Abstract
Pteropine orthoreovirus (PRV), potentially of bat origin, is reported to be a causative agent of emerging respiratory tract infections among humans in Southeast Asia. We evaluated the efficacy of serologic assays using the major outer capsid and cell attachment proteins (CAP) of PRV strains in the screening, confirmation and identification of three groups of human PRV infections; Indonesian/Japanese, Indonesian/Hong Kong and Malaysian strains. The different serologic assays were tested using rabbit polyclonal antisera raised against these proteins of selected PRV strains, and validation was carried out using sera from a Miyazaki-Bali/2007 PRV-infected patient and the patient’s contacts. The results of this study showed that rabbit polyclonal antisera raised against the CAP of the Miyazaki-Bali/2007 PRV strain showed the highest reactivity to the Miyazaki-Bali/2007 PRV and to a lesser extent, cross-reactivity with the HK23629/07 and Melaka PRVs, respectively. Neutralization activity against the Miyazaki-Bali/2007 PRV was observed using rabbit anti-Miyazaki-Bali/2007 PRV CAP (320) but not with rabbit anti-HK23629/07 (<20) and Melaka (<20) PRV CAP. This lack of cross-neutralization, suggests the potential for human reinfection with different strains. The use of sera collected from contacts of the Miyazaki-Bali/2007 PRV-infected patient suggested that human-to-human infections with PRV are unlikely. Previously reported cases of PRV infections among human have been mild. However, the expanding geographic distribution of these viruses, of which its virulence remains unknown, warrants close monitoring to enable the development of prevention and control strategies in the event that a change in virulence occurs.
Emerging Microbes and Infections (2016) 5, e44; doi:10.1038/emi.2016.35; published online 11 May 2016
INTRODUCTION
Pteropine orthoreovirus (PRV), which is potentially of bat origin, was previously reported as a novel cause of human respiratory tract infections that were centered in Southeast Asia, MalaysiaCitation1, Citation2, Citation3, Citation4, Citation5 and Indonesia, from which imported cases were subsequently reported in JapanCitation6 and Hong Kong.Citation7, Citation8 These human pathogenic strains, which show a phylogenetic relationship to orthoreoviruses of bat origin that have been isolated in Southeast Asia,Citation2, Citation9, Citation10 ChinaCitation11, Citation12 and Australia,Citation2, Citation13, Citation14 suggest that the contribution of various bat species to the spillover of the viruses to humans is causing novel and emerging PRV infections.Citation1, Citation2, Citation12 In addition, a serological study on PRV infections in Central Vietnam revealed that 4.5% of the population has PRV antibodies, suggesting that PRV infections may be more prevalent than previously thought.Citation15
The structural characteristics of PRV (that is, it is composed of 10 discrete gene segments) can facilitate genetic reassortment between PRV strains.Citation1, Citation2, Citation3, Citation4, Citation5, Citation6, Citation7, Citation8, Citation9, Citation10, Citation11, Citation12, Citation13, Citation14, Citation15, Citation16 The potential for genetic reassortment is a major determinant of the evolution of PRV.Citation2 Although the previously reported human cases have exhibited mild respiratory symptoms with limited human-to-human transmission,Citation1, Citation2, Citation3, Citation4, Citation5, Citation6, Citation7, Citation8 the recent increase in reported cases highlights the need for the surveillance of human PRV infections in Southeast Asia.
In the present study, we performed sensitive and specific serological assays to diagnose, screen and confirm emerging human PRV infections. The assays allowed for three strains to be identified: the Indonesian/Japanese,Citation6 Indonesian/Hong KongCitation7, Citation8 and MalaysianCitation2, Citation3, Citation4, Citation5 strains. The use of these assays provided valuable information on the nature of PRV. In addition, given the recent increase in the number of reported PRV infections in humansCitation1, Citation2, Citation3, Citation4, Citation5, Citation6, Citation7, Citation8 and bats,Citation2, Citation9, Citation10, Citation11, Citation12, Citation13, Citation14 these assays will be useful for determining the distribution of PRV.
MATERIALS AND METHODS
Recombinant baculoviruses and protein purification
The small segment genes of the Miyazaki-Bali/2007 PRV major outer capsid protein (MOCP) and the cell attachment protein (CAP) genes of the Miyazaki-Bali/2007, HK23629/07 and Melaka PRVs were inserted into recombinant baculoviruses with a histidine-tag gene downstream of the polyhedrin promoter (Ac-His), as previously described.Citation17, Citation18 The Miyazaki-Bali/2007 PRV genes were amplified by PCR using pre-designed primer sets (Supplementary Table S1), while those of the HK23629/07 and Melaka PRVs were chemically synthesized. The GenBank accession numbers of the nucleotide sequences corresponding to these genes are shown in Supplementary Table S1. The amplified DNA was digested with BamHI and then ligated to the BamHI site of the recombinant transfer vector pAcYM1-HisCitation18, Citation19 using T4 DNA ligase (Roche, Mannheim, Germany) to generate the recombinant pAcYM1-His constructs. Sf9 insect cells were then co-transfectedCitation20 with the recombinant pAcYM1-His constructs and linearized baculovirus DNA (BD Biosciences, Franklin Lakes, NJ, USA), according to the manufacturer’s instructions, to generate the recombinant baculoviruses.
The recombinant (r) proteins were purified from Tn5 insect cells infected with Ac-His-Miyazaki-Bali/2007 PRV-MOCP, Ac-His Miyazaki-Bali/2007 PRV-CAP, Ac-His HK23629/07 PRV-CAP and Ac-His Melaka PRV-CAP, and then they were solubilized in phosphate-buffered saline (PBS) containing 8 mol/L urea, as previously described.Citation17, Citation21 The expression of each recombinant protein was checked by SDS-PAGE and confirmed by western blot analysis using an anti-His antibody (Qiagen Inc, Valencia, CA, USA).
Polyclonal antibodies
To generate polyclonal antisera against the Miyazaki-Bali/2007 PRV-MOCP and the CAPs of the Miyazaki-Bali/2007, HK23629/07 and Melaka PRVs, two rabbits each were immunized with the following Ac-His purified proteins as previously described: the Miyazaki-Bali/2007 PRV-rMOCP and the rCAPs of Miyazaki-Bali/2007, HK23629/07 and Melaka PRV.Citation18, Citation22 The protocols of the animal experiments were approved by the Animal Care and Use Committee of the National Institute of Infectious Diseases, Tokyo, Japan (NO. 214078).
ELISA
A 2-step ELISA approach was established in the present study. As the first step for the screening of anti-PRV antibodies, Miyazaki-Bali/2007 PRV-rMOCP, a protein that is conserved among the different PRV strains, was used as the ELISA antigen. Miyazaki-Bali/2007 PRV-MOCP shares 96.6%–99.4% amino acid homology with the other PRV strains (Supplementary Table S2, Supplementary Figure S1).Citation2, Citation3, Citation4, Citation5, Citation6, Citation7, Citation8, Citation10, Citation11, Citation12, Citation13, Citation14 In the second step, which was established to identify the PRV strains, the rCAPs of Miyazaki-Bali/2007, HK23629/07 and Melaka PRV served as the ELISA antigens. The CAP is a less conserved protein among the different PRV strains (Supplementary Table S2, Supplementary Figure S2).Citation2, Citation3, Citation4, Citation5, Citation6, Citation7, Citation8, Citation9, Citation10, Citation11, Citation12, Citation13, Citation14, Citation15, Citation16 The negative antigen was produced by following the same methods as were used to produce the recombinant virus antigens by using Tn5 cells that were infected with the recombinant baculovirus without the polyhedrin gene (Δp).
Figure 1 The strain specificity of the rabbit anti-PRV rCAP antibodies against the rCAPs of the different PRVs. IgG ELISA shows the reactivity in the polyclonal antisera raised in rabbits for (A) Miyazaki-Bali/2007, (B) HK23629/07 and (C) Melaka PRV–CAP and (D) anti-His tag antibody control (which was used to confirm the antigen quantity) with the rCAPs of Miyazaki-Bali/2007 (○), HK23629/07 (□) and Melaka (Δ) PRVs as the ELISA antigen. ───────, hyperimmune serum; •••••••••••••••••, preimmunization serum; ─ •• ─ •• ─, anti-His tag antibody. OD405, optical density at 405 nm against a reference wavelength of 490 nm; Δp, recombinant baculovirus without the polyhedrin gene. The representative results of one rabbit per group are shown. An anti-His tag antibody control was used to confirm that equal quantities of rCAP antigens (800) were used. The y-axis represents OD405−ΔP. Abbreviations: PRV, Pteropine orthoreovirus; rCAP, recombinant cell attachment protein.
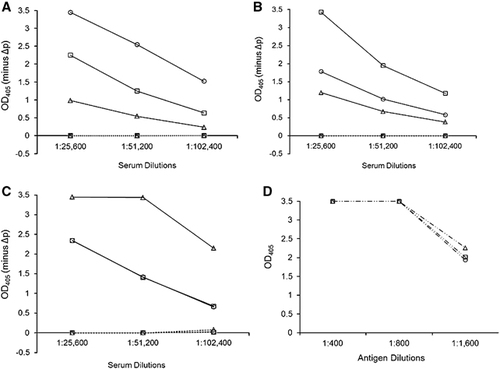
The standard ELISA protocol was followedCitation17, Citation23 using a predetermined optimal antigen quantity that was produced by diluting the antigen stock to 1:800 with PBS. The detection capacity of the MOCP-based ELISA system was tested by screening rabbit anti-Miyazaki-Bali/2007 PRV rMOCP hyperimmune and preimmunization sera for anti-PRV antibodies. The cross-reactivity between the rabbit polyclonal anti-rCAP sera and the rCAPs of the three PRVs was analyzed using the rCAP-based ELISA. The ΔP antigen was used for each of the MOCP and CAP-based ELISA assays. The optical density at 405 nm (OD405) value of ΔP was subtracted from the OD405 value obtained for each recombinant protein tested. Anti-His tag antibodies served as controls to confirm that the same quantity of rCAP antigens was used.
Neutralization test (NT)
Rabbit polyclonal antisera raised against the rCAP of each strain was used to determine the neutralization and cross-neutralization activity against the Miyazaki-Bali/2007 PRV strain as previously described.Citation15 Briefly, the serum samples were diluted four-fold from 1:20 to 1:5120 in Dulbecco’s modified Eagle’s medium (DMEM) containing 2% fetal calf serum. Each 50 μL sample was then mixed with 200 plaque forming units of Miyazaki-Bali/2007 PRV in 50 μL of DMEM, and the mixture was incubated for 1 h at 37 °C for neutralization. After incubation, Vero cell monolayers were inoculated with the mixtures for 1 h. The inoculants were removed, and the cells were cultured for five days with DMEM containing 2% FCS and 1% agarose. The plaques produced by Miyazaki-Bali/2007 PRV were counted under a light microscope. The end-point neutralizing antibody titer was determined by the highest dilution of serum to reduce the plaques by 50% in comparison to the controls (PRNT50).
Immunofluorescence assay
Antigen slides were prepared for an immunofluorescence assay (IFA) using Miyazaki-Bali/2007 PRV-infected 293T cells. The virus-positive cells and mock-infected cells were mixed at a ratio of 1:3, as previously described.Citation15 Briefly, rabbit polyclonal antiserum or human serum samples were diluted two-fold with PBS containing 0.05% Tween-20 from 1:10 to 1:2560. 20 μL of each dilution was spotted over each well. The slides were then incubated for 1 h at 37 °C. Following incubation, the slides were washed with PBS three times and then spotted with 20 μL of 1:400 Alexa Fluor 488 goat anti-rabbit or human IgG (Thermo Fisher Scientific, Inc, Waltham, MA, USA) in PBS containing 0.05% Tween-20. The slides were washed again in the manner described above and examined for signals under a fluorescence microscope. The end-point antibody titer was defined as the highest dilution of serum to show a positive signal under the fluorescence microscope.
Western blotting analysis
The western blotting antigen consisted of Miyazaki-Bali/2007 PRV-infected 293T cells and mock-infected cells. Briefly, Miyazaki-Bali/2007 PRV-infected 293T cells (multiplicity of infection: 3 per cell) or mock-infected cells were cultured for 24 h. The cells were then pelleted, washed with PBS and resuspended in PBS containing 1% Nonidet P-40. Western blotting was performed as previously describedCitation24, Citation25 using each of the different rabbit polyclonal antisera produced in this study or in patient serum.
Validation
The different assays established in the present study were validated to confirm their reactivity and specificity using serum that was previously collected from a Miyazaki-Bali/2007 PRV-infected patient and 46 of the patient’s contacts, which included the patient’s family members, healthcare providers and local health station staff.Citation6 Although the NT against Miyazaki-Bali/2007 PRV had previously been performed, we repeated the NT in the present study to confirm the validity of our assay.Citation6 For the detection of anti-PRV antibodies by MOCP-based ELISA (first-step screening), the mean and standard deviation was determined using a total of 18 serum samples from healthy donors. The cutoff value for the assay was defined as the mean plus three standard deviations.
Ethical statement
The serum samples that were used were collected under informed consent from a Miyazaki-Bali/2007-infected patient and the patient’s contacts.Citation6 The protocol of this study was approved by the Ethics Committee of the National Institute of Infectious Diseases, Tokyo, Japan (NO. 452).
RESULTS
The detection of anti-CAP antibodies
As expected, the rabbit anti-Miyazaki-Bali/2007 PRV-MOCP sera showed a positive reaction in the Miyazaki-Bali/2007 PRV MOCP-based ELISA (data not shown). The potential use of the CAP-based ELISA for the differentiation of PRV strains was also observed. Rabbit polyclonal antisera raised against the CAP of a particular strain (for example, Miyazaki-Bali/2007 PRV) showed the highest OD405 value to the rCAP antigen of that same strain and, to a lesser extent, cross-reactivity with the other strains (), while the anti-His tag antibody showed the same OD405 values for the three rCAP antigens (). The cross-reactivity of the rabbit polyclonal antisera, which were raised against the different PRVs, was also demonstrated by IFA and western blotting. The IFA titers of the rabbit sera raised against Miyazaki-Bali/2007 PRV-rMOCP and rCAP (1:280) were higher than those of the rabbit sera raised against the rCAPs of HK23629/07 and Melaka PRV (both 1:320) (). All of the rabbit polyclonal sera that were raised in this study were also found to have activity against Miyazaki-Bali/2007 PRV by western blotting (, Supplementary Figure S3).
On the other hand, the neutralization assays indicated no cross-reactivity between the rabbit anti-CAP sera of the other strains (HK23629/07 and Melaka) against Miyazaki-Bali/2007 PRV (). The rabbit anti-Miyazaki-Bali/2007 PRV CAP serum showed strong neutralization activity against the Miyazaki-Bali/2007 PRV strain (neutralization titer: 320); in contrast, the anti-Miyazaki-Bali/2007 PRV MOCP antibody had a neutralization titer of 20 ().
Table 1 Antigenicity and cross-antigenicity among the different rabbit anti-Pteropine orthoreovirus (PRV) hyperimmune sera raised in this study with Miyazaki-Bali/2007 PRVFootnotea
Validation
The convalescent phase serum of the patient infected with Miyazaki-Bali/2007 PRV showed a positive reaction in the MOCP-based ELISA, while the acute phase serum of the patient did not (). Similarly, the convalescent phase serum showed a positive reaction in the IFA at a titer of 320, while the acute phase serum was positive at a titer of 10 (). In addition, a significant increase in the NT score was observed between the acute and the convalescent phases, while all of the NT-negative healthy donor serum samples were found to be negative by the MOCP-based ELISA, IFA and western blotting (). Similarly, none of the Miyazaki-Bali/2007 PRV infected patient’s contacts showed the presence of anti-PRV antibodies by the MOCP-based ELISA, IFA or NT (). These results indicate the high specificity of the antibody detection assays; however, further studies are needed to evaluate their sensitivity.
The patient’s convalescent phase serum showed a stronger reaction against Miyazaki-Bali/2007 PRV-rCAP than against HK23629/07 PRV-rCAP or Melaka PRV-rCAP (). The detection of anti-PRV antibodies by western blotting using the convalescent serum of the Miyazaki-Bali/2007 PRV-infected patient and the absence of anti-PRV antibodies in the serum of the patient’s contacts further validates these assays (, ).
Figure 2 An IgG ELISA using serum from the Miyazaki-Bali/2007 PRV-infected patient in the convalescent phase with the recombinant cell attachment protein of Miyazaki-Bali/2007 (○), HK23629/07 (□) and Melaka (Δ) PRVs as ELISA antigens. OD405, optical density at 405 nm against a reference wavelength of 490 nm; Δp, recombinant baculovirus without the polyhedrin gene. The y-axis represents OD405−ΔP. Abbreviation: PRV, Pteropine orthoreovirus.
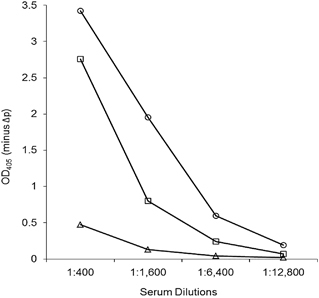
Figure 3 Serologic analysis (western blotting (IgG)) of the serum of the Miyazaki-Bali/2007 PRV-infected patient in the (A) acute and (B) convalescent phases, as well as the (C) healthy donor serum against Miyazaki-Bali/2007 PRV at a primary antibody dilution of 1:1000. Infected: Miyazaki-Bali/2007 PRV-infected 293T cell lysate; mock: mock-infected 293T cell lysate.
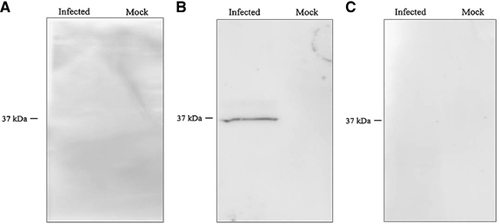
Table 2 Serologic assay validation using serum from a patient infected with Miyazaki-Bali/2007 Pteropine orthoreovirus (PRV)
DISCUSSION
The detection of the anti-PRV antibodies that were validated in this study using the Miyazaki-Bali/2007 PRV MOCP (a protein that is conserved among the different PRV strains), is useful for the screening and diagnosis of PRV infections and possible infections caused by other orthoreovirus species.Citation2, Citation6, Citation14, Citation26, Citation27, Citation28, Citation29 The absence of anti-PRV antibodies among the contacts of the Miyazaki-Bali/2007 PRV-infected patient, as shown in this study, suggests that human-to-human transmission of PRV was not likely, despite the development of symptoms among several of the patient’s contacts.Citation6
The results obtained in the present study suggested that antibodies against different PRVs (even closely related strains) could be distinguished by CAP-based ELISAs. The results of this study suggest that the simultaneous use of the three rCAP-based ELISAs may be useful for the detection of antibodies in human serum to each of the three groups of strains (Miyazaki-Bali/2007, HK46686/09, HK50842/10 and Kampar virus with the rCAP of Miyazaki-Bali/2007 PRV; Sikamat/MYS/2010, Melaka virus and HK23629/07 with the rCAP of Melaka PRV; and HK23629/07 with the rCAP of HK23629/07 PRV, respectively). The identification of the strain of PRV infection is useful in assessing the geographic distribution of infections among closely related strains of this diverse group of viruses. It is possible that PRV variants may exist in bats in Southeast Asia,Citation2 due to the marked divergence that is observed, even among closely related orthoreovirus strains.Citation27, Citation28 The circulation of the PRV variants in nature may cause emerging infections in humans. Although this study was limited by the availability of the different viral strains, it demonstrated (by ELISA and IFA), that the rabbit polyclonal antisera raised against the CAP of HK23629/07 and Melaka PRV had lower cross-reactivity with that of Miyazaki-Bali/2007 PRV. The CAP of Miyazaki-Bali/2007 PRV shares a 57.3 and 56.2% homology with the CAP of Melaka and HK23629/07 PRV, respectively.Citation6, Citation15
The CAP of orthoreoviruses has previously been reported to induce the production of neutralizing antibodies during an infection.Citation27 In this study, the neutralization assays suggested that the neutralizing antibodies raised against CAP do not offer cross-protection against different PRV strains. Because various PRVs may exist in Southeast Asia, reinfection episodes, with different PRV strains, may possibly occur in humans.Citation15
Although PRV infections in humans are generally mild and exhibit a limited capacity for human-to-human transmission, they exist as a diverse group, and their virulence remains unknown. The expanding geographic distribution of reported cases and the possibility of reinfection with different PRV strains suggest the need for close monitoring of PRV infections, especially in Southeast Asia.Citation2, Citation6, Citation8, Citation15, Citation27, Citation28 The development of an ELISA system using the rMOCP and rCAP of PRV as antigens, as shown in this study allows for the screening, confirmation and strain differentiation of infections with PRV and other orthoreovirus infections in humans. The NT, IFA and western blotting assays can also be used as confirmatory assays for samples that are reactive during ELISA screenings. The use of these assays in the diagnosis and surveillance of PRV infections in humans may contribute to the development of prevention and control strategies.
Supplementary Table S1
Download PDF (448.3 KB)Supplementary Table S2
Download PDF (359.4 KB)Supplementary Figure S1
Download PDF (977.6 KB)Supplementary Figure S2
Download PDF (263 KB)Supplementary Figure S3
Download PDF (268.4 KB)This study was supported financially by grants-in-aid from the Ministry of Health, Labor and Welfare (Japan) (H25-Shinko-Ippan-004 and H24-Shinko-Ippan 013).
Supplementary Information for this article can be found on the Emerging Microbes and Infections website (http://www.nature.com/emi)
- VoonK,TanYF,LeongPPet al.Pteropine orthoreovirus infection among out-patients with acute upper respiratory tract infection in Malaysia.J Med Virol2015; 87:2149–2153.
- VoonK,ChuaKB,YuMet al.Evolutionary relationship of the L- and M-class genome segments of bat-borne fusogenic orthoreoviruses in Malaysia and Australia.J Gen Virol2011; 92:2930–2936.
- ChuaKB,CrameriG,HyattAet al.A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans.Proc Natl Acad Sci USA2007; 104:11424–11429.
- ChuaKB,VoonK,CrameriGet al.Identification and characterization of a new orthoreovirus from patients with acute respiratory infections.PLoS One2008; 3:e3803.
- ChuaKB,VoonK,YuMet al.Investigation of a potential zoonotic transmission of orthoreovirus associated with acute influenza-like illness in an adult patient.PLoS One2011; 6:e25434.
- YamanakaA,IwakiriA,YoshikawaTet al.Imported case of acute respiratory tract infection associated with a member of species nelson bay orthoreovirus.PLoS One2014; 9:e92777.
- ChengP,LauCS,LaiAet al.A novel reovirus isolated from a patient with acute respiratory disease.J Clin Virol2009; 45:79–80.
- WongAH,ChengPKC,LaiMYYet al.Virulence potential of fusogenic orthoreoviruses.Emerg Infect Dis2012; 18:944–948.
- LorussoA,TeodoriL,LeoneAet al.A new member of the Pteropine Orthoreovirus species isolated from fruit bats imported to Italy.Infect Genet Evol2015; 30:55–58.
- PritchardLI,ChuaKB,CumminsDet al.Pulau virus; a new member of the Nelson Bay orthoreovirus species isolated from fruit bats in Malaysia.Arch Virol2006; 151:229–239.
- DuL,LuZ,FanYet al.Xi River virus, a new bat reovirus isolated in southern China.Arch Virol2010; 155:1295–1299.
- HuT,QiuW,HeBet al.Characterization of a novel orthoreovirus isolated from fruit bat, China.BMC Microbiol2014; 14:293.
- GardGP,MarshallID.Nelson Bay virus. A novel reovirus.Arch Gesamte Virusforsch1973; 43:34–42.
- ThalmannCM,CumminsDM,YuMet al.Broome virus, a new fusogenic Orthoreovirus species isolated from an Australian fruit bat.Virology2010; 402:26–40.
- SinghH,ShimojimaM,NgocTCet al.Serological evidence of human infection with Pteropine orthoreovirus in Central Vietnam.J Med Virol2015; 87:2145–2148.
- DermodyTS,ParkerJSL,SherryB.Orthoreovirus. In:KnipeDM,HowleyPM,editors.Fields Virology,6th edn.Philadelphia: Lippincott Williams & Wilkins.2013.pp1304–1346.
- FukushiS,NakauchiM,MizutaniTet al.Antigen-capture ELISA for the detection of Rift Valley fever virus nucleoprotein using new monoclonal antibodies.J Virol Methods2012; 180:68–74.
- SaijoM,QingT,NiikuraMet al.Recombinant nucleoprotein-based enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Crimean-Congo hemorrhagic fever virus.J. Clin. Microbiol2002; 40:1587–1591.
- MatsuuraY,PosseeRD,OvertonHAet al.Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins.J Gen Virol1987; 68:1233–1250.
- KittsPA,PosseeRD.A method for producing recombinant baculovirus expression vectors at high frequency.Biotechniques1993; 14:810–817.
- FukushiS,TaniH,YoshikawaTet al.Serological assays based on recombinant viral proteins for the diagnosis of arenavirus hemorrhagic fevers.Viruses2012; 4:2097–2114.
- KakuY,NoguchiA,MarshGAet al.A neutralization test for specific detection of Nipah virus antibodies using pseudotyped vesicular stomatitis virus expressing green fluorescent protein.J Virol Methods2009; 160:7–13.
- BukbukDN,FukushiS,TaniHet al.Development and validation of serological assays for viral hemorrhagic fevers and determination of the prevalence of Rift Valley fever in Borno State, Nigeria.Trans R Soc Trop Med Hyg2014; 108:768–773.
- SaijoM,TangQ,ShimayiBet al.Antigen-capture enzyme-linked immunosorbent assay for the diagnosis of crimean-congo hemorrhagic fever using a novel monoclonal antibody.J Med Virol2005; 77:83–88.
- NiikuraM,IkegamiT,SaijoMet al.Detection of Ebola viral antigen by enzyme-linked immunosorbent assay using a novel monoclonal antibody to nucleoprotein.J Clin Microbiol2001; 39:3267–3271.
- KohlC,LesnikR,BrinkmannAet al.Isolation and characterization of three mammalian orthoreoviruses from European bats.PLoS One2012; 7:e43106.
- DayJM,Pantin-JackwoodMJ,SpackmanE.Sequence and phylogenetic analysis of the S1 genome segment of turkey-origin reoviruses.Virus Genes2007; 35:235–242.
- DayJM.The diversity of the orthoreoviruses: Molecular taxonomy and phylogentic divides.Infect Genet Evol2009; 9:390–400.
- DuncanR.Extensive sequence divergence and phylogenetic relationships between the fusogenic and nonfusogenic orthoreoviruses: A species proposal.Virology1999; 260:316–328.
