Abstract
Emerging Microbes & Infections (2016) 5, e108; doi:10.1038/emi.2016.107; published online 12 October 2016
Dear Editor,
Spotted fever group (SFG) rickettsiae are obligate intracellular Gram-negative bacteria that are most commonly transmitted by a range of ticks and fleas. Recently, attention has become focused on mosquitoes as vectors of SFG rickettsiae with Rickettsia felis being identified by PCR in Aedes spp., Anopheles spp. and Mansonia uniformis in Africa,Citation1, Citation2, Citation3 and Anopheles sinensis and Culex pipiens in China.Citation4 Further, it has been shown that Anopheles gambiae can be infected with R. felis and transmit the organism during feeding, indicating it might be a potential vector.Citation5 Finally, a new Rickettsia species has been found in An. gambiae and Anopheles melas in Africa,Citation3 the blood of a patient from Senegal,Citation3 and in Cx. pipiens in China.Citation4 To gather more data on Rickettsia species in mosquitoes in China, we studied convenience samples of mosquitoes from Yangzhou and report our findings below.
In June 2015, convenience samples of mosquitoes were captured with hand nets in the environs of the halls of residence of Yangzhou University. The species and gender of the trapped mosquitoes were determined using standard morphological criteria (www.wrbu.org/morph_MQ.html) before the Cx. pipiens males and females and the Aedes albopictus were pooled separately (15 per pool) in 600 μL of RNA/DNA Stabilization Reagent for Blood/Bone Marrow (Roche Molecular Biochemicals, Indianapolis, IN, USA) and stored at −80 °C. The DNA was extracted from the pooled mosquitoes as described beforeCitation4 and tested for Rickettsia with a gltA-based FRET-PCR and a nested-PCR as described before.Citation4 To further characterize the Rickettsia found in the positive mosquito pools, we used multilocus sequence typing (MLST) with primers against the gltA which we developed (forward primer: 5'-ATG AGC AGA ATG CTT CTA CTT CAA CA-3'; reverse primer: 5'-ATT TTC TCT CAA TAA AAT ATT CAT CTT TAA G-3'), and those previously described for ompA, ompB and sca4.Citation3, Citation6, Citation7 A FRET-PCR targeting the mammalian hydroxymethylbilane synthase (HMBS) geneCitation8 and a regular PCR we designed to amplify the avian HMBS gene (forward primer: 5'-TTA GCA GTG GAA GTT CGT GCC AA-3'; reverse primer: 5'-AGG GAC ACT ACA GCC ACC CTC CT-3') were used to determine whether mosquitoes had ingested a blood meal.
In total, we captured 450 male and 345 female Cx. pipiens and found 30% (9/30) of the male pools and 9% (2/23) of the female pools positive by PCR for Rickettsia species. None of the male pools were positive by PCR for the HMBS, which was expected as males only feed from plants. The two female pools positive for Rickettsia species were negative for the HMBS suggesting that infected females were unfed. Ten of the remaining 21 female pools were positive for the mammalian and/or avian HMBS genes showing blood meals had been taken from mammals (n=2), birds (8) and both (3).
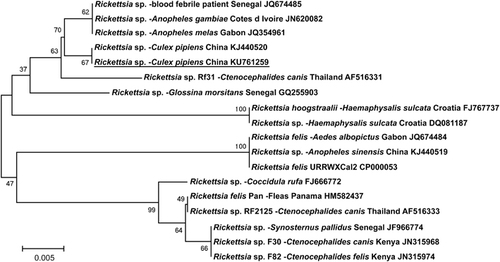
The MLST we performed showed the Rickettsia species in all 11 of our mosquito pools were identical and most closely related to the new Rickettsia species (JN620082/JQ354961) in An. gambiae and An. melas from Cote d’Ivoire, Africa,Citation3 and a Rickettsia sp. (JQ674485) in blood from a patient in SenegalCitation3 (). It was more distantly related to R. felis from cat fleas (Ctenocephalides felis; HM582437, CP000053, NC_007109, DQ408668), mosquitoes (An. melas; JQ674484),Citation1 people (KP318094) and book lice (Liposcelis bostrychophila; GQ329878).
Figure 1 Phylogenetic tree using a bootstrap analysis for the Rickettsia species in Culex pipiens from China. The nearest GenBank sequences (shown at the end of the sequence name) were aligned using the multi-sequence alignment program. The evolutionary tree was constructed using parsimony and maximum-likelihood methods. The Rickettsia sp. found in this study (underlined) was identical to the sequence we studied before, and was closest to the new Rickettsia species in An. gambiae and An. melas from Africa.
None of the male (10) or female (14) pools of Ae. albopictus we collected were positive for Rickettsia species with some of the female pools (29%; 4/14) having evidence of a mammalian and/or avian blood meal.
Our study adds to the growing evidence that mosquitoes might have a role in the epidemiology of infections with SFG rickettsiae. Since the first reports that rickettsiae could be propagated in mosquito cell lines,Citation9, Citation10 there has been growing evidence that mosquitoes can be infected with the organisms.Citation1, Citation2, Citation3, Citation4, Citation5 Our study provides further information on Rickettsia species in mosquitoes, in particular the common house mosquito Cx. pipiens that occurs widely in temperate areas of the world and feeds most commonly on a wide variety of bird species but also occasionally on people and a wide range of mammals.Citation11 We found relatively large numbers of pools of Cx. pipiens contained Rickettsia (11/53; 21%) indicating infections are common in this species. This is supported by unpublished data from our laboratory on individual Cx. pipiens which shows around 8% of females and 1% of males are infected. MLST indicated the Rickettsia in our pooled samples was the new Rickettsia species reported in An. gambiae and An. melas in Africa, or a very closely related species. As noted previously, the definitive characterization of this species will depend on isolates becoming available, which will enable more thorough analysis.Citation3
In a previous study,Citation4 we found DNA of R. felis in 5% of the Cx. pipiens we studied but all were negative in the current study. We were not able to determine a reason for this difference but it is known that numbers of mosquitoes infected with RickettsiaCitation2 and dengue virusCitation12 can vary with season and this might have been the case with our samples which were collected in spring (April to June) in the current study but in autumn (October and November) in the previously published study.
It is of note that we found the new Rickettsia species or a closely related organism in relatively large numbers of the pools of male Cx. pipiens. Male mosquitoes do not take blood meals, as confirmed by our negative mammalian and avian HMBS gene PCRs, and hence the organisms were not part of a blood meal ingested from a rickettsemic host.
Such vertical transmission of SFG rickettsiae is not unusual and has been demonstrated in ticks, R. africae in Amblyomma hebraeum for example,Citation13 and in fleas, R. felis in C. felis for example.Citation14 Studies on transmission in mosquitoes have been equivocal with vertical transmission not observed in An. gambiae experimentally infected with R. felis despite organisms being present in the ovaries.Citation5 On the other hand, R. felis was found in a male An. arabiensis, which suggested vertical transmission.Citation2
Our study shows a new Rickettsia species first described in An. gambiae and An. melas in Africa, or a closely related species, appears to also occur in China in Cx. pipiens. Detection of the Rickettsia in pools of male Cx. pipiens and unfed female Cx. pipiens suggests there might be transovarial transmission. Further studies are needed to characterize the organism, its transmission and its potential role as a pathogen, particularly as it might infect people.Citation3
This project was supported by grants from the National Natural Science Foundation of China (NO 31472225) and the National Key Research Project of China (no. 2016YFD0500804), and the priority Academic Program Development of Jiangsu Higher Education Institutions, Yangzhou, Jiangsu, China.
- SocolovschiC,PagésF,RaoultD.Rickettsia felis in Aedes albopictus mosquitoes, Libreville, Gabon.Emerg Infect Dis2012; 18:1687–1689.
- MediannikovO,SocolovschiC,EdouardSet al.Common epidemiology of Rickettsia felis infection and malaria, Africa.Emerg Infect Dis2013; 19:1775–1783.
- SocolovschiC,PagesF,NdiathMO,RatmanovP,RaoultD.Rickettsia species in African Anopheles mosquitoes.PLoS One2012; 7:e48254.
- ZhangJ,LuG,KellyPet al.First report of Rickettsia felis in China.BMC Infect Dis2014; 14:682.
- DiemeC,BechahY,SocolovschiCet al.Transmission potential of Rickettsia felis infection by Anopheles gambiae mosquitoes.Proc Natl Acad Sci USA2015; 112:8088–8093.
- RouxV,RaoultD.Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membraneprotein rOmpB (ompB.Int J Syst Evol Microbiol2000; 50(Pt 4):1449–1455.
- SekeyovaZ,RouxV,RaoultD.Phylogeny of Rickettsia spp. inferred by comparing sequences of 'gene D', which encodes an intracytoplasmicprotein.Int J Syst Evol Microbiol2001; 51(Pt 4):1353–1360.
- WeiL,KellyP,ZhangJet al.Use of a universal hydroxymethylbilane synthase (HMBS)-based PCR as an endogenous internal control and to enable typing of mammalian DNAs.Appl Microbiol Biotechnol2014; 98:5579–5587.
- HortaMC,LabrunaMB,DurigonEL,SchumakerTT.Isolation of Rickettsia felis in the mosquito cell line C6/36.Appl Environ Microbiol2006; 72:1705–1707.
- SakamotoJM,AzadAF.Propagation of arthropod-borne Rickettsia spp. in two mosquito cell lines.Appl Environ Microbiol2007; 73:6637–6643.
- HamerGL,KitronUD,GoldbergTLet al.Host selection by Culex pipiens mosquitoes and West Nile virus amplification.Am J Trop Med Hyg2009; 80:268–278.
- AngelB,JoshiV.Distribution and seasonality of vertically transmitted dengue viruses in Aedes mosquitoes in arid and semi-arid areas of Rajasthan, India.J Vector Borne Dis2008; 45:56–59.
- KellyPJ,MasonPR.Transmission of a spotted fever group rickettsia by Amblyomma hebraeum (Acari: Ixodidae).J Med Entomol1991; 28:598–600.
- WedincampJJr,FoilLD.Vertical transmission of Rickettsia felis in the cat flea (Ctenocephalides felis Bouché).J Vector Ecol2002; 27:96–101.
