Abstract
Scrub typhus, caused by Orientia tsutsugamushi infection, has been a serious public health issue in the Asia-Pacific region, with rising incidence and sporadic outbreaks. However, human protective immunity against specific antigens has been poorly characterized for this bacterium. In addition, immunity produced in early vaccine trials or even after natural infections, did not last long and had poor cross-reactivity among various genotypes. Here, we systematically investigated the kinetics and magnitude of specific adaptive immunity against two membrane antigens, 56 kDa type-specific antigen (TSA56) and surface cell antigen A (ScaA), that are involved in bacterial adhesion and invasion of the host in 64 recovered scrub typhus patients. Antibody responses to the bacterial antigens in patients were generally short-lived and waned to baseline levels 2 years after recovery. The anti-TSA56 IgG responses were predominantly composed of the IgG1 and IgG3 subclasses and persisted for up to 1 year after recovery, whereas IgG specific to ScaA primarily consisted of more transient IgG1, with limited responses by other subclasses. Cellular immunity, including CD4 and CD8 T-cells specific to membrane antigens, also rapidly declined from 1 year after infection, as measured by enzyme-linked immunospot (ELISPOT) assays and flow cytometry. The short longevity of antigen-specific adaptive immunity might be attributable to limited memory responses, as observed in earlier vaccine studies using whole bacterial antigens. Finally, we identified HLA-A*0201-restricted and highly conserved CD8 T-cell epitopes in the TSA56 antigen, which may be valuable tools for assessing cellular immunity against O. tsutsugamushi and developing an effective scrub typhus vaccine.
Emerging Microbes & Infections (2017) 6, e116 doi:10.1038/emi.2017.106; published online 20 December 2017
Introduction
Scrub typhus is an acute febrile infectious disease endemic in Asia and the western Pacific region.Citation1 The disease has been a serious public health problem, afflicting more than one million patients annually in the endemic area. In addition, a rapid increase in disease incidence, as well as sporadic local outbreaks, has been reported in several endemic countries within the last decade.Citation1 Recently, scrub typhus has emerged in unexpected geographic locations, including ChileCitation2 and Kenya,Citation3, Citation4 suggesting that the endemicity of scrub typhus may not be restricted to the traditional ‘tsutsugamushi triangle’ in the Asia-Pacific area.Citation5 Therefore, more attention needs to be paid to this neglected infectious disease.
Scrub typhus is a mite-borne infectious disease that is caused by Orientia tsutsugamushi, an obligate intracellular bacterium.Citation6 Bacteria are transmitted from infected chigger mites to humans and systemically spread to multiple organs, including the lung, kidney, liver and spleen.Citation7 Clinical presentations are typically characterized by eschar at the mite-biting site, fever, rashes, lymphadenopathy and myalgia. The disease severity can vary from a self-limiting flu-like syndrome to a life-threatening disease causing multi-organ failure and disseminated intravascular coagulation.Citation8 The mortality of untreated patients is ∼6%, with a wide range of 0–70% (min–max) depending on the endemic area and patient’s age.Citation9 Even though early diagnosis and subsequent proper treatment with antibiotics, such as doxycycline or chloramphenicol, usually results in rapid recovery from febrile illness, reports of poorly responsive cases to antibiotic therapy suggest possible drug resistance.Citation10, Citation11 In addition, recurrent human infection has often been observed in highly endemic regions,Citation12, Citation13 primarily due to antigenic heterogeneity and the short longevity of adaptive immunity against the bacterial pathogen.Citation8 Despite the wide range of preventative approaches that have been attempted in the past 70 years, all have failed to yield an effective prophylactic vaccine.Citation8
In earlier vaccine studies using inactivated bacterial antigens or live Orientia challenge followed by antibiotic treatment, protective immunity against the homologous strain lasted for only a few years, whereas heterologous protection generally disappeared within a few months in humans.Citation14 Although there is very little information about protective immunity in humans, antibodies generated from mice immunized with outer membrane proteins of O. tsutsugamushi, such as 56 kDa type-specific antigen (TSA56) and surface cell antigen A (ScaA), neutralized and significantly suppressed bacterial entry into host cells in vitro.Citation15, Citation16 In addition, we recently found that immunization with ScaA, when combined with TSA56, not only provides protective immunity against lethal challenges with the homologous strain but also confers significant protection against heterologous strains in a mouse infection model.Citation16 Recently, two other groups reported that CD8 T cells from infected mice protected naive mice from lethal challenges with O. tsutsugamushi.Citation17, Citation18 These results indicate that antigen-specific adaptive immunity, especially bacteria-specific CD8 T cells, may have a significant role in protecting against scrub typhus. Nevertheless, antigen-specific adaptive immune responses and their longevity are poorly characterized in scrub typhus patients. For example, immunoglobulin G (IgG) is classified into four subclasses (IgG1 ∼4) according to their constant regions and variable degree of effector functions, such as complement fixation, neutralization and opsonization.Citation19 However, a systemic study of IgG subtypes specific to O. tsutsugamushi antigens has not been conducted in human patients. Although a few studies have shown that IgG responses in recovered scrub typhus patients only persist for a short period,Citation12, Citation20, Citation21 the majority of these studies used whole bacteria as antigens for immunofluorescence assays (IFA), and antibody responses against specific protein antigens were measured by the total IgG or IgM level.Citation22 Moreover, CD4 or CD8 T-cell responses specific to protein antigens of O. tsutsugamushi have been poorly characterized in scrub typhus patients, despite their functional importance in protective immunity. In this report, we systematically investigated specific adaptive immunity against two Orientia membrane antigens, TSA56 and ScaA, in recovered patients to assess the dominant subtypes of the IgG and T-cell responses as well as their kinetic changes after recovery. We also screened and selected potential CD8 T-cell epitopes of TSA56 as they can be applied as a useful tool for monitoring antigen-specific CD8 T-cell immunity in humans. The types, magnitudes and kinetics of specific adaptive immunity in scrub typhus patients may provide valuable clues for assessing functional adaptive immunity in humans and for the development of an effective vaccine.
Materials and methods
Ethics statement
Ethical approval for this work was granted by the Institutional Review Board of both Seoul National University Hospital (IRB NO 1308-058-513) and Chungnam National University Hospital (IRB NO 2014-12-006). All patients and healthy volunteers provided written informed consent prior to sample collection.
Study subjects
Human peripheral blood was drawn from healthy volunteers (n=29) and recovered scrub typhus patients (n=64) after obtaining informed consent at Chungnam National University Hospital in Daejeon, South Korea. To analyze the humoral and cellular immune responses, scrub typhus patients were divided into four groups based on the time after recovery from the disease (Y0: within 2 weeks after recovery, Y1–Y3: 1–3 year(s) after recovery). Scrub typhus was confirmed based on clinical symptoms and a positive serology: a fourfold or greater rise in the titer of paired plasma or single cut-off titer of an IgM antibody ≥1:160 by an indirect immunofluorescence antibody assay (IFA) against O. tsutsugamushi antigens or passive hemagglutination assay (PHA) during hospital admission.Citation21 Healthy volunteers had never been previously diagnosed with scrub typhus and their sera were negative when examined by IFA. The mean age of the scrub typhus patients was 60.1 years (range: 17–88) and healthy control was 60.5 years (range: 52–71). The ratio of males to females was 45:55 in scrub typhus patients and 32:68 in healthy controls.
Preparation of peripheral blood mononuclear cells and plasma
Blood samples were collected in heparinized tubes and centrifuged at 800g for 20 min. The top layer containing plasma was cryopreserved to titrate antibodies against O. tsutsugamushi antigens. Peripheral blood mononuclear cells (PBMCs) were isolated by standard density centrifugation with Histopaque (GE Healthcare, Pittsburgh, PA, USA). Fresh PBMCs were used for Enzyme-Linked ImmunoSpot (ELISPOT) assays, and the remaining PBMCs were cryopreserved in liquid nitrogen for flow cytometric analysis.
Preparation of recombinant ScaA and TSA56 proteins
The scaA and tsa56 genes were amplified from genomic DNA of the O. tsutsugamushi Boryong strain (GenBank accession No. AM494475.1) by PCR using the primer pairs listed in Table . The PCR products were cloned into the pET-28a plasmid and sequenced to confirm successful in-frame cloning. Recombinant ScaA and TSA56 proteins were purified from E. coli BL21 (DE3) harboring a recombinant plasmid encoding each bacterial antigen. Following induction with isopropyl βthiogalactoside (IPTG, 0.1 mM, Duchefa, Zwijndrecht, Netherlands) at 16 °C for 16 h, the proteins were purified using Ni-nitrilotriacetic acid (NTA) His-resin (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions. The purified proteins were dialyzed against phosphate-buffered saline (PBS) in Slide-A-Lyzer Dialysis Cassettes (Thermo Scientific, Waltham, MA, USA) at 4 °C overnight. The identity and purity of the proteins were assessed by western blotting and Coomassie blue staining as previously described.Citation23
Table 1 List of primers used in this study
CD8+ T-cell epitope prediction and HLA typing
To identify potential HLA-A*0201-restricted CD8 T-cell epitopes within the TSA56 antigen of O. tsutsugamushi, four web-based programs were used: BIMAS (https://www-bimas.cit.nih.gov/),Citation24 SYFPEITHI (http://www.syfpeithi.de/),Citation25 IEDB (http://tools.immuneepitope.org/) and NetMHCpan (www.cbs.dtu.dk/services/NetMHCpan/).Citation26 We selected 17 potential peptide epitopes that were predicted with top 1% scores or percentile ranks in at least three of the four programs. The peptide sequences and their affinity (IC50) to HLA-A*0201 predicted by NetMHCpan are summarized in Table . Human leukocyte antigen (HLA) typing was performed to screen for the HLA-A2 type using a PE-labeled HLA-A2 antibody (MBL, Nagoya, Japan) by flow cytometric analysis.
Table 2 Predicted HLA-A*0201-restricted CD8+ T-cell epitopes of TSA56
Immunofluorescence assay (IFA)
An IFA was performed to measure the total IgG antibody titers against O. tsutsugamushi. L929 cells infected with three strains of O. tsutsugamushi (Boryong, Karp and Gilliam strain) were harvested, mixed in equal amounts and used as antigens for IFA as previously described.Citation23 Briefly, infected L929 cells were harvested, washed with PBS, spotted onto teflon-coated spot slides and fixed with cold acetone for 10 min. The slides were stored at -70 °C until use. Twofold serially diluted (1:40 to 1:5120 in PBS) patient plasma was added to the antigen-coated spot on the slide and incubated for 30 min in a moist chamber at room temperature. An Alexa Fluor 488-conjugated goat anti-human IgG antibody (Molecular Probes, Waltham, MA, USA), diluted 1:1000 in PBS, was used as the secondary antibody. The stained slides were examined under an Olympus FV1000 laser scanning confocal microscope (Olympus, Tokyo, Japan). The endpoint titer of IFA was defined as the highest titer showing a fluorescence signal above the background.
ELISA
To assess the ScaA- or TSA56-specific antibody responses, 96-well immunoassay plates (Nunc, Waltham, MA, USA) were coated with 100 μL of purified antigen at a concentration of 1 μg/mL at 4 °C overnight. The plates were then blocked for 2 h at room temperature (RT) with PBS containing 5% skim milk. One hundred microliters of twofold serially diluted plasma samples were incubated for 2 h at RT. After washing with PBS containing 0.05% Tween20 (0.05% PBST), horseradish peroxidase (HRP)-conjugated mouse anti-human IgG1, IgG2, IgG3, IgG4 or IgG antibody (Southern Biotech, Birmingham, AL, USA) was added and incubated for 1 h at RT. Wells were washed with 0.05% PBST and incubated with a 3,3′,5,5′-tetramethylbenzidine (TMB) peroxidase substrate solution (KPL, Gaithersburg, MD, USA) for 10 min. The reactions were stopped by addition of a 1 M phosphoric acid solution. Absorbance was measured at 450 nm using a microplate reader (Beckman Coulter, Brea, CA, USA). The cut-off titer for the ELISA was determined as the lowest titer showing an optical density (OD) over the mean OD plus 3 × standard deviation (s.d.) from 19 control plasma samples (diluted 1:100).
Flow cytometric analysis
Human PBMCs were cultured for 18–20 h in RPMI 1640 medium (Gibco, Gaithersburg, MD, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 nM β-mercaptoethanol, 100 U/mL penicillin/streptomycin and 2 mM glutamine (Welgene, Seoul, South Korea) in the presence of 10 μg/mL of purified ScaA or TSA56 antigens in 24-well, flat-bottomed culture plates (5 × 106 cells per well). For intracellular cytokine staining, GolgiPlug (BD Biosciences, San Jose, CA, USA) was added and cells were further incubated for 6 h. Cells were then washed 3 times with ice-cold FACS buffer (PBS containing 1% bovine serum albumin and 1 mM EDTA) and blocked on ice for 30 min with ultra-block solution (10% rat sera, 10% hamster sera, 10% mouse sera and 10 μg/mL of 2.4G2 monoclonal antibody). PBMCs were stained with Pacific blue-conjugated CD4 and FITC-conjugated CD8 antibodies (BD Pharmingen, Franklin Lakes, NJ, USA) at 4 °C for 30 min. After surface CD4 or CD8 staining, cells were washed three times with ice-cold FACS buffer and subjected to intracellular cytokine staining using the Cytofix/Cytoperm kit according to the manufacturer’s instructions (BD Biosciences). Cells were stained with an APC-conjugated IFN-γ antibody (BD Pharmingen) at 4 °C for 30 min. The stained cells were analyzed with a FACS LSRII flow cytometer (BD Immunocytometry Systems, San Jose, CA, USA). Data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Enzyme-linked immunospot (ELISPOT) assay
Human IFN-γ/IL-4 dual color ELISPOT assays were performed according to the manufacturer’s instructions (Abcam, Cambridge, UK). Briefly, polyvinylidene difluoride-backed microtiter plates (MSIP, Millipore, Temecula, CA, USA) were treated with 25 μl of 35% methanol for 30 s and washed 3 times with sterile PBS. Plates were coated with IFN-γ/IL-4 capture antibodies at 4 °C overnight. Human PBMCs (5 × 105 cells per well) were stimulated with 10 μg/mL ScaA, TSA56 or 9-mer epitope peptides (1 μM) in the presence of co-stimulatory anti-CD28 and anti-CD49d monoclonal antibodies (BD Pharmingen) at 37 °C for 18 h. The wells were incubated further with a secondary anti-IFN-γ FITC-conjugated antibody and biotinylated anti-IL-4 antibody at room temperature for 90 min, followed by incubation with anti-FITC HRP and streptavidin-alkaline phosphatase (AP) conjugates for 1 h. Immunospots were developed by sequential incubation of wells with an AEC or BCIP/NBT substrate and were counted using a CTL ImmunoSpot reader (Cellular Technology, Cleveland, OH, USA). The count settings (intensity, gradient and size of spot) were manually confirmed by the naked eye in each experimental set.
Statistical analysis
Data were analyzed using GraphPad Prism 5.01 software (GraphPad Software, La Jolla, CA, USA). Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by the Newman–Keuls t-test for comparisons of values among different groups. P<0.05 was considered statistically significant.
Results
Kinetics and magnitude of the anti-ScaA and TSA56 antibody responses in scrub typhus patients
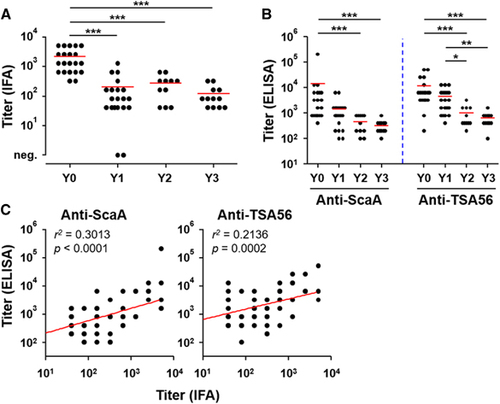
As measured by IFA, the mean titer of O. tsutsugamushi-specific IgG responses was 2194, ranging from 320 to 5120 (s.d.: ±1825), in 21 patients’ plasma collected within 2 weeks after recovery (Y0, ). The antibody titers significantly dropped 1 year after recovery (Y1) and generally waned over 3 years (mean titer: Y1=204, Y2=272 and Y3=120). Specific antibody responses against the ScaA and TSA56 antigens, as measured by ELISA using the bacterial antigens, also gradually declined (). The antibody titers against both antigens were significantly lower at 2 years after infection. It is also notable that the anti-ScaA responses (mean titers: Y0=14 421; Y1=1460; Y2=473 and Y3=325) more rapidly declined than the anti-TSA56 titers (mean titers: Y0=12 011; Y1=4500; Y2=1000 and Y3=642), indicating that anti-TSA56 antibodies are more persistent than the anti-ScaA responses. Both antibody responses measured by ELISA were significantly correlated with the IFA titer against whole bacterial antigens, although the anti-ScaA titers showed better correlation with the IFA titer ().
Differential kinetics and magnitude of specific IgG isotype responses against ScaA and TSA56
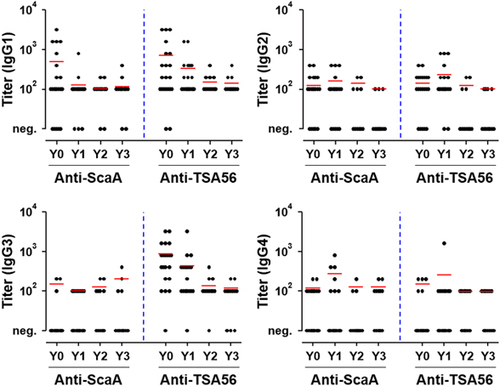
The distribution of IgG subclasses specific to ScaA and TSA56 was examined by ELISA. As seen in Figure , TSA56-specific IgG1 and IgG3 subclasses were dominant in Y0 patients, and the titers of both isotypes gradually declined thereafter. By contrast, IgG3 responses specific to ScaA antigen were barely detectable in the recovered patients, and only the IgG1 isotype was prevalent immediately after recovery (Y0). The ScaA-specific IgG1 responses also rapidly dropped at 1 year after recovery. IgG2 and IgG4 subclasses specific to the bacterial antigens were not significantly detected in recovered patients. Therefore, the dominant IgG isotype responses were IgG1 and IgG3 subclasses specific to TSA56, which declined gradually, as well as the IgG1 isotype against ScaA, which waned to basal levels within 1 year after infection.
Kinetics and magnitude of antigen-specific T-cell responses
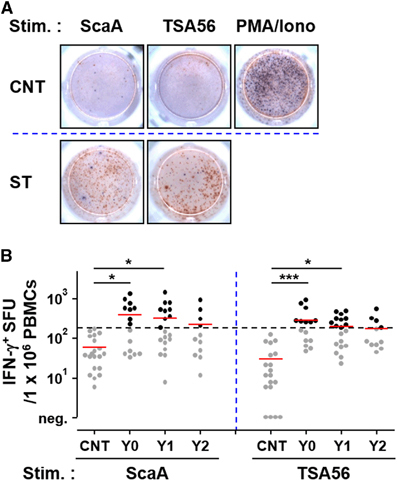
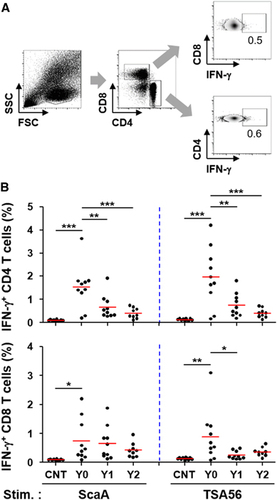
To assess the relative magnitude and longevity of antigen-specific cellular immunity, we performed ELISPOT and flow cytometric analysis using PBMCs after stimulation with TSA56 and ScaA antigens. The cellular counts of IFN-γ-positive PBMCs stimulated with either of the antigens were significantly elevated in Y0 patients (mean counts: 196 for ScaA and 145 for TSA56/5 × 105 PBMCs) and Y1 patients (mean counts: 167 for ScaA and 102 for TSA56/5 × 105 PBMCs) compared to those of control patients (mean counts: 98 for ScaA and 71 for TSA56/5 × 105 PBMCs) (Figure ). At Y0, 53% (8 out of 15) and 67% (10 out of 15) of the recovered patients showed positive ELISPOT results (counts over the mean plus 3 × s.d. of 19 control samples) when stimulated with ScaA and TSA56, respectively. The counts of IFN-γ-positive PBMCs stimulated with either of the antigens gradually declined thereafter and were not statistically significant in the Y2 group. The cellular counts of IL-4-positive PBMCs upon antigen stimulation were not significantly different among the patient and control groups (Supplementary Figure S1), demonstrating that Th1-biased cellular responses are dominant in scrub typhus patients. To examine the antigen-specific T-cell response profile in more detail, CD4 and CD8 T cells secreting IFN-γ upon antigen stimulation were further assessed by flow cytometric analysis (Figure ). Upon ScaA or TSA56 stimulation, cellular fractions of IFN-γ-positive CD4 T cells in PBMCs were significantly elevated in the Y0 group compared to those of the control, but were significantly reduced in recovered patients of the Y1 and Y2 groups. In the case of CD8 T cells, the IFN-γ-secreting population significantly increased upon antigen stimulation in the Y0 group. ScaA-specific, IFN-γ-positive CD8 T cells gradually declined without statistical significance in the Y1 and Y2 groups, whereas cells specific to TSA56 significantly decreased at 1 year after infection. These results indicate that antigen-specific T cells were significantly elevated immediately after recovery and gradually declined thereafter, as seen in the antibody responses. It is also notable that ScaA-specific CD8 T cells appear to be more persistent than those specific for TSA56.
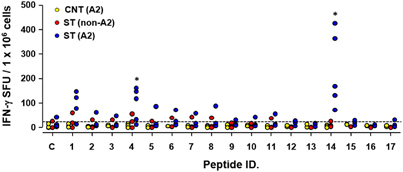
Screening of CD8 T-cell epitopes in the TSA56 antigen
TSA56, a major outer membrane protein of O. tsutsugamushi, has been a major target for vaccine development.Citation1 In addition, it was recently reported that CD8 T cells provide immune protection against O. tsutsugamushi infection.Citation17, Citation18 Therefore, assessment of O. tsutsugamushi-specific CD8 T-cell responses can be crucial for monitoring protective immunity in humans. To generate MHC class I-restricted epitopes in the TSA56 antigen as a tool for measuring CD8 T-cell responses, we screened potential HLA-A*0201-restricted CD8 T-cell epitopes in the TSA56 antigen using four web-based programs. Our analysis revealed 17 potential epitopes that were predicted with top 1% scores or percentile ranks in at least three of the four programs (Table ). When we performed ELISPOT assays using the 17 peptide epitopes, two epitope peptides (YLRNISAEV and QLYKDLVKL) elicited significantly more IFN-γ-positive cellular spots in PBMCs from recovered patients with HLA-A2 type compared to control patients with HLA-A2 type and/or recovered patients with non-HLA-A2 type. Although our screen included a limited number of human subjects (3–5 per group) and needs to be further evaluated, the selected epitopes may be useful tools for monitoring antigen-specific CD8 T-cell immunity in recovered scrub typhus patients or for future vaccine study.
Discussion
In this study, we investigated the kinetics and magnitude of adaptive immunity, including antibodies and T-cell responses specific to the ScaA and TSA56 antigens in scrub typhus patients. These two outer membrane proteins have been shown to have a significant role in bacterial adhesion and invasion.Citation16, Citation27, Citation28 In addition, immunization with the two antigens could provide protective immunity, potentially via generation of neutralizing antibodies and cellular immunity.Citation15, Citation16, Citation29, Citation30 As the nature of protective immunity against the intracellular pathogen has been primarily assessed in murine infection models, systemic characterization of the antibody and T-cell responses that are specific to the bacterial antigens in humans is urgently required for the development of an effective scrub typhus vaccine.
Antibody responses to ScaA and TSA56 in recovered scrub typhus patients were generally short-lived and waned to baseline levels 2 years after infection ( and ). The levels and trends of the adaptive immune responses were generally consistent regardless of patients’ age or gender (data not shown). This short longevity of IgG responses against the TSA56 antigen has also been reported in other studies.Citation22, Citation31 Nevertheless, specific antibodies against TSA56 were more robust and persistent than anti-ScaA antibodies. The relative robustness of the IgG responses against TSA56 compared to other autotransporter proteins, including ScaA, was also previously confirmed in scrub typhus patients.Citation23 These results might be because TSA56 is the most abundant major outer membrane protein, occupying ∼20% of the whole bacterial proteome.Citation32 However, the relative immunogenicity of the two membrane antigens needs to be further characterized. It is also interesting to note that anti-the TSA56 IgG responses were predominantly composed of IgG1 and IgG3 subclasses, whereas IgG responses specific to ScaA primarily consisted of transient IgG1 and limited responses by the other subclasses (Figure ). Considering that IgG1 and IgG3, particularly IgG3 due to its longer hinge region and greater flexibility, are more potent triggers of effector mechanisms such as neutralization, complement fixation and phagocytosis than IgG2 and IgG4,Citation19 stronger and more persistent induction of IgG1 and IgG3 against both of the membrane antigens might be required for better protection against O. tsutsugamushi infection. However, the role of antibody responses in protective immunity is still unclear in animal infection models. Passive transfer of convalescent sera did not protect the majority of mice challenged with O. tsutsugamushi, but pre-incubation of the bacterial pathogen with immune sera markedly increased the survival rate of infected mice.Citation33 In addition, the presence of specific antibodies did not terminate rickettsemia in a non-human primate infection model.Citation34 O. tsutsugamushi and specific antibodies circulated concurrently in infected primates, suggesting that a mechanism other than humoral immunity might be an important factor in the eradication of the pathogen in vivo.Citation34 Recently, two reports showed that genetic knockout or antibody-mediated depletion of CD8 T cells increased the bacterial loads and severity of illness in murine infection models.Citation17, Citation18 Adoptive transfer also confirmed the major role of immune CD8 T cells in protecting against O. tsutsugamushi infection. Although the relative contribution of CD4 T cells or antibody-secreting B cells has not been directly compared with CD8 T-cell activity, the critical importance of CD8 T cells in effective host immune responses during bacterial infection is convincing in murine infection models. Previously, we and others showed a preferential increase of CD8 T cells with activated phenotypes in scrub typhus patients during the convalescent phase,Citation6, Citation35 suggesting a potential role of cytotoxic T cells in clearing bacteria during human infection. However, the antigen-specificity of induced T cells has never been assessed in human infections. In this study, we demonstrate for the first time a significant elevation of ScaA- and TSA56-specific CD4 and CD8 T cells secreting IFN-γ in scrub typhus patients within 2 weeks after recovery (Figure ). Consistent with this result, dominance of Th1-biased immune responses, as represented by elevated IFN-γ (Figure ), has also been observed in scrub typhus patients.Citation36, Citation37 In summary, cellular immunity mediated by antigen-specific T cells may have a significant role in the eradication of bacterial pathogens from infected patients. ScaA- or TSA56-specific T cells, however, rapidly declined at 1 year after infection and were barely detectable at 2 years after recovery. This short longevity of antigen-specific T cells, as well as antibody responses, might be clinically associated with the limited memory responses observed in recovered or vaccinated patients.Citation38, Citation39, Citation40, Citation41
Finally, we also screened and selected epitope peptides for the analysis of TSA56-specific CD8 T cells. Potential HLA-A*0201-restricted CD8 T-cell epitopes on the TSA56 antigen were first screened bioinformatically as TSA56 is one of the most common HLA alleles in the human population, especially in the endemic region of scrub typhus (http://www.allelefrequencies.net/). Two epitope peptides (YLRNISAEV and QLYKDLVKL) elicited specific and a significantly higher number of IFN-γ-positive cellular spots in PBMCs from recovered patients with HLA-A2 type, as measured by the ELISPOT assay (Figure ). These peptide epitopes may therefore be useful for the analysis of TSA56-specific cellular immunity in humans with HLA-A2 type. Interestingly, the predicted T-cell epitopes are primarily located outside of the variable domains of TSA56 (Supplementary Figure S2).Citation32 By contrast, antigenic determinants for antibody responses generally overlap with variable domains of TSA56,Citation42, Citation43 and immunological pressure on the major outer membrane protein during vertebrate host infection might be a crucial driver of genetic diversification of O. tsutsugamushi.Citation1 Discovery of the preservation of T-cell epitopes in the major outer membrane protein among different genotypes represents a significant advancement toward generating universal and protective T-cell responses that encompass the diverse genotype strains of O. tsutsugamushi. The potential role of T-cell responses specific to the conserved epitopes in protective immunity against the bacterial infection needs to be further verified for the development of a universal scrub typhus vaccine.
Supplementary Figure 1
Download MS Word (32.5 KB)Supplementary Information
Download MS Word (761 KB)Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI16C0472). N-YH, YK, C-KM, H-IK and NTHY from Seoul National University received a scholarship from the BK21-plus education program provided by the National Research Foundation of Korea.
The authors declare no conflict of interest.
Supplementary Information for this article can be found on the Emerging Microbes & Infections website (http://www.nature.com/emi)
Notes
Restriction enzyme sites are italisized.
O. tsutsugamushi Boryong strain served as the template.Citation44
Amino acid position in TSA56 sequence (GeneBank accession No. CAM79668).
Peptide affinity predicted by NetMHCpan 3.0 Server.Citation26
References
- Kim G, Ha NY, Min CKet al.Diversification of Orientia tsutsugamushi genotypes by intragenic recombination and their potential expansion in endemic areas. PLoS Negl Trop Dis 2017;11: e0005408.
- Weitzel T, Dittrich S, Lopez Jet al.Endemic scrub typhus in South America. N Engl J Med 2016;375: 954–961.
- Maina AN, Farris CM, Odhiambo Aet al.Q fever, scrub typhus, and rickettsial diseases in children, Kenya, 2011-2012. Emerg Infect Dis 2016;22: 883–886.
- Thiga JW, Mutai BK, Eyako WKet al.High seroprevalence of antibodies against spotted fever and scrub typhus bacteria in patients with febrile illness, Kenya. Emerg Infect Dis 2015;21: 688–691.
- Walker DH.Scrub typhus— scientific neglect, ever-widening impact. New Engl J Med 2016;375: 913–915.
- Cho BA, Ko Y, Kim YSet al.Phenotypic characterization of peripheral T cells and their dynamics in scrub typhus patients. PLoS Negl Trop Dis 2012;6: e1789.
- Moron CG, Popov VL, Feng HMet al.Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol 2001;14: 752–759.
- Paris DH, Shelite TR, Day NPet al.Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg 2013;89: 301–307.
- Taylor AJ, Paris DH, Newton PN.A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi. PLoS Negl Trop Dis 2015;9: e0003971.
- Watt G, Chouriyagune C, Ruangweerayud Ret al.Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet 1996;348: 86–89.
- Mathai E, Rolain JM, Verghese GMet al.Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci 2003;990: 359–364.
- Bourgeois AL, Olson JG, Fang RCet al.Humoral and cellular responses in scrub typhus patients reflecting primary infection and reinfection with Rickettsia tsutsugamushi. Am J Trop Med Hyg 1982;31 (3 Pt 1): 532–540.
- Mahajan SK, Rolain JM, Kashyap Ret al.Scrub typhus in Himalayas. Emerg Infect Dis 2006;12: 1590–1592.
- Valbuena G, Walker DH.Approaches to vaccines against Orientia tsutsugamushi. Front Cell Infect Microbiol 2012;2: 170.
- Seong SY, Kim HR, Huh MSet al.Induction of neutralizing antibody in mice by immunization with recombinant 56 kDa protein of Orientia tsutsugamushi. Vaccine 1997;15: 1741–1747.
- Ha NY, Sharma P, Kim Get al.Immunization with an autotransporter protein of Orientia tsutsugamushi provides protective immunity against scrub typhus. PLoS Negl Trop Dis 2015;9: e0003585.
- Hauptmann M, Kolbaum J, Lilla Set al.Protective and pathogenic roles of CD8+ T lymphocytes in murine Orientia tsutsugamushi infection. PLoS Negl Trop Dis 2016;10: e0004991.
- Xu G, Mendell NL, Liang Yet al.CD8+ T cells provide immune protection against murine disseminated endotheliotropic Orientia tsutsugamushi infection. PLoS Negl Trop Dis 2017;11: e0005763.
- Vidarsson G, Dekkers G, Rispens T.IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014;5: 520.
- Saunders JP, Brown GW, Shirai Aet al.The longevity of antibody to Rickettsia tsutsugamushi in patients with confirmed scrub typhus. Trans R Soc Trop Med Hyg 1980;74: 253–257.
- Kim DM, Lee YM, Back JHet al.A serosurvey of Orientia tsutsugamushi from patients with scrub typhus. Clin Microbiol Infect 2010;16: 447–451.
- Chen HW, Zhang Z, Huber Eet al.Kinetics and magnitude of antibody responses against the conserved 47-kilodalton antigen and the variable 56-kilodalton antigen in scrub typhus patients. Clin Vaccine Immunol 2011;18: 1021–1027.
- Ha NY, Kim Y, Choi JHet al.Detection of antibodies against Orientia tsutsugamushi Sca proteins in scrub typhus patients and genetic variation of sca genes of different strains. Clin Vaccine Immunol 2012;19: 1442–1451.
- Parker KC, Bednarek MA, Coligan JE.Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol 1994;152: 163–175.
- Rammensee H, Bachmann J, Emmerich NPet al.SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 1999;50: 213–219.
- Andreatta M, Nielsen M.Gapped sequence alignment using artificial neural networks: application to the MHC class I system. Bioinformatics 2016;32: 511–517.
- Lee JH, Cho NH, Kim SYet al.Fibronectin facilitates the invasion of Orientia tsutsugamushi into host cells through interaction with a 56-kDa type-specific antigen. J Infect Dis 2008;198: 250–257.
- Cho BA, Cho NH, Seong SYet al.Intracellular invasion by Orientia tsutsugamushi is mediated by integrin signaling and actin cytoskeleton rearrangements. Infect Immun 2010;78: 1915–1923.
- Seong SY, Huh MS, Jang WJet al.Induction of homologous immune response to Rickettsia tsutsugamushi Boryong with a partial 56-kilodalton recombinant antigen fused with the maltose-binding protein MBP-Bor56. Infect Immun 1997;65: 1541–1545.
- Ha NY, Shin HM, Sharma Pet al.Generation of protective immunity against Orientia tsutsugamushi infection by immunization with a zinc oxide nanoparticle combined with ScaA antigen. J Nanobiotechnol 2016;14: 76.
- Ching WM, Wang H, Eamsila Cet al.Expression and refolding of truncated recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi and its use in enzyme-linked immunosorbent assays. Clin Diagn Lab Immunol 1998;5: 519–526.
- Ohashi N, Nashimoto H, Ikeda Het al.Diversity of immunodominant 56-kDa type-specific antigen (TSA) of Rickettsia tsutsugamushi. Sequence and comparative analyses of the genes encoding TSA homologues from four antigenic variants. J Biol Chem 1992;267: 12728–12735.
- Robinson DM, Huxsoll DL.Protection against scrub typhus infection engendered by the passive transfer of immune sera. Southeast Asian J Trop Med Public Health 1975;6: 477–482.
- Robinson DM, Gan E, Chan TCet al.Patterns of rickettsemia and antibody response in silvered leaf monkeys (Presbytis cristatus after inoculation with virulent and avirulent strains of Rickettsia tsutsugamushi. J Infect Dis 1977;135: 664–668.
- de Fost M, Chierakul W, Pimda Ket al.Activation of cytotoxic lymphocytes in patients with scrub typhus. Am J Trop Med Hyg 2005;72: 465–467.
- Sumonwiriya M, Paris DH, Sunyakumthorn Pet al.Strong interferon-gamma mediated cellular immunity to scrub typhus demonstrated using a novel whole cell antigen ELISpot assay in rhesus macaques and humans. PLoS Negl Trop Dis 2017;11: e0005846.
- Kang SJ, Jin HM, Cho YNet al.Increased level and interferon-gamma production of circulating natural killer cells in patients with scrub typhus. PLoS Negl Trop Dis 2017;11: e0005815.
- Smadel JE, Ley HL Jr, Diercks FHet al.Immunization against scrub typhus. I. Combined living vaccine and chemoprophylaxis in volunteers. Am J Hyg 1951;53: 317–325.
- Ley HL Jr, Diercks FH, Paterson PYet al.Immunization against scrub typhus. IV. Living Karp vaccine and chemoprophylaxis in volunteers. Am J Hyg 1952;56: 303–312.
- Smadel JE, Ley HL Jr, Diercks FHet al.Immunization against scrub typhus: duration of immunity in volunteers following combined living vaccine and chemoprophylaxis. Am J Trop Med Hyg 1952;1: 87–99.
- Smadel JE, Ley HL Jr, Diercks FHet al.Immunity in scrub typhus: resistance to induced reinfection. AMA Arch Pathol 1950;50: 847–861.
- Takahashi K, Urakami H, Tamura A.Purification of cell envelopes of Rickettsia tsutsugamushi. Microbiol Immunol 1985;29: 475–478.
- Seong SY, Park SG, Huh MSet al.Mapping of antigenic determinant regions of the Bor56 protein of Orientia tsutsugamushi. Infect Immun 1997;65: 5250–5256.
- Cho NH, Kim HR, Lee JHet al.The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host–cell interaction genes. Proc Natl Acad Sci USA 2007;104: 7981–7986.