Research Council of Canada (grant #154280 to Marcelo Gottschalk and 342150-07 to Mariela Segura) and by China–Canada Joint Health Research Initiative financed by Canadian Institutes of Health Research and the National Natural Science Foundation of China (812111251). This publication made use of the Multilocus Sequence Typing Website (http://www.mlst.net) at Imperial College London developed by David Aanensen and funded by the Welcome Trust.
- Gottschalk M, Segura M, Xu J. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim Health Res Rev2007; 8: 29–45.
- Gottschalk M, Xu J, Lecours MP, Grenier D, Fittipaldi N, Segura M. Streptococcus suis infections in humans: what is the prognosis for Western countries? (Part II). Clin Microbiol Newslett2010; 32: 97–102.
- Wertheim HF, Nghia HD, Taylor W, Schultsz C. Streptococcus suis: an emerging human pathogen. Clin Infect Dis2009; 48: 617–625.
- Fittipaldi N, Segura M, Grenier D, Gottschalk M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol2012; 7: 259–279.
- Baums CG, Valentin-Weigand P. Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim Health Res Rev2009; 10: 65–83.
- Higgins R, Gottschalk M. Streptococcal diseases. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ (eds.) Diseases of swine. Ames, IA: Iowa State University Press, 2006: 769–783.
- Sanford SE, Tilker ME. Streptococcus suis type II-associated diseases in swine: observations of a one-year study. J Am Vet Med Assoc1982; 181: 673–676.
- Staats JJ, Feder I, Okwumabua O, Chengappa MM. Streptococcus suis: past and present. Vet Res Commun1997; 21: 381–407.
- Perch B, Kristjansen P, Skadhauge K. Group R streptococci pathogenic for man. Two cases of meningitis and one fatal case of sepsis. Acta Pathol Microbiol Scand1968; 74: 69–76.
- Mai NT, Hoa NT, Nga TV et al. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis2008; 46: 659–667.
- Suankratay C, Intalapaporn P, Nunthapisud P, Arunyingmongkol K, Wilde H. Streptococcus suis meningitis in Thailand. Southeast Asian J Trop Med Public Health2004; 35: 868–876.
- Hui AC, Ng KC, Tong PY et al. Bacterial meningitis in Hong Kong: 10-years’ experience. Clin Neurol Neurosurg2005; 107: 366–370.
- Fongcom A, Pruksakorn S, Mongkol R, Tharavichitkul P, Yoonim N. Streptococcus suis infection in northern Thailand. J Med Assoc Thai2001; 84: 1502–1508.
- Arends JP, Zanen HC. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis1988; 10: 131–137.
- Huang YT, Teng LJ, Ho SW, Hsueh PR. Streptococcus suis infection. J Microbiol Immunol Infect2005; 38: 306–313.
- Tang J, Wang C, Feng Y et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med2006; 3: e151.
- Higgins R, Gottschalk M. An update on Streptococcus suis identification. J Vet Diagn Invest1990; 2: 249–252.
- Gottschalk M, Xu J, Calzas C, Segura M. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol2010; 5: 371–391.
- Lutticken R, Temme N, Hahn G, Bartelheimer EW. Meningitis caused by Streptococcus suis: case report and review of the literature. Infection1986; 14: 181–185.
- Michaud S, Duperval R, Higgins R. Streptococcus suis meningitis: first case reported in Quebec. Can J Infect Dis1996; 7: 329–331.
- Tsai HC, Lee SS, Wann SR, Huang TS, Chen YS, Liu YC. Streptococcus suis meningitis with ventriculoperitoneal shunt infection and spondylodiscitis. J Formos Med Assoc2005; 104: 948–950.
- Yen MY, Liu YC, Wang JH, Chen YS, Wang YH, Cheng DL. Streptococcus suis meningitis complicated with permanent perceptive deafness: report of a case. J Formos Med Assoc1994; 93: 349–351.
- Donsakul K, Dejthevaporn C, Witoonpanich R. Streptococcus suis infection: clinical features and diagnostic pitfalls. Southeast Asian J Trop Med Public Health2003; 34: 154–158.
- Okwumabua O, O’Connor M, Shull E. A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiol Lett2003; 218: 79–84.
- Gottschalk M, Lacouture S, Bonifait L, Roy D, Fittipaldi N, Grenier D. Characterization of Streptococcus suis isolates recovered between 2008 and 2011 from diseased pigs in Quebec, Canada. Vet Microbiol2013; 162: 819–825.
- Tien le HT, Sugiyama N, Duangsonk K, Tharavichitkul P, Osawa R. Phenotypic and PCR-based identification of bacterial strains isolated from patients with suspected Streptococcus suis infection in northern Thailand. Jpn J Infect Dis2012; 65: 171–174.
- Hill JE, Gottschalk M, Brousseau R, Harel J, Hemmingsen SM, Goh SH. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet Microbiol2005; 107: 63–69.
- Brousseau R, Hill JE, Prefontaine G, Goh SH, Harel J, Hemmingsen SM. Streptococcus suis serotypes characterized by analysis of chaperonin 60 gene sequences. Appl Environ Microbiol2001; 67: 4828–4833.
- Messier S, Lacouture S, Gottschalk M. Distribution of Streptococcus suis capsular types from 2001 to 2007. Can Vet J2008; 49: 461–462.
- Wang K, Sun X, Lu C. Development of rapid serotype-specific PCR assays for eight serotypes of Streptococcus suis. J Clin Microbiol2012; 50: 3329–3334.
- Tien le HT, Nishibori T, Nishitani Y, Nomoto R, Osawa R. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22, 26, and 33 based on DNA–DNA homology and sodA and recN phylogenies. Vet Microbiol2013; 162: 842–849.
- Segura M, Zheng H, Greeff Ad et al. Latest developments on Streptococcus suis: an emerging zoonotic pathogen: part 1. Future Microbiol2014; 9: 441–444.
- Van Calsteren MR, Gagnon F, Lacouture S, Fittipaldi N, Gottschalk M. Structure determination of Streptococcus suis serotype 2 capsular polysaccharide. Biochem Cell Biol2010; 88: 513–525.
- Van Calsteren MR, Gagnon F, Calzas C et al. Structure determination of Streptococcus suis serotype 14 capsular polysaccharide. Biochem Cell Biol2013; 91: 49–58.
- Gottschalk M, Higgins R, Jacques M, Mittal KR, Henrichsen J. Description of 14 new capsular types of Streptococcus suis. J Clin Microbiol1989; 27: 2633–2636.
- Gottschalk M, Higgins R, Jacques M, Beaudoin M, Henrichsen J. Isolation and characterization of Streptococcus suis capsular types 9–22. J Vet Diagn Invest1991; 3: 60–65.
- Gottschalk M, Higgins R, Boudreau M. Use of polyvalent coagglutination reagents for serotyping of Streptococcus suis. J Clin Microbiol1993; 31: 2192–2194.
- Perch B, Kjems E, Slot P, Pedersen KB. Biochemical and serological properties of R, S, and RS streptococci. Acta Pathol Microbiol Scand B1981; 89: 167–171.
- Wisselink HJ, Joosten JJ, Smith HE. Multiplex PCR assays for simultaneous detection of six major serotypes and two virulence-associated phenotypes of Streptococcus suis in tonsillar specimens from pigs. J Clin Microbiol2002; 40: 2922–2929.
- Marois C, Bougeard S, Gottschalk M, Kobisch M. Multiplex PCR assay for detection of Streptococcus suis species and serotypes 2 and 1/2 in tonsils of live and dead pigs. J Clin Microbiol2004; 42: 3169–3175.
- Liu Z, Zheng H, Gottschalk M et al. Development of multiplex PCR assays for the identification of the 33 serotypes of Streptococcus suis. PLoS One2013; 8: e72070.
- Okura M, Lachance C, Osaki M et al. Development of a two-step multiplex PCR assays for typing of capsular polysaccharide synthesis gene clusters of Streptococcus suis. J Clin Microbiol2014; 52: 1714–1719.
- Okura M, Takamatsu D, Maruyama F et al. Genetic analysis of capsular polysaccharide synthesis gene clusters from all serotypes of Streptococcus suis: potential mechanisms for generation of capsular variation. Appl Environ Microbiol2013; 79: 2796–2806.
- Kopic J, Paradzik MT, Pandak N. Streptococcus suis infection as a cause of severe illness: 2 cases from Croatia. Scand J Infect Dis2002; 34: 683–684.
- Vilaichone RK, Vilaichone W, Nunthapisud P, Wilde H. Streptococcus suis infection in Thailand. J Med Assoc Thai2002; 85: S109–S117.
- Prieto C, Garcia FJ, Suarez P, Imaz M, Castro JM. Biochemical traits and antimicrobial susceptibility of Streptococcus suis isolated from slaughtered pigs. Zentralbl Veterinarmed B1994; 41: 608–617.
- Bonifait L, Gottschalk M, Grenier D. Cell surface characteristics of nontypeable isolates of Streptococcus suis. FEMS Microbiol Lett2010; 311: 160–166.
- Lakkitjaroen N, Takamatsu D, Okura M, Sato M, Osaki M, Sekizaki T. Loss of capsule among Streptococcus suis isolates from porcine endocarditis and its biological significance. J Med Microbiol2011; 60: 1669–1676.
- Brehony C, Jolley KA, Maiden MC. Multilocus sequence typing for global surveillance of meningococcal disease. FEMS Microbiol Rev2007; 31: 15–26.
- Maiden MC. Multilocus sequence typing of bacteria. Annu Rev Microbiol2006; 60: 561–588.
- King SJ, Leigh JA, Heath PJ et al. Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: identification of virulent clones and potential capsular serotype exchange. J Clin Microbiol2002; 40: 3671–3680.
- Fittipaldi N, Xu J, Lacouture S et al. Lineage and virulence of Streptococcus suis serotype 2 isolates from North America. Emerg Infect Dis2011; 17: 2239–2244.
- Takamatsu D, Nishino H, Ishiji T et al. Genetic organization and preferential distribution of putative pilus gene clusters in Streptococcus suis. Vet Microbiol2009; 138: 132–139.
- Lachance C, Gottschalk M, Gerber PP, Lemire P, Xu J, Segura M. Exacerbated type II interferon response drives hypervirulence and toxic shock by an emergent epidemic strain of Streptococcus suis. Infect Immun2013; 81: 1928–1939.
- Fittipaldi N, Fuller TE, Teel JF et al. Serotype distribution and production of muramidase-released protein, extracellular factor and suilysin by field strains of Streptococcus suis isolated in the United States. Vet Microbiol2009; 139: 310–317.
- Martinez G, Pestana de Castro AF, Ribeiro Pagnani KJ, Nakazato G, Dias da Silveira W, Gottschalk M. Clonal distribution of an atypical MRP+, EF*, and suilysin+ phenotype of virulent Streptococcus suis serotype 2 strains in Brazil. Can J Vet Res2003; 67: 52–55.
- Costa AT, Lobato FC, Abreu VL, Assis RA, Reis R, Uzal FA. Serotyping and evaluation of the virulence in mice of Streptococcus suis strains isolated from diseased pigs. Rev Inst Med Trop Sao Paulo2005; 47: 113–115.
- Wei Z, Li R, Zhang A et al. Characterization of Streptococcus suis isolates from the diseased pigs in China between 2003 and 2007. Vet Microbiol2009; 137: 196–201.
- Chen L, Song Y, Wei Z, He H, Zhang A, Jin M. Antimicrobial susceptibility, tetracycline and erythromycin resistance genes, and multilocus sequence typing of Streptococcus suis isolates from diseased pigs in China. J Vet Med Sci2013; 75: 583–587.
- Li LL, Liao XP, Sun J et al. Antimicrobial resistance, serotypes, and virulence factors of Streptococcus suis isolates from diseased pigs. Foodborne Pathog Dis2012; 9: 583–588.
- Kim D, Han K, Oh Y et al. Distribution of capsular serotypes and virulence markers of Streptococcus suis isolated from pigs with polyserositis in Korea. Can J Vet Res2010; 74: 314–316.
- Schultsz C, Jansen E, Keijzers W et al. Differences in the population structure of invasive Streptococcus suis strains isolated from pigs and from humans in The Netherlands. PLoS One2012; 7: e33854.
- Vela AI, Goyache J, Tarradas C et al. Analysis of genetic diversity of Streptococcus suis clinical isolates from pigs in Spain by pulsed-field gel electrophoresis. J Clin Microbiol2003; 41: 2498–2502.
- Tarradas C, Perea A, Vela AI et al. Distribution of serotypes of Streptococcus suis isolated from diseased pigs in Spain. Vet Rec2004; 154: 665–666.
- Luque I, Blume V, Borge C et al. Genetic analysis of Streptococcus suis isolates recovered from diseased and healthy carrier pigs at different stages of production on a pig farm. Vet J2010; 186: 396–398.
- Wisselink HJ, Smith HE, Stockhofe-Zurwieden N, Peperkamp K, Vecht U. Distribution of capsular types and production of muramidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis strains isolated from diseased pigs in seven European countries. Vet Microbiol2000; 74: 237–248.
- Marois C, Le Devendec L, Gottschalk M, Kobisch M. Detection and molecular typing of Streptococcus suis in tonsils from live pigs in France. Can J Vet Res2007; 71: 14–22.
- Wang K, Zhang W, Li X et al. Characterization of Streptococcus suis isolates from slaughter swine. Curr Microbiol2013; 66: 344–349.
- Ngo TH, Tran TB, Tran TT et al. Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in southern Vietnam. PLoS One2011; 6: e17943.
- Kerdsin A, Dejsirilert S, Akeda Y et al. Fifteen Streptococcus suis serotypes identified by multiplex PCR. J Med Microbiol2012; 61: 1669–1672.
- Zhang CP, Ning YB, Zhang ZQ et al. [Distributions of pathogenic capsular types and in vitro antimicrobial susceptibility of different serotypes of Streptococcus suis isolated from clinically healthy sows from 10 provinces in China.] Zhonghua Liu Xing Bing Xue Za Zhi2009; 30: 235–238. Chinese.
- Nghia HD, Tu le TP, Wolbers M et al. Risk factors of Streptococcus suis infection in Vietnam. A case-control study. PLoS One2011; 6: e17604.
- Vilaichone RK, Mahachai V, Nunthapisud P. Streptococcus suis peritonitis: case report. J Med Assoc Thai2000; 83: 1274–1277.
- Lopreto C, Lopardo HA, Bardi MC, Gottschalk M. [Primary Streptococcus suis meningitis: first case in humans described in Latin America.] Enferm Infecc Microbiol Clin2005; 23: 110. Spanish.
- Koch E, Fuentes G, Carvajal R et al. [Streptococcus suis meningitis in pig farmers: report of first two cases in Chile.] Rev Chilena Infectol2013; 30: 557–561. Spanish.
- Alarcon P, Araya P, Aguayo C et al. [Laboratory confirmation of Streptococcus suis in Chile.] Rev Chilena Infectol2013; 30: 539–540. Spanish.
- Callejo R, Prieto M, Salamone F, Auger J, Goyette-Desjardins G, Gottschalk M. Atypical Streptococcus suis in man, Argentina, 2013. Emerg Infect Dis2014; 20: 500–502.
- Padungtod P, Tharavichitkul P, Junya S et al. Incidence and presence of virulence factors of Streptococcus suis infection in slaughtered pigs from Chiang Mai, Thailand. Southeast Asian J Trop Med Public Health2010; 41: 1454–1461.
- Hoa NT, Chieu TT, Do Dung S et al. Streptococcus suis and porcine reproductive and respiratory syndrome, Vietnam. Emerg Infect Dis2013; 19: 331–333.
- Kataoka Y, Sugimoto C, Nakazawa M, Morozumi T, Kashiwazaki M. The epidemiological studies of Streptococcus suis infections in Japan from 1987 to 1991. J Vet Med Sci1993; 55: 623–626.
- Ip M, Fung KS, Chi F et al. Streptococcus suis in Hong Kong. Diagn Microbiol Infect Dis2007; 57: 15–20.
- Ma E, Chung PH, So T et al. Streptococcus suis infection in Hong Kong: an emerging infectious disease? Epidemiol Infect2008; 136: 1691–1697.
- Marie J, Morvan H, Berthelot-Herault F et al. Antimicrobial susceptibility of Streptococcus suis isolated from swine in France and from humans in different countries between 1996 and 2000. J Antimicrob Chemother2002; 50: 201–209.
- Tian Y, Aarestrup FM, Lu CP. Characterization of Streptococcus suis serotype 7 isolates from diseased pigs in Denmark. Vet Microbiol2004; 103: 55–62.
- Aarestrup FM, Jorsal SE, Jensen NE. Serological characterization and antimicrobial susceptibility of Streptococcus suis isolates from diagnostic samples in Denmark during 1995 and 1996. Vet Microbiol1998; 60: 59–66.
- Princivalli MS, Palmieri C, Magi G et al. Genetic diversity of Streptococcus suis clinical isolates from pigs and humans in Italy (2003–2007). Euro Surveill2009; 14: pii: 19310.
- Stanojkovic A, Asanin R, Misic D, Asanin J, Stanojkovic-Sebic A. The presence and serological types of Streptococcus suis strains isolated from pigs originating from some farms in Serbia. Fresen Environ Bull2012; 21: 3558–3561.
- Seol B, Kelneric Z, Hajsig D, Madic J, Naglic T. Susceptibility to antimicrobial agents of Streptococcus suis capsular type 2 strains isolated from pigs. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg1996; 283: 328–331.
- Buddle JR, Jones JE, Pass DA, Robertson J. The isolation of Streptococcus suis type II from a pig with meningitis. Aust Vet J1981; 57: 437–438.
- Mwaniki CG, Robertson ID, Trott DJ, Atyeo RF, Lee BJ, Hampson DJ. Clonal analysis and virulence of Australian isolates of Streptococcus suis type 2. Epidemiol Infect1994; 113: 321–334.
- Dickie AS, Bremner DA, Wong PY, North JD, Robertson ID. Streptococcus suis bacteraemia. N Z Med J1987; 100: 677–678.
- Kohler W, Queisser H, Kunter E, Sawitzki R, Frach G. [Type 2 Streptococcus suis (R-Streptococci) as pathogens of occupational diseases. Report of a case and a review of the literature.] Z Gesamte Inn Med1989; 44: 144–148. German.
- Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis2007; 7: 201–209.
- Trottier S, Higgins R, Brochu G, Gottschalk M. A case of human endocarditis due to Streptococcus suis in North America. Rev Infect Dis1991; 13: 1251–1252.
- Haleis A, Alfa M, Gottschalk M, Bernard K, Ronald A, Manickam K. Meningitis caused by Streptococcus suis serotype 14, North America. Emerg Infect Dis2009; 15: 350–352.
- Willenburg KS, Sentochnik DE, Zadoks RN. Human Streptococcus suis meningitis in the United States. N Engl J Med2006; 354: 1325.
- Fittipaldi N, Collis T, Prothero B, Gottschalk M. Streptococcus suis meningitis, Hawaii. Emerg Infect Dis2009; 15: 2067–2069.
- Fowler HN, Brown P, Rovira A et al. Streptococcus suis meningitis in swine worker, Minnesota, USA. Emerg Infect Dis2013; 19: 330–331.
- Nagel A, Manias V, Busquets N, Sniadowsky S, Anzardi J, Mendez Ede L. [Streptococcus suis meningitis in an immunocompetent patient.] Rev Argent Microbiol2008; 40: 158–160. Spanish.
- Nunez JM, Marcotullio M, Rojas A, Acuna L, Caceres M, Mochi S. [First case of meningitis by Streptococcus suis in the norwest area of Argentina.] Rev Chilena Infectol2013; 30: 554–556. Spanish.
- Demar M, Belzunce C, Simonnet C et al. Streptococcus suis meningitis and bacteremia in man, French Guiana. Emerg Infect Dis2013; 19: 1545–1546.
- Vlieghe E. The microbiological spectrum of invasive bacterial infections in Cambodian adults and implications for standard treatment guidelines. MD thesis, University of Leuven, Leuven, Belgium, 2014.
- Cheng AF, Oo KT, Li EK, French GL. Septic arthritis caused by Streptococcus suis serotype 2. J Infect1987; 14: 237–241.
- Hu X, Zhu F, Wang H et al. [Studies on human streptococcal infectious syndrome caused by infected pigs.] Zhonghua Yu Fang Yi Xue Za Zhi2000; 34: 150–152. Chinese.
- Yu H, Jing H, Chen Z et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis2006; 12: 914–920.
- Feng Y, Shi X, Zhang H et al. Recurrence of human Streptococcus suis infections in 2007: three cases of meningitis and implications that heterogeneous S. suis 2 circulates in China. Zoonoses Public Health2009; 56: 506–514.
- Zhu Y, Tan Z, Zhu L et al. Streptococcus suis serotype 2 caused streptococcal toxic shock syndrome (STSS) in a patient. J Nanjing Med Univ2008; 22: 313–316.
- Chau PY, Huang CY, Kay R. Streptococcus suis meningitis. An important underdiagnosed disease in Hong Kong. Med J Aust1983; 1: 414–416, 417.
- Ho AK, Woo KS, Tse KK, French GL. Infective endocarditis caused by Streptococcus suis serotype 2. J Infect1990; 21: 209–211.
- Kay R, Cheng AF, Tse CY. Streptococcus suis infection in Hong Kong. QJM1995; 88: 39–47.
- Woo J. Streptococcus suis meningitis in man in Hong Kong. Trans R Soc Trop Med Hyg1986; 80: 848–849.
- Woo J, Li EK. Streptococcus suis meningitis requires prolonged treatment with penicillin. Infection1987; 15: 129–130.
- Ng HW. Overwhelming postsplenectomy sepsis presenting as purpura fulminans due to Streptococcus suis infection. Hong Kong J Emerg Med2013; 20: 155–160.
- Ibaraki M, Fujita N, Tada M, Ohtaki O, Nagai H. [A Japanese case of Streptococcus suis meningitis associated with lumbar epidural abscess.] Rinsho Shinkeigaku2003; 43: 176–179. Japanese.
- Matsuo H, Sakamoto S. [Purulent meningitis caused by Streptococcus suis in a pig breeder.] Kansenshogaku Zasshi2003; 77: 340–342. Japanese.
- Chang B, Wada A, Ikebe T et al. Characteristics of Streptococcus suis isolated from patients in Japan. Jpn J Infect Dis2006; 59: 397–399.
- Ishigaki K, Nakamura A, Iwabuchi S et al. [A case of Streptococcus suis endocarditis, probably bovine-transmitted, complicated by pulmonary embolism and spondylitis.] Kansenshogaku Zasshi2009; 83: 544–548. Japanese.
- Mori K, Ishii N, Mochizuki H, Taniguchi A, Shiomi K, Nakazato M. Bilateral sensorineural hearing impairment due to Streptococcus suis meningitis 20 days after swine bite. Rinsho Shinkeigaku2013; 53: 732–735.
- Lee GT, Chiu CY, Haller BL, Denn PM, Hall CS, Gerberding JL. Streptococcus suis meningitis, United States. Emerg Infect Dis2008; 14: 183–185.
- Tambyah PA, Kumarasinghe G, Chan HL, Lee KO. Streptococcus suis infection complicated by purpura fulminans and rhabdomyolysis: case report and review. Clin Infect Dis1997; 24: 710–712.
- Chan YC, Wilder-Smith A, Ong BK, Kumarasinghe G, Wilder-Smith E. Adult community acquired bacterial meningitis in a Singaporean teaching hospital. A seven-year overview (1993–2000). Singapore Med J2002; 43: 632–636.
- Tan JH, Yeh BI, Seet CS. Deafness due to haemorrhagic labyrinthitis and a review of relapses in Streptococcus suis meningitis. Singapore Med J2010; 51: e30–33.
- Kim H, Lee SH, Moon HW et al. Streptococcus suis causes septic arthritis and bacteremia: phenotypic characterization and molecular confirmation. Korean J Lab Med2011; 31: 115–117.
- Huh HJ, Park KJ, Jang JH et al. Streptococcus suis meningitis with bilateral sensorineural hearing loss. Korean J Lab Med2011; 31: 205–211.
- Choi SM, Cho BH, Choi KH et al. Meningitis caused by Streptococcus suis: case report and review of the literature. J Clin Neurol2012; 8: 79–82.
- Oh YJ, Song SH. A case of Streptococcus suis infection causing pneumonia with empyema in Korea. Tuberc Respir Dis2012; 73: 178–181.
- Tsai HY, Liao CH, Liu CY, Huang YT, Teng LJ, Hsueh PR. Streptococcus suis infection in Taiwan, 2000–2011. Diagn Microbiol Infect Dis2012; 74: 75–77.
- Takamatsu D, Wongsawan K, Osaki M et al. Streptococcus suis in humans, Thailand. Emerg Infect Dis2008; 14: 181–183.
- Kerdsin A, Dejsirilert S, Puangpatra P et al. Genotypic profile of Streptococcus suis serotype 2 and clinical features of infection in humans, Thailand. Emerg Infect Dis2011; 17: 835–842.
- Takeuchi D, Kerdsin A, Pienpringam A et al. Population-based study of Streptococcus suis infection in humans in Phayao Province in northern Thailand. PLoS One2012; 7: e31265.
- Hanterdsith B, Tharavichitkul P, Mahanupab P, Raksamat W. Postmortem diagnosis of sudden unexpected death from Streptococcus suis type 2 infection: a case report. J Forensic Leg Med2013; 20: 347–349.
- Kerdsin A, Oishi K, Sripakdee S et al. Clonal dissemination of human isolates of Streptococcus suis serotype 14 in Thailand. J Med Microbiol2009; 58: 1508–1513.
- Kerdsin A, Dejsirilert S, Sawanpanyalert P et al. Sepsis and spontaneous bacterial peritonitis in Thailand. Lancet2011; 378: 960.
- Leelarasamee A, Nilakul C, Tien-Grim S, Srifuengfung S, Susaengrat W. Streptococcus suis toxic-shock syndrome and meningitis. J Med Assoc Thai1997; 80: 63–68.
- Chotmongkol V, Janma J, Kawamatawong T. Streptococcus suis meningitis: report of a case. J Med Assoc Thai1999; 82: 922–924.
- Teekakirikul P, Wiwanitkit V. Streptococcus suis infection: overview of case reports in Thailand. Southeast Asian J Trop Med Public Health2003; 34 (Suppl 2): 178–183.
- Wangkaew S, Chaiwarith R, Tharavichitkul P, Supparatpinyo K. Streptococcus suis infection: a series of 41 cases from Chiang Mai University Hospital. J Infect2006; 52: 455–460.
- Wangsomboonsiri W, Luksananun T, Saksornchai S, Ketwong K, Sungkanuparph S. Streptococcus suis infection and risk factors for mortality. J Infect2008; 57: 392–396.
- Rusmeechan S, Sribusara P. Streptococcus suis meningitis: the newest serious infectious disease. J Med Assoc Thai2008; 91: 654–658.
- Navacharoen N, Chantharochavong V, Hanprasertpong C, Kangsanarak J, Lekagul S. Hearing and vestibular loss in Streptococcus suis infection from swine and traditional raw pork exposure in northern Thailand. J Laryngol Otol2009; 123: 857–862.
- Fongcom A, Pruksakorn S, Netsirisawan P, Pongprasert R, Onsibud P. Streptococcus suis infection: a prospective study in northern Thailand. Southeast Asian J Trop Med Public Health2009; 40: 511–517.
- Laohapensang K, Rutherford RB, Arworn S. Mycotic abdominal aortic aneurysm due to Streptococcus suis: a case report. Surg Infect2010; 11: 179–181.
- Pachirat O, Taksinachanekit S, Mootsikapun P, Kerdsin A. Human Streptococcus suis endocarditis: echocardiographic features and clinical outcome. Clin Med Insights Cardiol2012; 6: 119–123.
- Hampson DJ, Trott DJ, Clarke IL, Mwaniki CG, Robertson ID. Population structure of Australian isolates of Streptococcus suis. J Clin Microbiol1993; 31: 2895–2900.
- Nga TV, Nghia HD, Tu le TP et al. Real-time PCR for detection of Streptococcus suis serotype 2 in cerebrospinal fluid of human patients with meningitis. Diagn Microbiol Infect Dis2011; 70: 461–467.
- Nghia HD, Hoa NT, Linh le D et al. Human case of Streptococcus suis serotype 16 infection. Emerg Infect Dis2008; 14: 155–157.
- Wertheim HF, Nguyen HN, Taylor W et al. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS One2009; 4: e5973.
- Taylor WR, Nguyen K, Nguyen D et al. The spectrum of central nervous system infections in an adult referral hospital in Hanoi, Vietnam. PLoS One2012; 7: e42099.
- Ho Dang Trung N, Le Thi Phuong T, Wolbers M et al. Aetiologies of central nervous system infection in Viet Nam: a prospective provincial hospital-based descriptive surveillance study. PLoS One2012; 7: e37825.
- Spiss HK, Kofler M, Hausdorfer H, Pfausler B, Schmutzhard E. [Streptococcus suis meningitis and neurophysiology of the acoustic system. First case report from Austria.] Nervenarzt1999; 70: 738–741. German.
- Colaert J, Allewaert M, Magerman H, Vandeven J, Vandepitte J. Streptococcus suis meningitis in man. First reported observation in Belgium. Acta Clin Belg1985; 40: 314–317.
- Cammaert T, Verstraete W, Baeck E. [Deafness–blindness caused by Streptococcus suis meningitis—epidemiology and rehabilitation.] Acta Otorhinolaryngol Belg1990; 44: 37–41. Dutch.
- Hantson P, Vekemans MC, Gautier P et al. Fatal Streptococcus suis meningitis in man. Acta Neurol Belg1991; 91: 165–168.
- Coolen L, Dens J, Baeck E et al. Streptococcus suis meningitis, permanent perceptive deafness and endophthalmitis. Intensive Care Med1989; 15: 545.
- Perch B, Kjems E. Group R Streptococcus infections in man. JAMA1971; 216: 1484.
- Koldkjaer O, Nielsen G. Haemolytic Streptococcus group R infection. Br Med J1972; 3: 765.
- Kjems E, Perch B. [Serious infections in man caused by group R streptococci.] Ugeskr Laeger1975; 137: 682–683. Danish.
- Poggenborg R, Gaini S, Kjaeldgaard P, Christensen JJ. Streptococcus suis: meningitis, spondylodiscitis and bacteraemia with a serotype 14 strain. Scand J Infect Dis2008; 40: 346–349.
- Hedegaard SS, Zaccarin M, Lindberg J. [Septic arthritis caused by Streptococcus suis.] Ugeskr Laeger2013; 175: 1574–1575. Danish.
- Avril MF, Ghanassia JP, Leger JM, Bure A, Modai J. [Meningitis by group R Streptococcus. About two observations.] Med Maladies Infect1977; 7: 531–534. French.
- Paul G, Ancelle JP, Lionsquy G, Brochard C, Brassac R, Nevot P. [Human meningitis by group R Streptococcus: an unknown anthropozoonosis?] Med Maladies Infect1977; 7: 525–529. French.
- Faucqueur B, Proust J. [Streptococcus suis meningitis. An occupational disease.] Presse Med1983; 12: 1821. French.
- Bonmarchand G, Massari P, Humbert G et al. Group R streptococci: wild boars as a second reservoir. Scand J Infect Dis1985; 17: 121–122.
- Desjars P, Reynaud AE, Tasseau F, Courtieu AL. [Streptococcus suis infections: about a meningitis in a slaughterhouse worker.] Med Maladies Infect1987; 17: 469–471. French.
- Dupas D, Vignon M, Geraut C. Streptococcus suis meningitis. A severe noncompensated occupational disease. J Occup Med1992; 34: 1102–1105.
- Bezian MC, Diallo BS, Chachia A, Gabinski C, Beyloi J. [Purpura fulminans during Streptococcus suis meningitis and septicemia.] Med Maladies Infect1996; 26: 349–351. French.
- Tayoro J, Besnier JM, Laudat P, Cattier B, Choutet P. Infective endocarditis due to Streptococcus suis serotype 2. Eur J Clin Microbiol Infect Dis1996; 15: 765–766.
- Caumont H, Gerard N, Depernet B, Brasme L, Eschard JP, Etienne JC. [Streptococcus suis L3–L4 spondylodiscitis in a butcher.] Presse Med1996; 25: 1348. French.
- Bouchaud L, Bourgain C, Aouar M, Grise G, Morcamp D, Deséchalliers JP. [Streptococcus suis type II meningitis. About a case.] Med Maladies Infect1997; 27: 317–318. French.
- François B, Gissot V, Ploy MC, Vignon P. Recurrent septic shock due to Streptococcus suis. J Clin Microbiol1998; 36: 2395.
- Durand F, Périno CL, Recule C et al. Bacteriological diagnosis of Streptococcus suis meningitis. Eur J Clin Microbiol Infect Dis2001; 20: 519–521.
- Bensaid T, Bonnefoi-Kyriacou B, Dupel-Pottier C, Bellon O, Lagier E, Chardon H. [Streptococcus suis meningitis following wild boar hunting.] Presse Med2003; 32: 1077–1078. French.
- Pedroli S, Kobisch M, Beauchet O, Chaussinand JP, Lucht F. [Streptococcus suis bacteremia.] Presse Med2003; 32: 599–601. French.
- Fauveau L, Mourtada Y, Hazouard E. [Meningoencephalitis related-to Streptococcus suis in a butcher: relevance of occupational questionnaire at emergency room.] Ann Fr Anesth Reanim2007; 26: 814–815. French.
- Bahloul H, Mofredj A, Mrabet A, Gineyt G, Rousselier P. [Streptococcus suis meningitis after oral contamination?] Med Maladies Infect2008; 38: 281–282. French.
- Rosenstingl S, Rouede Y, Galanti MJ, Gatfosse M. [Streptococcus suis septicaemia in a hunter's wife.] Med Maladies Infect2008; 38: S177. French.
- Piech C, Guettrot-Imbert G, Delevaux I, André M, Lhoste A, Aumaître O. [Streptococcus suis spondylodiscitis and oligoarthritis in a hunter.] Rev Med Intern2009; 30: S142. French.
- Kaufhold A, Lutticken R, Litterscheid S. [Systemic infection caused by Streptococcus suis.] Dtsch Med Wochenschr1988; 113: 1642–1643. German.
- Bungener W, Bialek R. Fatal Streptococcus suis septicemia in an abattoir worker. Eur J Clin Microbiol Infect Dis1989; 8: 306–308.
- Grebe T, Bergenthal D, Fahr AM, Scheja HW. [Meningitis caused by Streptococcus suis type 2 in an adult.] Dtsch Med Wochenschr1997; 122: 1244–1247. German.
- Strangmann E, Froleke H, Kohse KP. Septic shock caused by Streptococcus suis: case report and investigation of a risk group. Int J Hyg Environ Health2002; 205: 385–392.
- Rosenkranz M, Elsner HA, Sturenburg HJ, Weiller C, Rother J, Sobottka I. Streptococcus suis meningitis and septicemia contracted from a wild boar in Germany. J Neurol2003; 250: 869–870.
- Heidt MC, Mohamed W, Hain T, Vogt PR, Chakraborty T, Domann E. Human infective endocarditis caused by Streptococcus suis serotype 2. J Clin Microbiol2005; 43: 4898–4901.
- Braun S, Jechart G, Emmerling U, Ehret W. [Meningitis caused by Streptococcus suis.] Dtsch Med Wochenschr2007; 132: 1098–1100. German.
- Mazokopakis EE, Kofteridis DP, Papadakis JA, Gikas AH, Samonis GJ. First case report of Streptococcus suis septicaemia and meningitis from Greece. Eur J Neurol2005; 12: 487–489.
- Voutsadakis IA. Streptococcus suis endocarditis and colon carcinoma: a case report. Clin Colorectal Cancer2006; 6: 226–228.
- Baddeley PG. Streptococcus suis infection. Occup Med1995; 45: 222.
- Manzin A, Palmieri C, Serra C et al. Streptococcus suis meningitis without history of animal contact, Italy. Emerg Infect Dis2008; 14: 1946–1948.
- Camporese A, Tizianel G, Bruschetta G, Cruciatti B, Pomes A. Human meningitis caused by Streptococcus suis: the first case report from north-eastern Italy. Infez Med2007; 15: 111–114.
- Perseghin P, Bezzi G, Troupioti P, Gallina M. Streptococcus suis meningitis in an Italian blood donor. Lancet1995; 346: 1305–1306.
- Kloppenburg M, Mulder NH, Houwerzijl J. Septicaemia due to porcine streptococci. Lancet1975; 306: 1218.
- Zanen HC, Engel HW. Porcine streptococci causing meningitis and septicaemia in man. Lancet1975; 305: 1286–1288.
- van Jaarsveld BC, van Kregten E, van Kesteren RG, Rozenberg-Arska M, Bartelink AK. [Fulminant sepsis caused by Streptococcus suis.] Ned Tijdschr Geneeskd1990; 134: 1462–1464. Dutch.
- Bartelink AK, van Kregten E. Streptococcus suis as threat to pig-farmers and abattoir workers. Lancet1995; 346: 1707.
- Halaby T, Hoitsma E, Hupperts R, Spanjaard L, Luirink M, Jacobs J. Streptococcus suis meningitis, a Poacher’s risk. Eur J Clin Microbiol Infect Dis2000; 19: 943–945.
- Peetermans WE, Moffie BG, Thompson J. Bacterial endocarditis caused by Streptococcus suis type 2. J Infect Dis1989; 159: 595–596.
- Arend SM, van Buchem MA, van Ogtrop ML, Thompson J. Septicaemia, meningitis and spondylodiscitis caused by Streptococcus suis type 2. Infection1995; 23: 128.
- van de Beek D, Spanjaard L, de Gans J. Streptococcus suis meningitis in the Netherlands. J Infect2008; 57: 158–161.
- de Ceuster LM, van Dillen JJ, Wever PC, Rozemeijer W, Louwerse ES. [Streptococcus suis meningitis in a meat factory employee.] Ned Tijdschr Geneeskd2012; 156: A5080. Dutch.
- Zalas-Wiecek P, Michalska A, Grabczewska E, Olczak A, Pawlowska M, Gospodarek E. Human meningitis caused by Streptococcus suis. J Med Microbiol2013; 62: 483–485.
- Taipa R, Lopes V, Magalhaes M. Streptococcus suis meningitis: first case report from Portugal. J Infect2008; 56: 482–483.
- Dragojlovic J, Milosevic B, Sasic N, Pelemis M, Sasic M. [Streptococcus suis infection–clinical manifestations.] Med Pregl2005; 58: 236–239. Serbian.
- Tarradas C, Luque I, de Andres D et al. Epidemiological relationship of human and swine Streptococcus suis isolates. J Vet Med B Infect Dis Vet Public Health2001; 48: 347–355.
- Vela AI, Aspiroz C, Fortuno B et al. Meningitis caused by an unusual genotype (ST3) of Streptococcus suis. Infection2013; 41: 701–703.
- Martinez Aviles P, Jusdado Ruiz-Capillas JJ, Gomez Rodrigo J, Solis Villa J. [Sacroiliitis caused by Streptococcus suis type II.] An Med Interna1994; 11: 309. Spanish.
- Juncal AR, Pardo F, Rodriguez I, Perez del Molino ML. [Meningitis by Streptococcus suis.] Enferm Infecc Microbiol Clin1997; 15: 120–121. Spanish.
- de la Hoz Adame ME, de la Rubia Martin F, Dominguez Fuentes B, Garcia Gil D. [Acute meningitis caused by Streptococcus suis: review of a case in a splenectomized patient.] An Med Interna2005; 22: 507. Spanish.
- Alonso-Socas Mdel M, Aleman-Valls R, Roldan-Delgado H, Gomez-Sirvent JL. [Endocarditis and spondylodiscitis caused by Streptococcus suis.] Enferm Infecc Microbiol Clin2006; 24: 354–355. Spanish.
- Luengo-Alvarez J, Martin-Ruiz C, Sanchez Munoz-Torrero JF, Iniguez-Ovando R. [Meningitis due to Streptococcus suis: a case report.] Enferm Infecc Microbiol Clin2006; 24: 352–354. Spanish.
- Hidalgo A, Ropero F, Palacios R, Garcia V, Santos J. Meningitis due to Streptococcus suis with no contact with pigs or porcine products. J Infect2007; 55: 478.
- Riquelme E, Escribano E, Blanch JJ, Crespo MD. [Acute Streptococcus suis meningitis in a woman working in a meat market.] Enferm Infecc Microbiol Clin2008; 26: 256–257. Spanish.
- Aspiroz C, Vela AI, Pascual MS, Aldea MJ. [Acute infective endocarditis due to Streptococcus suis serotype 2 in Spain.] Enferm Infecc Microbiol Clin2009; 27: 370–371. Spanish.
- Galbarro J, Franco-Alvarez de Luna F, Cano R, Angel Castano M. [Acute meningitis and spondylodiscitis due to Streptococcus suis in a patient who had no contact with pigs or porcine products.] Enferm Infecc Microbiol Clin2009; 27: 425–427. Spanish.
- Fernandez-Ferro J, Lopez-Gonzalez FJ, Pardo F, Pias-Peleteiro JM. [Acute Streptococcus suis meningitis in a pig breeder.] Enferm Infecc Microbiol Clin2011; 29: 396–397. Spanish.
- Christensen P, Kronvall G. [A case of Streptococcus suis meningitis—a new occupational disease in Sweden?] Lakartidningen1985; 82: 119. Swedish.
- Hickling P, Cormack FC. Meningitis caused by group R haemolytic streptococci. Br Med J1976; 2: 1299–1300.
- Agass MJ, Willoughby CP, Bron AJ, Mitchell CJ, Mayon-White RT. Meningitis and endophthalmitis caused by Streptococcus suis type II (group R). Br Med J1977; 2: 167–168.
- McLendon BF, Bron AJ, Mitchell CJ. Streptococcus suis type II (group R) as a cause of endophthalmitis. Br J Ophthalmol1978; 62: 729–731.
- Joynson DH. Infections of man and Group R Streptococci. Br J Clin Pract1980; 34: 147–149, 154.
- Shneerson JM, Chattopadhyay B, Murphy MF, Fawcett IW. Permanent perceptive deafness due to Streptococcus suis type II infection. J Laryngol Otol1980; 94: 425–427.
- Twort CH. Group R streptococcal meningitis (Streptococcus suis type II): a new industrial disease? Br Med J (Clin Res Ed)1981; 282: 523–524.
- Clements MR, Hamilton DV, Clifton-Hadley FA, O’Reilly JF. Streptococcus suis type II infection. A new industrial disease? Practitioner1982; 226: 323–325.
- McNeil NI, Gordon T. Meningitis caused by Streptococcus suis type II. Postgrad Med J1986; 62: 743–744.
- Hay PE, Cunniffe JG, Kramer G, France AJ, Gray JA, Watt B. Two cases of Streptococcus suis meningitis. Br J Ind Med1989; 46: 352–353.
- Meecham JS, Worth RC. Persistent diplopia following Streptococcus suis type 2 meningitis. J R Soc Med1992; 85: 579–580.
- Watkins EJ, Brooksby P, Schweiger MS, Enright SM. Septicaemia in a pig-farm worker. Lancet2001; 357: 38.
- Doube A, Calin A. Bacterial endocarditis presenting as acute monoarthritis. Ann Rheum Dis1988; 47: 598–599.
- Maher D. Streptococcus suis septicaemia presenting as severe acute gastro-enteritis. J Infect1991; 22: 303–304.
- Bronstein AM, Morland AB, Ruddock KH, Gresty MA. Recovery from bilateral vestibular failure: implications for visual and cervico-ocular function. Acta Otolaryngol Suppl1995; 520 (Pt 2): 405–407.
- Rao SS, Mariathas A, Teare L. Meningitis in a butcher. Emerg Med J2008; 25: 607–608.
- Kennedy KJ, Jadeer AA, Ong CW, Senanayake SN, Collignon PJ. Two cases of Streptococcus suis endocarditis in Australian piggery workers. Med J Aust2008; 189: 413.
- Tramontana AR, Graham M, Sinickas V, Bak N. An Australian case of Streptococcus suis toxic shock syndrome associated with occupational exposure to animal carcasses. Med J Aust2008; 188: 538–539.
- Rojas MT, Gottschalk M, Velazquez Ordonez V. [Virulence evaluation of serotypes of Streptococcus suis isolated in slaughterhouse workers in the valley of Toluca, State of Mexico, Mexico.] Vet Mex2001; 32: 201–205. Spanish.
- Quessy S, Dubreuil JD, Caya M, Higgins R. Discrimination of virulent and avirulent Streptococcus suis capsular type 2 isolates from different geographical origins. Infect Immun1995; 63: 1975–1979.
- Robertson ID, Blackmore DK. Occupational exposure to Streptococcus suis type 2. Epidemiol Infect1989; 103: 157–164.
- Elbers AR, Vecht U, Osterhaus AD et al. Low prevalence of antibodies against the zoonotic agents Brucella abortus, Leptospira spp., Streptococcus suis serotype II, hantavirus, and lymphocytic choriomeningitis virus among veterinarians and pig farmers in the southern part of The Netherlands. Vet Q1999; 21: 50–54.
- Smith TC, Capuano AW, Boese B, Myers KP, Gray GC. Exposure to Streptococcus suis among US swine workers. Emerg Infect Dis2008; 14: 1925–1927.
- Sala V, Colombo A, Gerola L. [Infection Risks of Streptococcus suis Type 2 Localizations in Slaughtered Swines.] Arch Vet It1989; 40: 180–184. Italian.
- Walsh B, Williams AE, Satsangi J. Streptococcus suis type 2: pathogenesis and clinical disease. Rev Med Microbiol1992; 3: 65–71.
- de Greeff A, Wisselink HJ, de Bree FM et al. Genetic diversity of Streptococcus suis isolates as determined by comparative genome hybridization. BMC Microbiol2011; 11: 161.
- Ye C, Bai X, Zhang J et al. Spread of Streptococcus suis sequence type 7, China. Emerg Infect Dis2008; 14: 787–791.
- Li W, Ye C, Jing H et al. Streptococcus suis outbreak investigation using multiple-locus variable tandem repeat number analysis. Microbiol Immunol2010; 54: 380–388.
- Rehm T, Baums CG, Strommenger B, Beyerbach M, Valentin-Weigand P, Goethe R. Amplified fragment length polymorphism of Streptococcus suis strains correlates with their profile of virulence-associated genes and clinical background. J Med Microbiol2007; 56: 102–109.
- Blume V, Luque I, Vela AI et al. Genetic and virulence-phenotype characterization of serotypes 2 and 9 of Streptococcus suis swine isolates. Int Microbiol2009; 12: 161–166.
- Tang Y, Zhao H, Wu W, Wu D, Li X, Fang W. Genetic and virulence characterization of Streptococcus suis type 2 isolates from swine in the provinces of Zhejiang and Henan, China. Folia Microbiol (Praha)2011; 56: 541–548.
- Zhu W, Wu C, Sun X et al. Characterization of Streptococcus suis serotype 2 isolates from China. Vet Microbiol2013; 166: 527–534.
- Ye C, Zhu X, Jing H et al. Streptococcus suis sequence type 7 outbreak, Sichuan, China. Emerg Infect Dis2006; 12: 1203–1208.
- Onishi H, Sugawara M, Okura M, Osaki M, Takamatsu D. Prevalence of Streptococcus suis genotypes in isolates from porcine endocarditis in East Japan. J Vet Med Sci2012; 74: 1681–1684.
- Luey CK, Chu YW, Cheung TK et al. Rapid pulsed-field gel electrophoresis protocol for subtyping of Streptococcus suis serotype 2. J Microbiol Methods2007; 68: 648–650.
- Chatellier S, Gottschalk M, Higgins R, Brousseau R, Harel J. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J Clin Microbiol1999; 37: 362–366.
- Chen C, Tang J, Dong W et al. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS One2007; 2: e315.
- Vecht U, Wisselink HJ, Jellema ML, Smith HE. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect Immun1991; 59: 3156–3162.
