Abstract
Moxifloxacin (MOX) and gatifloxacin (GAT) have exhibited promising mycobactericidal activity, and a number of clinical trials have been conducted in recent decades to compare the treatment efficacy of MOX-containing and/or GAT-containing regimens with the standard regimen. The aim of this meta-analysis for clinical trials of MOX- or GAT-containing regimens was to evaluate their treatment efficacy and safety in initial therapy for drug-sensitive tuberculosis (TB). Databases were searched for randomized controlled trials, and nine studies with 6980 patients were included. We found that fluoroquinolone substitution for isoniazid or ethambutol in short-course regimens might result in more frequent unfavorable treatment outcomes compared with the standard regimen—in particular, an increased incidence of relapse. In a per-protocol analysis, MOX-containing regimens had slightly higher rates of sputum culture conversion at two months than the standard regimen (RR 1.08, 95% CI 1.04–1.11, P <0.001); there was no significant difference in the rate of sputum conversion between the GAT-containing regimens and the standard regimen (RR 1.13, 95% CI 0.96–1.33, P = 0.13). There were no significant differences in the incidence of death from any cause, including TB, nor were there serious adverse events between the MOX- or GAT-containing regimens and the standard regimen. In conclusion, MOX or GAT might not have the ability to shorten treatment duration in the initial therapy for tuberculosis despite the non-inferiority or even slightly better efficacy in the early phase of treatment compared with the standard regimen. Furthermore, it is safe to include MOX or GAT in initial TB treatment.
INTRODUCTION
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis, and it remains a major global health problem. The World Health Organization estimated approximately 9.0 million new TB cases and 1.5 million TB-related deaths in 2013.Citation1 The current recommended therapy for new cases of drug-susceptible TB is a standard 6-month regimen with four first-line drugs: isoniazid, rifampicin, ethambutol and pyrazinamide. The treatment success rate can reach up to 86% among newly diagnosed TB cases.Citation1 However, the treatment duration is too long to achieve excellent compliance, which contributes to relapse and drug resistance. Therefore, new drugs or new drug combinations are required to simplify the regimen or to shorten the course of treatment with a non-inferior therapeutic effect.Citation2,Citation3,Citation4
Both the anti-mycobacterial activity in vitroCitation5,Citation6,Citation7,Citation8,Citation9 and the promising early sterilizing effect in animalsCitation7,Citation10,Citation11,Citation12 led to the suggestion that fluoroquinolones (FQs) might reduce the duration of treatment in new TB cases. The fourth-generation FQs gatifloxacin (GAT) and moxifloxacin (MOX) have shown promising mycobactericidal activity,Citation13,Citation14,Citation15,Citation16 and a number of clinical trials have been conducted in recent decades to compare the treatment efficacy of MOX-containing and/or GAT-containing regimens with the standard regimen. In these regimens, MOX or GAT was used as the substitution for or as an addition to the established standard regimen. Previous reviews have indicated that insufficient evidence was available to assess the efficacy and safety of MOX or GAT in the first-line regimen for primary pulmonary TB and that much larger trials were required.Citation17,Citation18 A recent meta-analysis concerning the efficacy and safety of MOX plus first-line therapy failed to include relevant newly published randomized clinical trials (RCTs) and did not evaluate the relapse rates of these regimens.Citation19 Given the inferiority of four-month FQ-containing regimens compared with the six-month standard regimen for drug-susceptible TB in recent large-scale trials,Citation20,Citation21,Citation22 we speculate whether these FQ-containing regimens have sufficient anti-TB efficacy in vivo. Because the evidence obtained via RCTs seemed contradictory and insufficient to address this issue, we summarized the clinical trials of MOX- or GAT-containing regimens and conducted a meta-analysis to evaluate their treatment efficacy and safety in drug-susceptible TB patients.
MATERIALS AND METHODS
Search strategy and selection criteria
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)Citation23 compliant literature search strategy was performed. The electronic databases MEDLINE (1966 to November 2014) and EMBASE (1974 to November 2014) were searched by two reviewers (R. and L.). We attempted to identify all relevant trials regardless of language. All of the titles and abstracts generated by this search strategy were independently reviewed. After the initial screening process, the full texts of the eligible articles were reviewed against the predefined inclusion criteria. In addition, we scrutinized the reference lists of each eligible paper for any omitted studies.
Studies were eligible for inclusion if they were RCTs that evaluated the MOX- or GAT-containing regimen for drug-sensitive pulmonary tuberculosis. Studies were excluded if they were not RCTs or if they lacked desired outcomes. Ethical approval was waived for this meta-analysis because the data we extracted were open-access from individual studies that had obtained ethics approval.
Data extraction and outcome management
Two researchers (R. and L.) independently extracted data from studies using a pre-defined data extraction form and resolved any discrepancies in the extracted data through discussion. The basic characteristics of the studies were recorded, including author; year of publication; country; number of participants in each treatment group; mean age and gender of participants; HIV prevalence; detailed treatment regimens of each arm; and duration of follow-up.
The rate of unfavorable outcomes was set as the primary outcome. The unfavorable outcomes were treatment failure, relapse, and death or withdrawal from the trial at the end of follow-up. The rate of sputum culture conversion at the end of the intensive phase (2-month) was set as the secondary efficacy outcome. Considering the possible impact of HIV status, we had planned to assess the primary and secondary outcomes in subgroups according to HIV status, but approximately half of the studies that included HIV-infected patients did not report the relevant data, and for those that reported data according to HIV status, the outcomes of HIV-positive patients were similar to those of HIV-negative patients. We therefore did not perform a subgroup analysis. The safety outcomes were the rates of death from any cause, TB-related death and serious adverse events. We extracted the number of treatment failures, relapses, deaths, sputum culture conversions and serious adverse events, as well as the number of participants in each treatment group. We presented dichotomous data and combined them using risk ratios (RR) with 95% confidence intervals (CIs).
Quality assessment and statistical analysis
The risk of bias for each study was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias.Citation24 We determined whether the selected studies were appropriate for inclusion in the meta-analysis by considering six domains: sequence generation, allocation concealment, blinding (of patients, personnel and outcome assessors), incomplete outcome data, selective outcome reporting and other sources of bias (Supplementary Table). Data analysis was performed using Review Manager 5.2 (The Cochrane Collaboration, 2008; The Nordic Cochrane Centre, Copenhagen, Denmark). We assessed heterogeneity among the studies by inspecting the forest plots and by applying the χ2 test and inconsistency (I2) statistic.Citation25 The fixed-effects model was used to calculate the RR for I2 <50%, and the random-effects model was used for I2 ≥ 50%. P values <0.05 suggested statistical significance.
RESULTS
Study selection and characteristics
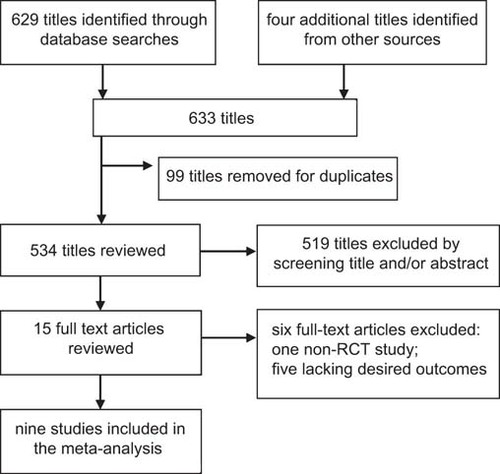
A total of 629 articles were identified from the databases, and four articles were identified from other sources. After duplicates were removed and titles and abstracts were reviewed, the full text of 15 articles was reviewed. Ultimately, nine studies met our inclusion criteria, and their data were extractedCitation20,Citation21,Citation22,Citation26,Citation27,Citation28,Citation29,Citation30,Citation31 ().
The basic characteristics of eligible RCTs are summarized in . The study locations were diverse, encompassing multiple countries. Trials were conducted mainly in Africa (Benin, Guinea, Kenya, Senegal, South Africa, Zambia, Zimbabwe, Botswana and Uganda), Asia (India, China, Malaysia and Thailand), North America (United States and Mexico), South America (Brazil) and Europe (Spain). These RCTs involved 6980 participants with a range of 170 to 1931 participants per trial. Male patients comprised 62%–74% of population, and the mean age was approximately 30 years old. Seven out of nine trials included both HIV-positive and HIV-negative patients, with the total HIV prevalence ranging from 3% to 58.5%.
Table 1 Basic characteristics of the studies included in this meta-analysis
Among these trials, the experimental treatment regimens could be categorized into three types: MOX/GAT as a substitute for ethambutol, MOX/GAT as a substitute for isoniazid and MOX/GAT as an addition to the standard regimen. Eight trials contained MOX in the experimental treatment regimens: five trials substituted MOX for ethambutol,Citation20,Citation26,Citation27,Citation28,Citation30 three trials substituted MOX for isoniazidCitation20,Citation21,Citation29 and one trial added MOX to the standard treatment regimen.Citation31 In Gillespie’s study, two MOX-containing regimens were tested in comparison with a controlled regimen.Citation20 Three trials contained GAT in experimental treatment regimens, and all of these trials substituted GAT for ethambutol.Citation22,Citation27,Citation30 All of the controlled regimens included standard doses of isoniazid, rifampicin, pyrazinamide and ethambutol. Notably, five of the nine studies used shortened regimens of four months in the experimental groups compared with the standard regimen of six months. The mean duration of follow-up ranged from two months of treatment to 24 months after the end of treatment.
Study quality
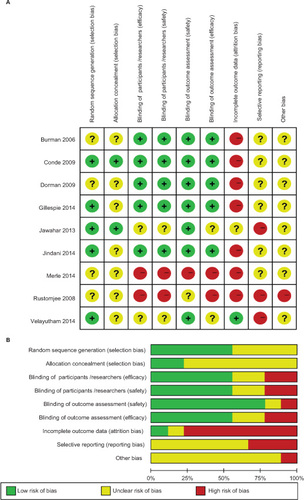
According to the Cochrane methodology, the risk of bias of the included studies was assessed as summarized in . Five trials were judged to be at low risk considering the detailed information regarding the generation of randomized sequence, whereas the remaining trials were judged to be unclear. Most studies had an unclear risk of allocation concealment in that no evidence could be found in their methodology. Five trials used blinding and a placebo and were judged to pose a low risk of bias. Seven trials were at a high risk of bias due to incomplete outcome data stemming from moderate dropout with the potential to alter results. Due to insufficient information regarding reporting and other sources of bias, most trials were judged to be of unclear risk.
Treatment outcome
Unfavorable outcomes
We aimed to use the rate of unfavorable outcomes at the end of follow-up as the primary efficacy outcome. After extracting data from the included studies, we found that four studies provided data regarding the desired outcome. However, due to differences in the duration of treatment and follow-up among the studies, we could not calculate a pooled estimate. All of the relevant information is summarized in .
Table 2 Summary of the rates of unfavorable outcomes, including treatment failure and relapse, between MOX-/GAT-containing regimens and the standard regimen in an intention-to-treat analysis or a modified intention-to-treat analysis
Three studies examined the efficacy of short-course MOX-containing regimens, and all of the unfavorable outcome rates for these 4-month regimens were higher than those of the standard regimen.Citation20,Citation21,Citation30 Similarly, higher rates of unfavorable outcomes were obtained for short-course GAT-containing regimens compared to the standard regimen.Citation22,Citation30 Considering that treatment failure rates and relapse rates were important components of assessing treatment efficacy, we also summarize detailed data in . For treatment failures at the end of treatment, a pooled estimate was performed, and we found that there was no difference between the standard regimen and short-course regimens (RR = 0.73, 95% CI: 0.34–1.56, P value 0.42) (Supplementary Figure 1). In contrast, the shortened regimens led to an increased incidence of relapse during follow-up, although the data were not pooled by variation in follow-up duration among these studies. The relapse rate following the 4-month MOX-containing regimens were approximately twice as high as that of the standard regimen in Gillespie’s study and Jindani’s study.Citation20,Citation21 Similarly, the short-course GAT-containing regimen resulted in a higher relapse rate compared to the standard regimen (RR = 2.01, 95% CI 1.44–2.79).Citation22
We aimed to conduct subgroup analysis according to HIV status to measure the possible impact of HIV co-infection. Among the seven studies that enrolled patients who were co-infected with HIV, only four reported detailed data. Two MOX-containing studiesCitation20,Citation21 and one GAT-containing studyCitation22 compared the unfavorable outcome rate between HIV-positive patients and HIV-negative patients, and another MOX-containing studyCitation26 set the two-month sputum conversion rate as the end point. We therefore did not conduct this subgroup analysis. However, all seven of these studies reported no difference in effect based on HIV status.
Sputum conversion at two months
The rate of sputum conversion was reported in each study included in our analysis and was used as the primary efficacy point in several studies. In this meta-analysis, the pooled rates of sputum culture conversion at two months of treatment were calculated as a secondary outcome. Because a considerable number of randomized patients failed to meet the inclusion criteria, were lost to follow-up, or withdrew, a per-protocol analysis was conducted.
In the per-protocol analysis, we found that MOX-containing regimens resulted in higher sputum culture conversion at two months [2235 (86.6%) of 2580 patients] than did the standard regimen [1011 (84.3%) of 1306 patients] with statistical significance (RR = 1.08, 95% CI: 1.04–1.11, P value <0.00001) (). A similar effect was observed via subgroup analysis when MOX was substituted for ethambutol or isoniazid in the experimental treatment regimens. In addition, most of the studies included did not show a significant difference. In Velayutham’s study, MOX was added to the standard regimen, and the five-drug daily regimen resulted in significantly higher sputum culture conversion after the first two months compared with the standard four-drug regimen (RR = 1.18, 95% CI 1.09–1.28,P value <0.001).Citation31 We detected moderate heterogeneity, with I2 = 41%. In addition, solid culture medium seemed to produce more negative culture results than liquid culture. In the per-protocol analysis of data from solid culture results, we found that MOX-containing regimens resulted in a much higher sputum culture conversion rate at two months [1934 (90.0%) of 2148 patients] than did the standard regimen [800 (78.1%) of 1024 patients] with statistical significance (RR = 1.16, 95% CI: 1.05–1.28, P value <0.00001) (Supplementary Figure 2). However, the data for liquid culture were heretofore insufficient.
Figure 3 Forest plots comparing the rates of sputum conversion at two months for (A) moxifloxacin-containing regimens versus the standard regimen, and (B) gatifloxacin-containing regimens versus the standard regimen. MOX, moxifloxacin; GAT, gatifloxacin
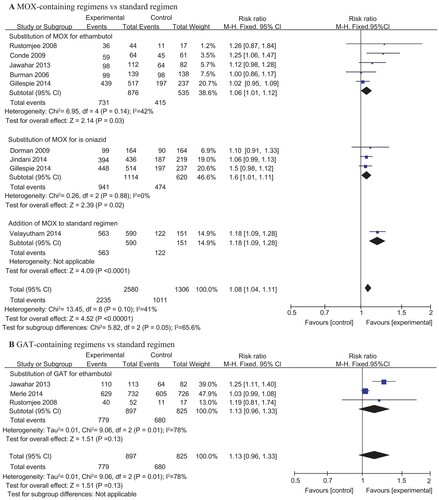
For GAT-containing regimens, the pooled rate of sputum conversion at two months of the experimental groups [779 (86.8%) of 897 patients] was slightly higher than that in the control groups [680 (82.4%) of 825 patients], but no significant difference was found (RR: 1.13, 95% CI: 0.96–1.33, P value = 0.13) (). However, heterogeneity was found between these studies, with I2 = 78%.
Safety outcomes
We also performed an analysis of safety outcomes and found no difference in the rates of death from any cause, TB-related death, or serious adverse events between MOX-containing regimens and the standard regimen ( and ). No heterogeneity was found, and the I2 value was very low. As with MOX, there was no significant difference in the number of serious adverse events between GAT-containing regimens and the standard regimen (). Two trials reported data on the number of deaths from any cause as well as TB-related deaths and found no significant difference between the experimental and control groups.Citation22,Citation27
Figure 4 Forest plots comparing the rates of death from any cause and TB-related deaths between MOX-containing regimens and the standard regimen. MOX, moxifloxacin
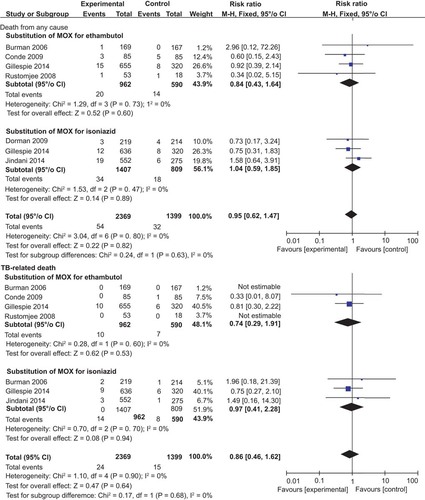
Figure 5 A forest plot comparing the rates of serious adverse events between MOX-containing regimens and the standard regimen. MOX, moxifloxacin
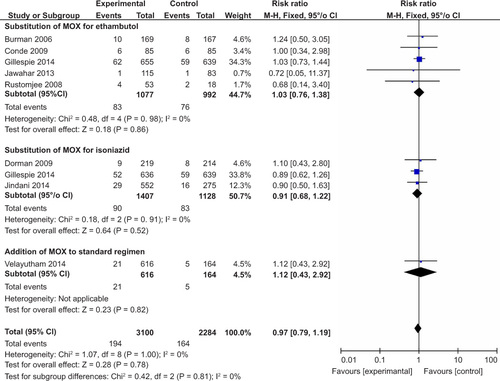
Table 3 Summary of the safety outcomes of the GAT-containing regimens and the standard regimen in the included studies
DISCUSSION
In the present study, we summarized the efficacy and safety of MOX and GAT as a part of first-line regimens for the treatment of drug-sensitive TB cases. Nine RCTs were eligible for our meta-analysis, and a total of 6980 participants were included. Our results indicate that FQ substitution for isoniazid or ethambutol in short-course regimens resulted in more frequent unfavorable treatment outcomes compared with the standard regimen, especially in terms of relapse. The MOX-containing regimens had a slightly higher sputum culture conversion rate at two months than did the standard regimen, but the GAT-containing regimens failed to achieve a similar result. There were no significant differences in the incidence of death from any cause, in TB-related death, or in serious adverse events between the MOX- or GAT-containing regimens and the standard regimen.
MOX, as is the case with isoniazid, has greater early bactericidal activity than other standard drugs. Previous studies have indicated that MOX greatly accelerated the speed of culture conversion and produced a stable cure when combined with rifampin and pyrazinamide in a mouse model.Citation11,Citation12 It also exhibited early bactericidal activity that was comparable with that of isoniazid in TB patients.Citation15 In this meta-analysis, we found a slight increase in the sputum culture conversion rate at two months when either ethambutol or isoniazid was replaced by MOX. The equivalence of the sputum conversion results may suggest an important role of MOX in the treatment of TB, particularly in patients intolerant to isoniazid or carrying isoniazid-resistant strains. However, the highly significant differences in sputum conversion (P <0.00001) corresponded to only slight differences in the conversion rates (86.6% vs. 84.3%). Of the phase two trials included in our meta-analysis, most investigators used 2-month sputum culture conversions as their primary efficacy endpoint, which is a surrogate marker for the final treatment outcome. Two-month culture status has been demonstrated in earlier studies to be a good marker for the efficacy of TB treatment regimen, but the observed correlation among populations is not strong enough to reliably predict treatment outcomes such as relapse. Based on the detailed data provided in , it seems that the 6-month MOX-containing regimen resulted in similar relapse rates compared to the standard regimen used in Jindani’s study,Citation21 whereas a higher relapse rate was reported when the regimens were shortened to 4 months in the three phase three clinical trials.Citation20–Citation22 The higher relapse rate indicated that MOX or GAT substitution might not permit a reduction in treatment duration. This result could be influenced by the shorter therapeutic period. In future studies, more robust surrogate markers of treatment efficacy are needed to select suitable regimens for shortening tuberculosis treatment that can be further assessed in phase three studies. Interestingly, Wallis and his colleagues developed a meta-regression model in 2013 to predict relapse risk using treatment duration and the 2-month sputum culture positive rate as predictors, and the analysis using this model of published2-month data for MOX-containing regimens indicated that it would result in relapse rates similar to those of standard therapy only if administered for over five months.Citation32,Citation33 They further assessed and refined the model in 2015 using more recent RCTs. This finding may provide information regarding treatment duration for future TB trials. Regretfully, a number of RCTs comparing non-shortened FQ-containing regimens with the standard regimen lacked data describing relapses and deaths due to an insufficient duration of follow-up.Citation26,Citation27,Citation28,Citation29 Thus, we could not determine whether non-shortened FQ-containing regimens resulted in better outcomes than the standard regimen. One study indicated that supplementing the controlled regimen with MOX could significantly hasten culture conversion.Citation31 However, this study was an interim analysis, and research regarding the rate of treatment success and relapse is in progress. Whether adding MOX or GAT to the standard regimen successfully shortens the initial treatment regimen remains unknown, and more studies are needed. Furthermore, the contradictory outcomes of animal studies and human trials indicate that current available mouse models do not fully recapitulate human TB infection; caution should be exercised when interpreting these studies.
In the safety analysis, there were no significant differences in the incidence of death from any causes, TB-related deaths between FQ-containing regimens and the standard regimen. Adverse events were recorded in each study, especially for certain symptoms: GAT had been reported to cause incidents of hypoglycemia and hyperglycemia,Citation34 and MOX was shown to prolong the QTc interval.Citation35 Our meta-analysis revealed that there were no significant differences between the groups regarding the percentage of serious adverse events that were considered relevant to the studied medications. However, studies of the use of GAT to treat TB were insufficient compared to those for MOX. shows the rates of death (from TB or from unrelated causes) and of serious side effects from three studies of GAT. However, one of these studies did not provide information regarding deaths, and another had a follow-up of only two months, rendering them insufficient to evaluate long-term safety. Thus, a precise safety assessment of GAT-containing regimens requires more study.
Furthermore, the use of FQs for initial tuberculosis therapy raises concerns regarding whether the application of FQs in drug-sensitive TB will increase the rate of FQ-resistant M. tuberculosis isolates, thereby hampering the treatment of multi-drug resistant TB (MDR-TB) in more difficult situations. FQs currently play a very important role in the treatment of MDR-TBCitation18,Citation36 and are strongly recommended by the WHO guidelines for the programmatic management of drug-resistant tuberculosis.Citation1,Citation37 They are significantly associated with cure—an effect that is more pronounced for later-generation FQs, such as levofloxacin, MOX and GAT.Citation37 However, several studies have reported a high prevalence of FQ-resistant TB in China,Citation38 IndiaCitation39 and the Philippines.Citation40 In Migliori’s meta-analysis, TB patients were found to have a three-fold higher risk of acquiring FQ-resistant TB when prescribed FQs before TB diagnosis compared to non-FQ-exposed patients (OR 2.81, 95% CI 1.47–5.39).Citation41 In another meta-analysis, previous exposure to FQs was determined to be a risk factor for FQ-resistant TB; it was found that 20.8% of patients exposed to FQs for more than 10 days, 60 days prior to a TB diagnosis had FQ-resistant TB compared to only 1.6% of those taking an FQ for less than 10 days.Citation42 Thus, if we use MOX for initial therapy, it is possible that FQ-resistant MDR-TB strains will flourish, leading to the development of extensively drug-resistant tuberculosis.
This meta-analysis has several limitations. First, variation in treatment duration and the follow-up period among the studies resulted in the inability to calculate a pooled estimate of the rate of unfavorable outcomes. Studies that use FQ as a component in the first-line non-shortened regimen with an adequate follow-up duration are needed to fully evaluate the efficacy of FQs. Second, although culture conversion is the most widely supported surrogate endpoint, the discrepancy between sputum culture conversion rate at two months and treatment outcome indicated that the relapse rate after the completion of therapy could be a precise indicator of effectiveness. However, among the nine included studies, only four studies reported the number of relapses. Third, only a handful of studies using GAT to treat TB were identified, and the duration of treatment and follow-up varied, which contributed to the moderate heterogeneity when we calculated the pooled estimates. Thus, the calculation of the rates of death and adverse events between GAT-containing regimens and the standard regimen may be crude, and the precise efficacy and safety of GAT in initial therapy of TB requires additional study. Fourth, although pooling the results using different culture methods may result in greater heterogeneity, the results should not impact the main finding.
In conclusion, MOX or GAT might not be able to shorten treatment duration in the initial therapy for TB, despite their equivalent or even slightly better efficacy in early phase of treatment compared with the standard regimen. Nevertheless, it is safe to include MOX or GAT in initial TB treatment.
Tuberculosis: the search for improved treatment continues
Two later-generation fluroquinolones (FQs) undergoing trials for initial treatment of tuberculosis (TB) may not reduce duration of therapy as scientists had hoped. The evidence obtained via randomized clinical trials (RCTs) seemed contradictory and insufficient. Wenhong Zhang and co-workers at Fudan University in Shanghai, China, statistically analyzed nine randomized clinical trials to investigate the efficacy and safety of two FQs: moxifloxacin (MOX) and gatifloxacin (GAT). The length of current TB treatment regimens is too long to achieve excellent compliance, so, quick-acting drugs are now sought. The team selected trials where the MOX/GAT had been used to replace or supplement existing TB therapies, and explored whether they helped reduce the duration of the standard 6-month treatment regimen for newly diagnosed TB. They found that, although MOX and GAT were safe and effective in the initial stages of therapy, their use in shorter treatment courses appeared to result in unfavorable outcomes including higher incidence of relapse.
Supplementary Table
Download MS Word (21.3 KB)Supplementary Figure 1
Download GIF Image (109.9 KB)Supplementary Figure 2
Download GIF Image (150 KB)This work was supported in part by the Key Technologies Research and Development Program for Infectious Diseases of China (2013ZX10003008-003).
- World Health Organization.Global tuberculosis report 2014. Geneva: WHO, 2015.Available at http://www.who.int/tb/publications/global_report/archive/en/ accessed 31 August 2015).
- MaZ,LienhardtC,McIlleronH,NunnAJ,WangX.Global tuberculosis drug development pipeline: the need and the reality.Lancet2010; 375:2100–2109.
- GinsbergAM.Tuberculosis drug development: progress, challenges, and the road ahead.Tuberculosis (Edinb)2010; 90:162–167.
- GospodarevskayaE,TullochO,BungaCet al.Patient costs during tuberculosis treatment in Bangladesh and Tanzania: the potential of shorter regimens.Int J Tuberc Lung Dis2014; 18:810–817.
- GillespieSH,BillingtonO.Activity of moxifloxacin against mycobacteria.J Antimicrob Chemother1999; 44:393–395.
- HuY,CoatesAR,MitchisonDA.Sterilizing activities of fluoroquinolones against rifampin-tolerant populations of Mycobacterium tuberculosis.Antimicrob Agents Chemother2003; 47:653–657.
- JiBH,LounisN,MasloC,Truffot-PernotC,BonnafousP,GrossetJ.In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis.Antimicrob Agents Chemother1998; 42:2066–2069.
- RodriguezJC,RuizM,LopezM,RoyoG.In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis.Int J Antimicrob Agents2002; 20:464–467.
- SulochanaS,RahmanF,ParamasivanCN.In vitro activity of Fluoroquinolones against Mycobacterium tuberculosis.J of Chemother2005; 17:169–173.
- MiyazakiE,MiyazakiM,ChenJM,ChaissonRE,BishaiWR.Moxifloxacin (BAY12-8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis.Antimicrob Agents Chemother1999; 43:85–89.
- NuermbergerEL,YoshimatsuT,TyagiSet al.Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis.Am J Respir Crit Care Med2004; 169:421–426.
- NuermbergerEL,YoshimatsuT,TyagiSet al.Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis.Am J Respir Crit Care Med2004; 170:1131–1134.
- GoslingRD,UisoLO,SamNEet al.The bactericidal activity of moxifloxacin in patients with pulmonary tuberculosis.Am J Respir Crit Care Med2003; 168:1342–1345.
- JohnsonJL,HadadDJ,BoomWHet al.Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis.Int J Tuberc Lung Dis2006; 10:605–612.
- PletzMW,De RouxA,RothA,NeumannKH,MauchH,LodeH.Early bactericidal activity of moxifloxacin in treatment of pulmonary tuberculosis: a prospective, randomized study.Antimicrob Agents Chemother2004; 48:780–782.
- WangJY,WangJT,TsaiTHet al.Adding moxifloxacin is associated with a shorter time to culture conversion in pulmonary tuberculosis.Int J Tuberc Lung Dis2010; 14:65–71.
- ZiganshinaLE,TitarenkoAF,DaviesGR.Fluoroquinolones for treating tuberculosis (presumed drug-sensitive).Cochrane Database Syst Rev2013; 6:Cd004795.
- RubinsteinE,KeynanY.Quinolones for mycobacterial infections.Int J Antimicrob Agents2013; 42:1–4.
- ChenZ,LiangJQ,WangJH,FengSS,ZhangGY.Moxifloxacin plus standard first-line therapy in the treatment of pulmonary tuberculosis: a meta-analysis.Tuberculosis (Edinb)2015; 95:490–496.
- GillespieSH,CrookAM,McHughTDet al.Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis.N Engl J Med2014; 371:1577–1587.
- JindaniA,HarrisonTS,NunnAJet al.High-dose rifapentine with moxifloxacin for pulmonary tuberculosis.N Engl J Med2014; 371:1599–1608.
- MerleCS,FieldingK,SowOBet al.A four-month gatifloxacin-containing regimen for treating tuberculosis.N Engl J Med2014; 371:1588–1598.
- MoherD,LiberatiA,TetzlaffJ,AltmanDG,Prisma Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.Ann Intern Med2009; 151:264–269, W64.
- HigginsJP,AltmanDG,GotzschePCet al.The cochrane collaboration's tool for assessing risk of bias in randomised trials.BMJ2011; 343:d5928.
- HigginsJP,ThompsonSG,DeeksJJ,AltmanDG.Measuring inconsistency in meta-analyses.BMJ2003; 327:557–560.
- BurmanWJ,GoldbergS,JohnsonJLet al.Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis.Am J Respir Crit Care Med2006; 174:331–338.
- RustomjeeR,LienhardtC,KanyokTet al.A Phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis.Int J Tuberc Lung Dis2008; 12:128–138.
- CondeMB,EfronA,LoredoCet al.Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial.Lancet2009; 373:1183–1189.
- DormanSE,JohnsonJL,GoldbergSet al.Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis.Am J Respir Crit Care Med2009; 180:273–280.
- JawaharMS,BanurekhaVV,ParamasivanCNet al.Randomized clinical trial of thrice-weekly 4-month moxifloxacin or gatifloxacin containing regimens in the treatment of new sputum positive pulmonary tuberculosis patients.PLoS One2013; 8:e67030.
- VelayuthamBV,AllaudeenIS,SivaramakrishnanGNet al.Sputum culture conversion with moxifloxacin-containing regimens in the treatment of patients with newly diagnosed sputum-positive pulmonary tuberculosis in South India.Clin Infect Dis2014; 59:E142–E149.
- WallisRS,WangC,MeyerD,ThomasN.Month 2 culture status and treatment duration as predictors of tuberculosis relapse risk in a meta-regression model.PLoS One2013; 8:e71116.
- WallisRS,PeppardT,HermannD.Month 2 culture status and treatment duration as predictors of recurrence in pulmonary tuberculosis: model validation and update.PLoS One2015; 10:e0125403.
- MohrJF,McKinnonPS,PeymannPJ,KentonI,SeptimusE,OkhuysenPC.A retrospective, comparative evaluation of dysglycemias in hospitalized patients receiving gatifloxacin, levofloxacin, ciprofloxacin, or ceftriaxone.Pharmacotherapy2005; 25:1303–1309.
- GrosjeanP,UrienS.Reevaluation of moxifloxacin pharmacokinetics and their direct effect on the QT interval.J Clin Pharmacol2012; 52:329–338.
- CamineroJA,SotgiuG,ZumlaA,MiglioriGB.Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis.Lancet Infect Dis2010; 10:621–629.
- FalzonD,JaramilloE,SchunemannHJet al.WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update.Eur Respir J2011; 38:516–528.
- ZhaoY,XuS,WangLet al.National survey of drug-resistant tuberculosis in China.N Engl J Med2012; 366:2161–2170.
- AgrawalD,UdwadiaZF,RodriguezC,MehtaA.Increasing incidence of fluoroquinolone-resistant Mycobacterium tuberculosis in Mumbai, India.Int J Tuberc Lung Dis2009; 13:79–83.
- GrimaldoER,TupasiTE,RiveraABet al.Increased resistance to ciprofloxacin and ofloxacin in multidrug-resistant Mycobacterium tuberculosis isolates from patients seen at a tertiary hospital in the Philippines.Int J Tuberc Lung Dis2001; 5:546–550.
- MiglioriGB,LangendamMW,D'AmbrosioLet al.Protecting the tuberculosis drug pipeline: stating the case for the rational use of fluoroquinolones.Eur Respir J2012; 40:814–822.
- DevasiaRA,BlackmanA,GebretsadikTet al.Fluoroquinolone resistance in Mycobacterium tuberculosis the effect of duration and timing of fluoroquinolone exposure.Am J Respir Crit Care Med2009; 180:365–370.