Abstract
In 2014, a serious dengue outbreak in Guangzhou occurred, consisting of 37 354 laboratory confirmed cases of infection. In this study, the clinical picture of dengue fever due to dengue virus (DENV) type 1 in Guangzhou was described. Clinical and laboratory data collected by studying 726 sera of suspected clinical cases from hospitals and 328 sera of healthy persons from two residence communities were analyzed during the outbreak, and 484 patients were diagnosed with an acute dengue infection. Fever, headache, congestion of the throat, and myalgia were the most typical symptoms in DENV-infected patients. Thrombocytopenia, leukopenia, and an increase in liver enzymes were significantly more common in the infected patients than in the healthy controls. Fourteen cases of silent infection were discovered among the 328 healthy persons, suggesting a DENV inapparent infection rate of 4.27% among healthy individuals. The data obtained by analyzing 212 positive sera with three methods indicated different results with different detection methods. DENV RNA should be used for early diagnoses during days 1–6 after symptom onset, immunoglobulin M (IgM) can be easily recognized after four days have passed since symptom onset and DENV isolation has a peak positive rate during days 1–3 after the onset of symptoms. A phylogenetic analysis of viral NS1 gene sequences from this outbreak indicated that the predominant isolates could be categorized as DENV-1 genotype III and had the highest homology with the India genotypes from 2009 to 2011. However, this analysis also revealed a co-epidemic of the 2013 Zhongshan and 2003 Singapore genotypes, both belonging to DENV-1 genotype I, which suggested multiple geographic origins for the 2014 epidemic of dengue 1 strains in Guangzhou.
Introduction
Dengue fever (DF) is one of the most common mosquito-borne viral infections and is caused by four antigenically distinct serotypes of dengue virus (DENV).Citation1,Citation2 DENV is a positive-sense RNA virus with a genotype of approximately 11 kb that encodes three structural proteins (C, prM, and E) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5).Citation3 Aedes aegypti and Aedes albopictus are the two main vectors responsible for the transmission of DENV.Citation4 DENV infection in humans is often inapparent but can lead to a wide range of clinical manifestations ranging from mild fever to the potentially fatal dengue shock syndrome (DSS) or dengue hemorrhagic fever (DHF).Citation5 To date, DF is the most important and rapidly spreading vector-borne viral disease in the tropics. It is estimated that more than 390 million (95% credible interval 284–528 million) dengue infections occur per year, of which 96 million (67–136 million) are symptomatic (with any level of disease severity).Citation6 In the past 50 years, the expansion in geographic distribution of both the viruses and the mosquito vectors has led to the rapid escalation of a dengue pandemic across the world.Citation7 The World Health Organization (WHO) estimated that there has been a 30-fold increase in DF incidence and that 2.5 billion people, more than 40% of the world population, are at risk across the world. The regions of Southeast Asia, Africa, the Eastern Mediterranean, the Americas, and the Western Pacific are the most affected.Citation8
In China, dengue epidemics have been reported sequentially in Hainan, Guangdong, Guangxi, Fujian, Zhejiang, and Yunnan since the first outbreak of dengue occurred in Foshan, Guangdong in 1978.Citation9 Among all of these regions, Guangzhou, the provincial capital of Guangdong, is a representative city that has experienced annual DENV transmission and more than 50% of the DENV cases in mainland China. With four serotypes of DENV reported, Guangzhou contains DENV strains of rapidly increasing genetic diversity as imported cases emerge every year.Citation10 Therefore, scientists have shown concern that a major DENV outbreak may occur in Guangzhou in the near future. In July 2014, a severe outbreak of dengue occurred in Guangzhou, proving the merits of this concern. It developed with an amazing rate of growth in the number of cases, which rose to 1000 per week. The total number of cases finally exceeded 37 000, seven times the historical record, confirming the epidemic as the most serious dengue outbreak in history. Simultaneously, dengue cases were also confirmed in 18 other cities of Guangdong province in succession, although these cities experienced much lower infection rates than Guangzhou.
In this study, clinical and laboratory data obtained from 726 sera of suspected clinical cases and 328 sera of healthy persons from two DF-impacted residence communities were collected and analyzed. The results obtained using real-time reverse transcript polymerase chain reaction (RT-PCR), immunoglobulin M (IgM) enzymelinked immunosorbent assay (ELISA) and virus isolation detection during different phases of the disease were also analyzed. Finally, the phyletic, evolutionary relationships between strains were also constructed based on the viral NS1 gene sequences of 10 DENV isolates obtained in Guangzhou in 2014.
Materials and methods
Case presentations
Seven hundred and twenty-six blood samples were obtained from patients presenting with acute febrile illness with at least one of the following accompanying symptoms: headache, retro-orbital pain, myalgia, arthralgia, rash, and hemorrhage. All samples were transported while refrigerated and were collected separately between June and November 2014 from four public hospitals of Guangzhou, China: the 458th hospital of PLA, the 421st hospital of PLA, the Guangdong province armed police hospital, and the General Hospital of Guangzhou Military Command. Four hundred and eighty-four of the 726 suspected cases were confirmed as DENV infection by lab detection. Sixty-four of the 484 patients were inpatients, and the rest were ambulatory. Clinical symptoms were classified retrospectively. Most of the patients were classified as having DF. Seventeen patients had the severe manifestations of DHF or DSS, according to the criteria of the WHO.Citation8 Among the 484 confirmed cases of infection, we collected 31 second sera samples from patients living in two residence communities in the Yuexiu district of Guangzhou, China. In these two residence communities, 328 blood samples from healthy persons were collected separately in August and November and were later used for the epidemiological investigation.
Real-time RT-PCR (TaqMan) assay
Viral RNA for the real-time RT-PCR assays was extracted from 200 µL of serum specimens by the MiniBEST Viral DNA/RNA Extraction kit (TaKaRa, Dalian, Liaoning Province, China) according to the manufacturer’s instructions. RNA was eluted in 50 µL of RNase-free ultrapure water. One-step real-time RT-PCR assays were performed in the Roche LightCycler 2.0 system (Roche, Rotkreuz, Switzerland). Samples were assayed in a 20 µL reaction mixture containing 2 µL of extracted RNA, 0.4 µL of TaKaRa Ex Taq HS, 0.4 µL of PrimeScript RT enzyme Mix II, 10 µL of one-step RT-PCR buffer III (TaKaRa) and 200 nM of each specific primer and fluorogenic probe (primers: DV1-001R, 5′-GTG GAG AGG AAC CTT GTG AAA CC-3′; DV1-001F, 5′-CTG TGA CTT TCT TCA GCC CAG C-3′; DV1-001 Probe: 5′-6-FAM-TGA CCA GCC ACC TCT TCC ACA ACC GA-BHQ-1-3′). Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control (primers: GAPDH-F6, 5′-GGT GGA CCT GAC CTG CCG TCT A-3′; GAPDH-R6, 5′-AGT GTA GCC CAG GAT GCC CTT GAG-3′; GAPDH-probe, 5′-6-FAM-CCT CCG ACG CCT GCT TCA CCA CCT TCT-TAMRA-1-3′).Citation11
Anti-DENV IgM and IgG antibodies assay
Two hundred and twelve sera samples from patients presenting with dengue-like syndrome (temperature of ≥38.5°C, arthralgia, headache, and/or myalgia) were tested using the commercially available dengue IgM capture ELISA (Cat. NO EDEN01M) and dengue IgG capture ELISA (Cat. NO E-DEN02G) from Panbio (Brisbane, Australia). Samples were collected during the acute phase of the disease (days 1–4, with onset of fever taken as day 1, i.e., the first 24 h) and/or the convalescent phase of the disease (at least 7 days after fever onset). The results were classified as positive, negative, or equivocal according to the manufacturer’s instructions. Any initial equivocal result was retested to confirm the result.
Virus isolation
A total of 46 samples positive for DENV RNA were prepared for virus isolation by inoculation into monolayers of a C6/36 A. albopictus cell line.Citation12 Cell suspensions were collected when cells were visibly mature and a cytopathic effect was clearly observed after five days and were centrifuged to remove any cells and debris. DENV isolates were identified by real-time RT-PCR as described above.
Blood biochemistry and routine complete blood count
Cystatin C (CYS-C) was measured by nephelometry (Biaojia CYS-C reagent kit, Guangzhou Biaojia Technology Co Ltd, Guangzhou, China). Creatine kinase isoenzyme MB (CK-MB) and lactate dehydrogenase isozyme (LDHI) were measured by an immunosuppressive method (CK and LDHI isoenzymes assay kit, Guangzhou Biaojia Technology Co Ltd, China). Alanine transaminase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatinine (Cr), creatine kinase (CK), α-hydroxybutyrate dehydrogenase (α-HBDH), and angiotensin-converting enzyme (ACE) were measured by a kinetic method (ALT, AST, LDH, Cr, CK and α-HBDH reagent kit, Guangzhou Biaojia Technology Co Ltd). All blood biochemistries were performed on an Aeroset modular autoanalyzer (Abbott Diagnostics, Chicago, IL, USA). The routine complete blood count was measured with routine laboratory methods using an autoanalyzer (Mindray Medical, Shenzhen, China).
Viral NS1 gene sequencing
The viral NS1 genes (1236 nt each) from the sera of 10 patients were amplified. All primers were synthesized by Sangon (Sangon Biotech, Shanghai, China) according to the reference sequence data of the candidate dengue 1 vaccine strain 45AZ5 (GenBank: 9626685). Amplification was done using the SuperScript One-Step kit (Invitrogen, Carlsbad, California, USA). The one-step RT-PCR reaction was performed in a volume of 50 µL containing 4 µL RNA template, 21.5 µL ddH2O, 22.5 µL RT-PCR buffer (5X), 2 µL enzyme mix, and 200 nM of each specific primer (primers: DenNS1-F, 5′-AGC GGT GTT TCT TGG ACC AT-3′; DenNS1-R, 5′-TTA CCA TCT GGA TCT CAT TAC CTC T-3′). The amplification program was performed as follows: reverse transcription at 55°C for 30 min, inactivation of the RT enzyme at 94°C for 2 min, followed by 40 cycles of 94°C for 30 s, 55°C for 30 s, 68°C for 2 min, and a final step at 68°C for 5 min. Amplified products were detected by agarose gel electrophoresis and were subcloned into a pMD 19-T vector (TaKaRa) and sequenced.
Sequence and phylogenetic analyses
Multiple alignments of the viral NS1 genes of 10 Guangzhou isolates were generated with the ClustalW program. For a comprehensive phylogenetic analysis, 50 of the closest reference strains of DENV-1 were obtained from GenBank. Neighbor-joining trees were constructed using MEGA version 5.1 with the Kimura-2 parameter corrections for multiple substitutions. The reliability of nodes was assessed by bootstrap resampling with 2000 replications.
Results
Clinical picture of the outbreak
Over the research period, 726 samples from patients matching the case definition were investigated. Four hundred and eighty-four patients, with a mean age of 34.8 years (range: 5 months to 92 years), were confirmed to be infected with DENV during the outbreak. The ratio of sexes among these patients was 1.64:1 (62.2% males; 37.8% females), and adults aged 20–34 years had a comparatively higher incidence of infection (). The majority of the patients went to a hospital 2–6 days after the onset of symptoms (371/484). The peak of the epidemic occurred in September–October and corresponded to 127 infections per week. In November 2014, the number of suspected cases dramatically decreased and the positivity rate of real-time RT-PCR surveillance was limited to a subset (4/9) of the weekly confirmed cases. Detailed age and sex distributions of the DF cases are shown in .
Table 1 Distribution of DF cases by age and sex
Characteristics of DF patients
The major symptoms that patients presented with were fever (93.39%), myalgia and polyarthralgia (34.71%), headache and retro-orbital pain (45.45%), and skin rash (27%). Additionally, some patients suffered from other symptoms including chills, asthenia, vomiting, diarrhea, itching, and congestion of the throat (). In some severe cases, hematemesis, melena, and gingival hemorrhage were observed. Detailed symptoms and signs observed in the DF patients are described in .
Table 2 Symptoms and signs in DF patients (n = 484)
Characteristics of DHF and DSS patients
According to the case definitions of the WHO, among the 484 confirmed DENV-infected patients, 15 cases experienced DHF and 2 cases experienced DSS. Of the 15 DHF patients, 15 had thrombocytopenia, 15 had leukopenia, three had urethremorrhage, three had alimentary tract hemorrhage, one had colporrhagia, and one had gingival hemorrhage. During the onset of disease, some blood biochemistry markers in the serum samples of DHF patients were markedly elevated. CK, LDH, and α-HBDH were markedly elevated in two patients, but CK-MB was normal, signifying the presence of muscle damage. Levels of ALT in six patients and AST in eight patients were moderately increased, which was associated with liver damage. The increase in levels of liver enzymes (ALT or AST) was found in 8 of the 20 analyzed DF cases and 8 of the 15 analyzed DHF cases, and there were no significant differences in the ALT and AST levels observed in these two groups. However, in two DSS patients (who presented chiefly with neurological symptoms such as extreme weakness and confusion and were sent directly to the intensive care unit), a mean ALT level of 93 U/L and mean AST level of 189 U/L were found, compared to normal ALT levels of 0–50 U/L and normal AST levels of 0–40 U/L. Cr and CYS-C were detected in two serum samples, which signified the presence of renal damage. The two DSS patients both presented with alimentary tract hemorrhage and shock in addition to all of the symptoms of DF. CK, LDH, α-HBDH, ALT, AST, Cr, and CYS-C were markedly elevated in these patients, signifying multiple organ injury.
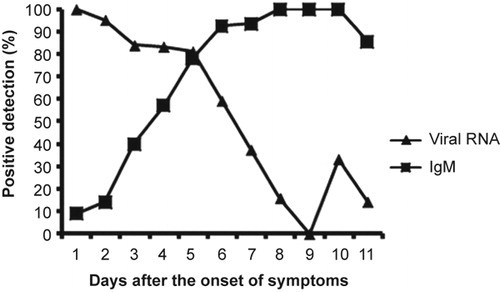
Kinetics of DENV RNA, IgM antibodies, and virus isolates in dengue type 1 infections
Of the 484 patient serum specimens, 212 acute- and convalescent-phase serum specimens were confirmed for viral RNA detection by real-time RT-PCR and for serological IgM diagnosis by capture ELISA as well. Disease day 1 was designated as the day of symptom onset. As shown in , dengue RNA was detectable as early as the first day after the onset of illness and was detected with a high positive rate of 100%. The overall positive predictive value of RNA detection was 89.0% (113 of 131) for samples taken during the first five days after symptom onset and 34.5% (28 of 81) for samples taken 6–11 days after the onset of symptoms. In contrast to RNA, anti-dengue IgM antibodies were detectable before the third day of illness and were detected with a low positive rate of 10.5% (4 of 32). The positive rate of IgM detection was 40% by the third day of illness and rapidly increased to 100% by day 8 of the illness. Combining the results of RNA and IgM detection allowed for a positive diagnosis in 100% of samples taken after the third day after symptom onset (). Thirty-four virus isolates were obtained from 46 DENV RNA-positive samples, representing a positive isolation rate of 73.91%. The overall sensitivity of the positive rate of virus isolation was 96% (24 of 25) for samples taken during the first 3 days after symptom onset and 50% (10 of 20) for samples taken 4–6 days after the onset of symptoms.
Results for DENV RNA and serological detection in samples from two impacted residence communities
Three hundred and twenty-eight blood samples from healthy persons from two residence communities in the Yuexiu district of Guangzhou, China (where the DF patients lived) were collected after the first cases appeared and were analyzed using RT-PCR, virus isolation, and/or IgM analysis via capture ELISA. Four silent infections without typical symptoms that were positive for DENV nucleic acids and 10 such infections that were positive for IgM antibodies were screened from among those 328 healthy persons. IgG seroconversions were detectable in 66.67% (six out of nine cases) of IgM antibody-positive healthy carriers (one case lacked information) within two months of exposure. Four IgM seroconversions but only two IgG seroconversions were detectable in the four DENV nucleic acid-positive cases. Infection rates among healthy persons may be underestimated in this study, because seroconversion was only tested for in the minority of the total number of samples. Samples from 31 cases that were previously lab confirmed to be DENV infection by virus isolation and/or RT-PCR and/or IgM analysis by capture ELISA were collected from those communities one month after they were cured. Among the 31 confirmed cases, IgM antibodies were detected in plasma samples from 24 cases, and IgG antibodies were detected in plasma samples from 14 cases. One case was IgM-equivocal but IgG-positive, one case was IgM-negative but IgG-positive, 10 cases were IgM-positive but IgG-negative, four cases were IgM-positive but IgG- equivocal, and eight cases were RT-PCR-positive previously but IgM- and IgG-negative.
Sequence analysis
To identify whether the 2014 outbreak strains were previously circulating in Guangzhou or were newly introduced, we initially analyzed the nucleotide sequences of the complete NS1 genes of 10 isolates. A more detailed comparison of the sequences of these 10 isolates revealed that two subgroups were present. There are 19 nucleotide changes in the NS1 nucleotide sequences between nine samples (GZ2014001-GZ2014005 and GZ2014007-GZ2014010), resulting in an identity of 98.20%–100%, and these samples showed a close relationship (99.51%–99.60%) with isolates obtained from India in 2009–2011 (GenBank: JQ917404, JQ692085, and KF289072). However, one isolate (GZ2014006) showed obvious differences; this isolate shared 92.31% identity in the NS1 gene (95 nucleotide changes) with the above nine isolates, 99.60% identity with the 2013 Zhongshan, China (a city of Guangdong province adjacent to Guangzhou) prototype (GenBank: KF179869.1) and 99.03% identity with the 2003 Singapore prototype (GenBank: FJ469907.1). Detailed information regarding the DENV isolates used in the sequence analysis is provided in .
Table 3 Dengue virus isolates used in sequence analysis
Phylogenetic tree of DENV-1 by E gene sequences
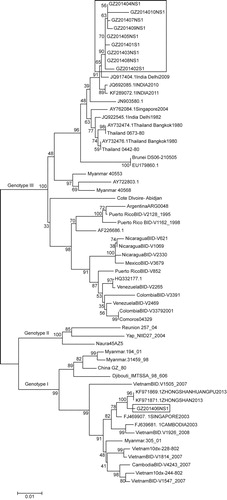
Subsets of 10 Guangzhou DENV-1 NS1 gene sequences were used in the initial phylogenetic analysis. Sequences were compared to 50 reference sequences of different DENV-1 genotypes available in GenBank. In the phylogenetic analysis using the NS1 gene sequences, two major clades of 2014 Guangzhou strains were clearly evident. The nine isolates of 2014 Guangzhou DENV-1 with identical sequences clustered together () within the 2009–2011 India group, representing the major viral population in the outbreak. However, the isolate GZ201406 clustered with the 2013 Zhongshan isolates, forming a subclade within the Singapore and Cambodia clade.
Figure 2 Phylogenetic relationships of DENV-1 isolates based on the complete NS1 nucleotide sequences (1236 nt) of 57 isolates, including seven Guangzhou isolates. The red boxes represent 2014 Guangzhou DENV-1 strains. A maximum-likelihood tree was constructed using MEGA version 6, based on the Tamura-Nei model. Bootstrap resampling values are indicated at major nodes. The scale bar indicates the number of base substitutions per site.
Discussion
During 2014, one of the most serious dengue outbreaks ever occurred in Guangdong province, China. The outbreak began in June 2014 in the Yuexiu district of Guangzhou, the provincial capital of Guangdong province, and later spread to the entire city and to 20 other cities of Guangdong province, resulting in 45 171 confirmed cases. Of these cases, 37 354 occurred in Guangzhou, accounting for 90% of all of the reported dengue cases in this outbreak (statistics from Guangdong province Health and Family Planning Commission). The peak month of the outbreak spanned from September to October (1189 cases per day at the peak), and the epidemic came to an end by December 2014. The epidemiological characteristics were consistent with our surveys. Typically, younger individuals and those aged over 60 years had an increased risk of acquiring a dengue infection in dengue-endemic areas.Citation13,Citation14 However, in this study, a comparatively higher incidence of infection was observed in adults aged 20–34 years, which suggested that Guangdong province still maintained a low prevalence of DENV and that immunity to DENV was still at low levels in adults, which was consistent with the reports by Guo et al.Citation10 It has also been reported that in southeast Asian countries, DHF is mainly a disease of childhood while in the tropical Americas, all age groups are susceptible.Citation15,Citation16 However, no DHF cases in children were observed in this study. All 22 DHF/DSS cases reported in this study were adults (between 18 and 30 years old).
According to ALT and AST data, liver injury was an important risk factor for DENV infections and the development of DSS, which agrees with the findings of other authors.Citation17,Citation18 An increase in levels of LDH was also found in 8 of 20 analyzed DF cases and in 9 of 15 analyzed DHF cases, whereas normal levels of LDH1 were observed in all analyzed cases, signifying the presence of muscle damage. The other prominent manifestations were thrombocytopenia (occurring in 77.78% of DF cases with a mean platelet count of 85.90 × 109/L, compared to a mean platelet count of 81.88 × 109/L in DHF cases) and leucopenia (occurring in 100% of DF cases and 94.74% of DHF cases), and fever (mean temperature of 39.2°C) and rash were noted in most of the DHF cases. However, all of the DSS cases in this study showed evidence of primary dengue infection by serology. This is in contrast to other reports that have described mortality and severe disease occurring predominantly in secondary infections.Citation19,Citation20 Serologic and pathogenic monitoring of healthy populations was also implemented in this study. The positive detection rate for IgM antibodies was 3.05% (10/328) and the positive rate of viremia was 1.22% (4/328) in healthy populations, which suggested many inapparent infections were not detected.
Although the first mention of the occurrence of dengue in mainland China is said to date to 1917, the first confirmed outbreak occurred in 1978 in Foshan of Guangdong province. Following that outbreak, dengue in China has dramatically expanded over the past few decades. By 1998, all four serotypes were prevalent. During this period, the epidemiology of dengue in China was very complex and ever changing. Serotypes of the virus changed year after year, and each year either the serotype or the genotype showed a change.Citation9 In this study, the molecular phylogeny based on the complete NS1 gene grouped the 2014 DENV-1 in Guangzhou into two genotypes, as previously reported.Citation21 Most viruses isolated (GZ2014001-GZ2014005 and GZ2014007-GZ2014010) in this epidemic diverged little from the DENV-1 genotype III associated with the 2009–2011 India outbreak, which suggested that the predominant 2014 cases appeared to be a continuation of the 2009 outbreak and were imported from neighboring countries. However, one DENV-1 isolate isolated in this study, called GZ2014006, diverged from the genotype 1 strain. There is only 92.31% sequence identity between the genotypes of the two DENV-1 strains in this epidemic. Thus, this analysis indicated that at least two genotypes of DENV-1 co-circulated in this outbreak, with genotype III as the predominant strain. In contrast to outbreaks caused by other dengue serotypes, the frequency of outbreaks of DENV-1 increased in the Chinese population after 1999. A series of isolates in Guangdong (1999, AY376738; 2004, EF032589; 2007, EU359008; 2008, FJ196848; 2011, JQ048541 and 2013, KF971871, KJ438296) also diverged from the multiple distinct lineages of genotype 1 and were strongly related to GZ2014001 (over 98% sequence identity). A thorough investigation into whether genotype 1 has become an endemic disease or an endogenous epidemic in Guangdong province in recent years is warranted.
Finally, the application of three distinct assays (virus isolation, IgM ELISA, and real-time RT-PCR) provided a better understanding of the viral mechanism in cases of DENV infection. As shown in , viremia was seen in patients 5–6 days after the onset of symptoms. The assessment of viremia performed best during the first 5 days after symptom onset; during this period, an 89.0% positivity rate was observed among the 131 serum samples, then the rate of positive results decreased dramatically (64% at day 6, 40% at day 7, 16% at day 8, 0% at day 9), whereas one positive result was observed in each of two older persons at days 10 and 11. IgM had a transient period for detection by ELISA during the first few days of illness (days 3–5), which is in agreement with previous reports.Citation22 The combination of RNA and IgM testing facilitates enhanced diagnosis rates in the acute- and early convalescent phases of infection.
This study helps illustrate the epidemiological situation of dengue infection that was experienced in 2014 in Guangzhou, China. Our findings describing the endemic nature will have important implications for the prevention and control of DF in Guangzhou. Well-designed studies of genetic determinants of virulence of viral isolates from other outbreak localities should be considered.
This work was financially supported by the Science and Technology Support Project of PLA (CGZ14J006, CWS11C267), the Guangdong Science and Technology Support Project (2013B021800163), and the Science and Technology Support Project of Guangzhou (2014J4100003).
- Brady OJ, Gething PW, Bhatt S et al.Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 2012;6: e1760.
- Gubler DJ.Emerging vector-borne flavivirus diseases: are vaccines the solution? Expert Rev Vaccines 2011;10: 563–565.
- Holmes EC, Twiddy SS.The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol 2003;3: 19–28.
- Sirisena PD, Noordeen F.Evolution of dengue in Sri Lanka – changes in the virus, vector, and climate. Int J Infect Dis 2014;19: 6–12.
- World Health Organization. Dengue and severe dengue. Fact Sheet No. 117.Geneva: WHO, 2013.Available at http://www.who.int/mediacentre/factsheets/fs117/en/ (accessed 20 December 2013).
- Bhatt S, Gething PW, Brady OJ et al.The global distribution and burden of dengue. Nature 2013;496: 504–507.
- Andraud M, Hens N, Marais C, Beutels P.Dynamic epidemiological models for dengue transmission: a systematic review of structural approaches. PLoS One 2012;7: e49085.
- World Health Organization.Handbook for clinical management of dengue. Geneva: WHO, 2012.Available at www.who.int/denguecontrol/9789241504713/en/ (accessed 14 December 2013).
- Wu W, Bai Z, Zhou H et al.Molecular epidemiology of dengue viruses in southern China from 1978 to 2006. Virol J 2011;8: 322.
- Guo RN, Lin JY, Li LH et al.The prevalence and endemic nature of dengue infections in Guangdong, South China: an epidemiological, serological, and etiological study from 2005–2011. PLoS One 2014;9: e85596.
- Bai Z, Liu L, Tu Z et al.Real-time PCR for detecting circulating dengue virus in the Guangdong Province of China in 2006. J Med Microbiol 2008;57: 1–6.
- Igarashi A.Isolation of a Singh’s Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol 1978;40: 531–544.
- Rodriguez-Barraquer I, Cordeiro MT, Braga C, de Souza WV, Marques ET, Cummings DA.From re-emergence to hyperendemicity: the natural history of the dengue epidemic in Brazil. PLoS Negl Trop Dis 2011;5:e935.
- Gupta E, Dar L, Kapoor G, Broor S.The changing epidemiology of dengue in Delhi, India. Virol J 2006;3: 92.
- Rigau-Pérez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV.Dengue and dengue haemorrhagic fever. Lancet 1998;352: 971–977.
- Ooi EE, Goh KT, Chee Wang DN.Effect of increasing age on the trend of dengue and dengue hemorrhagic fever in Singapore. Int J Infect Dis 2003;7: 231–232.
- Wahid SF, Sanusi S, Zawawi MM, Ali RA.A comparison of the pattern of liver involvement in dengue hemorrhagic fever with classic dengue fever. Southeast Asian J Trop Med Public Health 2000;31: 259–263.
- Mohan B, Patwari AK, Anand VK.Hepatic dysfunction in childhood dengue infection. J Trop Pediatr 2000;46: 40–43.
- Vaughn DW, Green S, Kalayanarooj S et al.Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 2000;181: 2–9.
- Halstead SB.Pathogenesis of dengue: challenges to molecular biology. Science 1988;239: 476–481.
- A-Nuegoonpipat A, Berlioz-Arthaud A, Chow V et al.Sustained transmission of dengue virus type 1 in the Pacific due to repeated introductions of different Asian strains. Virology 2004;329: 505–512.
- Hu D, Di B, Ding X et al.2011.Kinetics of non-structural protein 1, IgM and IgG antibodies in dengue type 1 primary infection. Virol J 2011;8: 47.