Abstract
The emerging human fungal pathogen Candida auris has been recognized as a multidrug resistant species and is associated with high mortality. This fungus was first described in Japan in 2009 and has been reported in at least 18 countries on five continents. In this study, we report the first isolate of C. auris from the bronchoalveolar lavage fluid (BALF) of a hospitalized woman in China. Interestingly, this isolate is susceptible to all tested antifungals including amphotericin B, fluconazole, and caspofungin. Copper sulfate (CuSO4) also has a potent inhibitory effect on the growth of this fungus. Under different culture conditions, C. auris exhibits multiple morphological phenotypes including round-to-ovoid, elongated, and pseudohyphal-like forms. High concentrations of sodium chloride induce the formation of a pseudohyphal-like form. We further demonstrate that C. auris is much less virulent than Candida albicans in both mouse systemic and invertebrate Galleria mellonella models.
These authors contributed equally: Xiaojuan Wang, Jian Bing, Qiushi Zheng
Introduction
The incidence of non-albicans Candida infections has risen dramatically in recent yearsCitation1. These species are often drug-resistant and difficult to eradicate from the human body, as well as the hospital environment. Candida auris, an emerging fungal pathogen of humans, is often resistant to multiple currently used drugsCitation2–Citation4. Fungemia caused by C. auris is associated with a high mortality rate and therapeutic failureCitation5–Citation7. Since the first report of C. auris infection in Japan in 2009, this fungus has been isolated on five continentsCitation2,Citation4. In a national survey of intensive care units (ICUs), C. auris was reported to account for >5% of candidemia in IndiaCitation5,Citation8. The ecological niches for this fungus remain unidentified. However, their survival and persistence ability on dry surfaces and within hospital environments may contribute to the prevalence and outbreaks of C. auris worldwide.
The morphological diversity and secreted aspartyl proteinases (Saps) are important virulence features of pathogenic Candida speciesCitation9–Citation12. For example, Candida albicans, the major fungal pathogen of humans, has multiple morphological cell types, including the yeast form, hyphae, pseudohyphae, and white, gray, and opaque cell typesCitation9,Citation13. C. albicans can switch among different morphologies under certain culture conditions or during infectionCitation13. Moreover, it has 10 genes encoding Saps that are the major virulence factors responsible for host tissue damageCitation10.
Although C. auris has attracted great attention in the clinical and scientific fields and more than 80 related research and review papers have been published in the past several years, knowledge of the biology and virulence features of this organism is still limited. In this study, we report the first isolate of C. auris in China. We further investigate its morphological characteristics under different culture conditions and its virulence in both mouse and invertebrate Galleria mellonella models.
Results
The first isolate of C. auris in China and analysis of its antifungal susceptibility
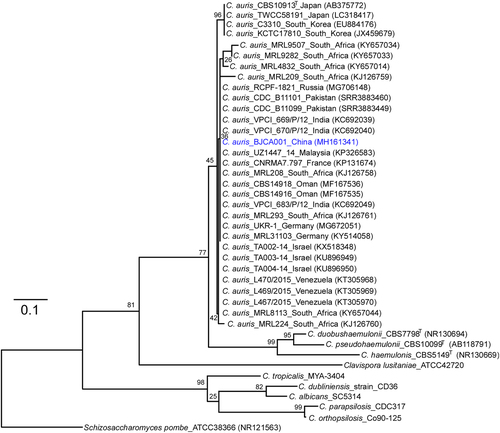
A 76-year-old woman with hypertension and nephritic syndrome was admitted to Peking University People’s Hospital. An isolate of C. auris (BJCA001) was identified from the bronchoalveolar lavage fluid (BALF). We did not isolate C. auris cells from the hospital facility and the other parts of the patient’s body. The strain was initially identified as C. auris by applying in-house MALDI-TOF MS database (with confidence > 1.8). Genomic DNA was extracted for further verification by molecular identification methods. The sequence of the internal transcribed spacers (ITS) of BJCA001 showed 99.9% identity to those of several reported C. auris isolatesCitation3,Citation8. To further verify this finding, we sequenced a C. auris-specific ORF (XM_018314828.1) in strain BJCA001. We then performed a phylogenetic analysis using ITS sequences (Fig. ). To our surprise, strain BJCA001 had very low minimal inhibitory concentration (MIC) values of all the tested drugs (Table ). The MICs of amphotericin B, fluconazole, and anidulafungin were 0.25, 2.0, and 0.12 μg/mL, respectively, whereas the MICs of flucytosine, itraconazole, caspofungin, micafungin, posaconazole, and voriconazole were less than 0.1 μg/mL.
Internal transcribed spacer (ITS) sequences of nuclear rDNA of Candida auris, C. auris closely related species, and Schizosaccharomyces pombe were used. The GenBank accession numbers are shown in the brackets. Candida species used: C. duobushaemulonii, C. pseudohaemulonii, C. haemulonis, C. tropicalis, C. dubliniensis, C. albicans, C. parapsilosis, and C. orthopsilosis. The Maximum-Likelihood phylogenetic tree was generated using RAxML based on the General Time Reversible (GTR) model and Gamma distribution with Invariant sites (G + I). The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are indicated at the branches. The scale bar indicates the nucleotide substitutions per site. Strain BJCA001 is highlighted in blue
Antifungal susceptibility testing of Candida auris BJCA001
Inhibitory effect of copper sulfate (CuSO4) on C. auris
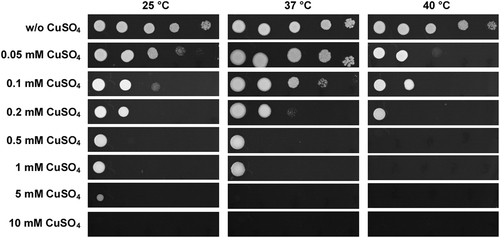
Copper is a potent antimicrobial agentCitation14,Citation15 and copper-based compounds have long been used as biocontrol agents. Therefore, we examined whether CuSO4 had antifungal activity toward C. auris. As shown in Fig. , serial dilution assays demonstrated that CuSO4 exhibited a potent inhibitory effect on the cell growth of C. auris, especially at high temperatures (>37 °C). At 40 °C, 0.5 mM of CuSO4 completely inhibited the growth of C. auris on YPD medium, whereas no growth was observed in the presence of 5 mM and 10 mM of CuSO4 at 37 °C and 25 °C, respectively.
Morphological analysis of C. auris
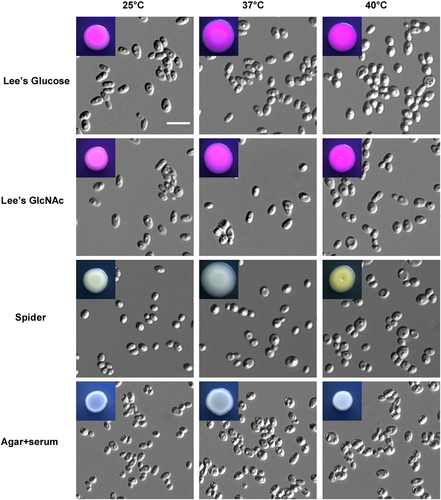
Morphological diversity is a key virulence feature of Candida speciesCitation9,Citation12,Citation16. To investigate whether C. auris had multiple cellular morphologies, we cultured cells using several growth media at 25 °C, 37 °C, and 40 °C (Fig. ). On Lee’s glucose and Lee’s GlcNAc media, C. auris cells exhibited an oval shape at 25 °C and 37 °C and a relative round shape at 40 °C. On Spider and agar plus serum media, cells were round and relatively small. No hyphal and pseudohyphal cells were observed under these conditions.
Cells (1 × 105) were spotted onto different medias and cultured at 25 °C, 37 °C, and 40 °C for five days. Scale bar, 10 μm
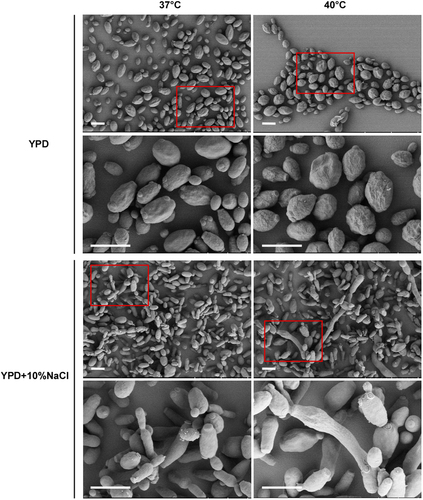
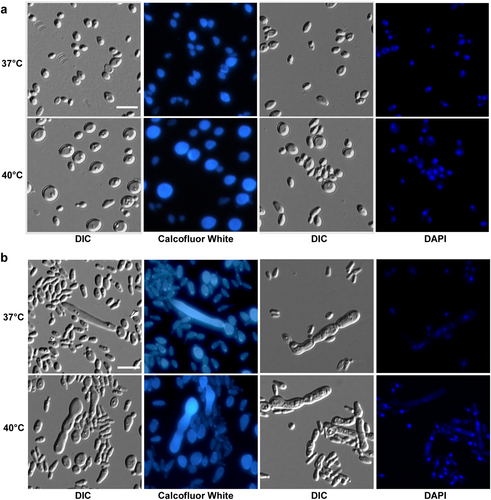
Since it has been reported that C. auris can grow at high salt concentrations and temperaturesCitation17, we next examined the morphology of C. auris on the rich medium YPD and YPD plus 10% NaCl. As shown in Figs. and , C. auris cells were round on regular YPD medium, but they showed an elongated shape on YPD plus 10% NaCl. Elongated cells of C. auris resembled opaque cells of C. albicans in shapeCitation18. Interestingly, we observed a small portion of highly elongated and pseudohyphal-like cells when they were grown on YPD plus 10% NaCl. Multiple nuclei were observed in the elongated cells by staining with DAPI. However, no septin/chitin rings were observed between conjoint cells when stained with Calcofluor white (Fig. ). These results suggest that the high-salt stress could lead to incomplete cell division and the formation of pseudohyphal-like cells.
Cells (1 × 105) were spotted onto different medias and cultured at 37 °C and 40 °C for five days. Cells were collected and stained with DAPI or Calcofluor white. Scale bar, 10 μm. DIC, differential interference contrast
Sap activity of C. auris at different temperatures
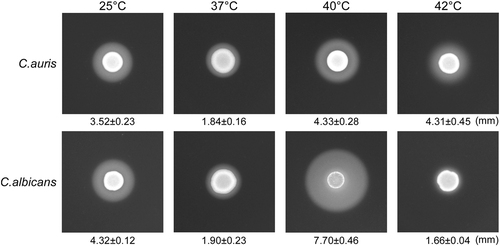
Secreted aspartyl proteinase (Saps) are important virulence factors that cause host tissue damages in Candida speciesCitation10. To uncover this virulence feature, we examined Sap activity in C. auris using YCB-BSA assays. As shown in Fig. , C. auris exhibited high Sap activity at 25 °C, 37 °C, 40 °C, and even 42 °C. C. albicans showed high Sap activity at 25 °C, 37 °C, and 40 °C, but showed significantly reduced Sap activity at 42 °C.
We spotted 5 × 106 cells of C. auris or C. albicans (SC5314) in 5 µL ddH2O onto YCB-BSA medium plates, followed by growth at 25 °C, 37 °C, 40 °C, and 42 °C for five days. The white precipitation zones (halos) around the cell spots indicate Sap-mediated BSA hydrolysis. The width of the precipitation zones is indicated below the corresponding image. Average values of three biological repeats and standard deviations are presented
Virulence of C. auris in mouse systemic infection models
To evaluate the infectious ability of C. auris, we performed both survival and fungal burden assays using mouse systemic infection models. C. albicans (SC5314) was used as a control strain. As shown in Fig. , all mice died at the sixth day post infection when injected with 1 × 106 C. albicans cells per mouse via the vein tail. However, no mice died even at 14 days post-infection after injection 1 × 107 C. auris cells per mouse. Consistent with a previous studyCitation19, our results suggest that C. auris is much less virulent than C. albicans.
a Survival curves of mice injected with C. auris (1 × 107 cells/mouse) and C. albicans (1 × 106 cells/mouse) via the lateral tail vein. Ten mice were used for each strain. b–d Fungal burden assays. Five mice were used for each infection group. Mice were killed for CFU assays at 24 h post-infection. b Each mouse was injected with 2 × 106 cells of C. auris or C. albicans. c Each mouse was injected with 2 × 107 cells of C. auris. d, Each mouse was injected with 1 × 105 cells of C. albicans. * Indicates a significant difference (P vale < 0.01, Student’s t-test, two-tailed) compared with the fungal burden in other organs
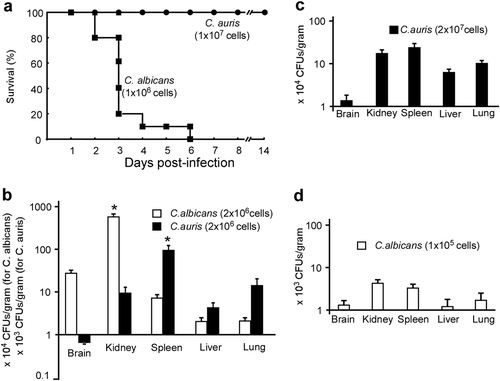
We further found that the fungal burden in kidney, spleen, lung, and liver were comparable when 2 × 107 C. auris cells were injected into each mouse, while fungal burden in the spleen was significantly higher than that in the kidney, lung, and liver when 2 × 106 C. auris cells were injected (Fig. ). For C. albicans, the fungal burden in the kidney was significantly higher than those in the spleen, lung, and liver following injection of 2 × 106 cells. However, the fungal burden in the kidney and spleen were comparable following injection of 2 × 105 C. albicans cells (Fig. ).
Virulence of C. auris in a G. mellonella infection model
To further characterize the virulence features of C. auris, we next performed infection assays using a G. mellonella model. As shown in Figure S1a and S1b, C. auris exhibited reduced virulence compared with C. albicans when 2 × 105 cells were injected into each G. mellonella larva, although the two fungi resulted in comparable survival rates following injection of 1 × 106 cells were injected. Interestingly, C. glabrata was much less virulent than C. auris and C. albicans in the G. mellonella infection model (Figure S1c).
Discussion
The novel fungal species, C. auris, is becoming a serious threat to global health. Since its first description in Japan in 2009Citation2, C. auris infections have been reported in at least 18 countriesCitation4. In the present study, we report the first isolate of C. auris from the bronchoalveolar lavage fluid of a hospitalized woman in Beijing, China. This fungus had not been isolated in China previously perhaps due to technical reasons. C. auris is often misidentified as C. haemulonii using conventional methodsCitation20. We further investigated the morphological phenotypes, susceptibility to antifungal agents and CuSO4, and virulence of C. auris using both mouse and G. mellonella infection models.
Previous investigations have demonstrated multiple geographic origins of C. auris infectionsCitation7. Although most of the previously reported C. auris strains exhibit multidrug resistance, to our surprise, BJCA001 was susceptible to all the tested antifungals (Table ). Therefore, it is unknown whether the drug resistance of previously reported C. auris isolates is a recently evolved feature or whether strain BJCA001 has lost antifungal resistance.
C. auris is difficult to eradicate from the hospital environment due to its ability to survive on surfacesCitation21. Some C. auris strains can even tolerate sanitizers such as sodium hypochlorite and peracetic acidCitation21. Our discovery of its susceptibility to CuSO4 may provide a new avenue to eradicate this organism from the hospital environment (Fig. ).
Consistent with a previous studyCitation19, we did not observe hyphal growth of C. auris under a variety of culture conditions. However, it did exhibit multiple cellular morphologies including round, elongated, and pseudohyphal-like forms (Figs. –). High concentrations of NaCl induce the development of elongated and pseudohyphal-like cells in C. auris (Figs. and ). The mechanism of this induction and the roles of different cell types during infection remain to be investigated. It is unknown whether the elongated cell type of C. auris is similar to the opaque phenotype of C. albicansCitation18.
Virulence assays demonstrated that C. auris exhibited a much lower virulence than C. albicans in both mouse and G. mellonella models (Fig. and S1). However, in the G. mellonella model, C. auris was much more virulent than C. glabrata in terms of survival rates (Figure S1). These results are largely consistent with previous reports that also showed that C. albicans was much more virulent than C. aurisCitation19,Citation22. The discrepancy between the Fakhim studyCitation22 and ours in fungal burden assays could be due to that the different mouse strains and Candida isolates were used. C. albicans and C. auris have comparative Sap activities at both 25 °C and 37 °C. However, the two species differ in their filamentous growth ability, which may contribute to their different abilities to cause infections.
Materials and methods
Strains and culture conditions
Candida auris, Candida albicans, and Candida glabrata strains were routinely grown in YPD medium (20 g/L peptone, 10 g/L yeast extract, 20 g/L glucose; for solid medium, 20 g/L agar was added). YPD, YPD + 10% NaCl, SpiderCitation23, agar (2%) + serum, and modified Lee’s glucose and Lee’s GlcNAc mediaCitation24,Citation25 were used for the morphological assays. Solid media were supplemented with 5 μg/mL phloxine B. For the morphological assays, approximately 1 × 105 cells of C. auris were spotted on the different media and cultured at 25 °C, 37 °C, or 40 °C for five days. YPD medium containing serial concentrations of copper sulfate (CuSO4) was used for the CuSO4 inhibition assays. The C. auris strain was adjusted to 5 × 108 cells/mL, and then 10-fold serial dilutions of cells (2 μL) were spotted onto plates containing different media.
Phylogenetic analysis
The internal transcribed sequences (ITS) of C. auris BJCA001 and previously reported isolates were aligned using mafft v7.015bCitation26. The Maximum- Likelihood phylogenetic tree was generated using RAxML v7.3.2Citation27. The General Time Reversible (GTR) model and Gamma distribution with Invariant sites (G + I) were adopted. Schizosaccharomyces pombe strain ATCC 38366 was used as an outgroup, whereas Candida pseudohaemulonii_CBS10099T, Candida duobushaemulonii_CBS7798T, and C. haemulonis_CBS5149T served as comparators. To better illustrate the phylogenetic position of C. auris, several other Candida species were also included. The ITS sequences of the reported strains were acquired from the GenBank (https://www.ncbi.nlm.nih.gov/) or CGD (http://www.candidagenome.org/) databases directly or extracted from the genome sequencesCitation3.
Minimal inhibitory concentration (MIC) assay
Antifungal susceptibility testing was performed using the Sensititre YeastOneTM methodology (Thermo Scientific, Inc., Cleveland, OH, USA) in accordance with the manufacturer’s instructions. MICs were determined after 24 h of incubation at 35 °C. Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality controls.
Microscopy assay
Cells grown on nutrient agar were collected and used for morphological analysis. Differential interference contrast (DIC) optics was used for standard cellular morphology assays. The 4’, 6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, Inc., Beijing) was used for nuclear staining, and Calcofluor white (Sigma-Aldrich, Inc., Beijing) was used for septa/chitin staining as described previouslyCitation28. Scanning electron microscopy (SEM) assays were performed as described in our previous publicationCitation29. The cell growth conditions are described in the figure legends.
Secreted aspartyl proteinase (Sap) activity assay
Sap activity was tested using the YCB-BSA method as described previouslyCitation30. Briefly, cells of C. auris or C. albicans were initially grown on YPD medium at 30 °C for 24 h. Next, 5 × 106 cells of each strain in 5 μL ddH2O were spotted onto YCB-BSA plates and cultured at 25 °C, 37 °C, 40 °C, or 42 °C for five days. The width of the BSA precipitation rings (halos), reflecting the activity of Saps, was examined on the fifth day. Three biological repeats were performed.
Mouse systemic infection models
All the animal experiments were performed according to the guidelines approved by the Animal Care and Use Committee of the Institute of Microbiology, Chinese Academy of Sciences (approval number: SQIMCAS2018009). Mouse systemic infection experiments were performed as described in our previous reports with slight modificationsCitation30. Five-week-old female BALB/c mice were used in this study. For the survival rate test, 10 mice were used for infection of each strain. 1 × 106 cells of C. albicans or 1 × 107 cells of C. auris in 250 μL 1 × PBS were injected into each mouse via the lateral tail vein.
For fungal burden assays, five mice were used for each intravenous infection of C. auris or C. albicans. The mice were humanely killed by cervical dislocation at 24 h post-infection. The brain, kidney, spleen, liver, and lung of each infected mouse were removed, weighed, and homogenized for colony-forming unit (CFU) analysis on YPD medium.
Galleria mellonella infection model
Galleria mellonella in the final instar larval stage were purchased from Tianjin Huiyu biological technology Co. LTD. (Tianjin, China). Larvae with a similar size (0.3~0.4 g) were used for infection assays. Cells of C. auris, C. albicans, and C. glabrata were cultured on YPD medium at 30 °C for 24 h. Cells were then collected and washed twice with 1 × PBS, and 5 × 106, 1 × 106, or 5 × 105 cells in 10 µL 1 × PBS were injected into each larva using a syringe as described previouslyCitation31. After injection, the larvae were placed in plastic culture dishes and incubated at 30 °C in the dark.
Figure S1
Download TIFF Image (171.6 KB)Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31625002 and 31370175 to GH and 31570139 to LT) and National Science and Technology Major Project (2018ZX10101004-003-002).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41426-018-0095-0).
References
- PfallerMADiekemaDJEpidemiology of invasive candidiasis: a persistent public health problemClin. Microbiol. Rev.200720 133 16310.1128/CMR.00029-061797637
- SatohKCandida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospitalMicrobiol. Immunol.200953414410.1111/j.1348-0421.2008.00083.x
- LockhartSRSimultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological AnalysesClin. Infect. Dis.20176413414010.1093/cid/ciw691
- Spivak, E. S. & Hanson, K. E. Candida auris: an Emerging Fungal Pathogen. J. Clin. Microbiol.56, 10.1128/JCM.01588-17 (2018).
- ClancyCJNguyenMHEmergence of Candida auris: An International Call to ArmsClin. Infect. Dis.20176414114310.1093/cid/ciw696
- ChowdharyASharmaCMeisJFCandida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globallyPLoS Pathog.201713e100629010.1371/journal.ppat.10062905436850
- BormanAMSzekelyAJohnsonEMIsolates of the emerging pathogen Candida auris present in the UK have several geographic originsMed. Mycol.20175556356710.1093/mmy/myw147
- ChowdharyAMultidrug-resistant endemic clonal strain of Candida auris in IndiaEur. J. Clin. Microbiol. Infect. Dis.20143391992610.1007/s10096-013-2027-1
- HuangGRegulation of phenotypic transitions in the fungal pathogen Candida albicansVirulence2012325126110.4161/viru.200103442837
- NaglikJRChallacombeSJHubeBCandida albicans secreted aspartyl proteinases in virulence and pathogenesisMicrobiol Mol. Biol. Rev.20036740042810.1128/MMBR.67.3.400-428.2003193873
- SchallerMSecreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosisMol. Microbiol19993416918010.1046/j.1365-2958.1999.01590.x
- WhitewayMBachewichCMorphogenesis in Candida albicansAnnu Rev. Microbiol20076152955310.1146/annurev.micro.61.080706.0933414452225
- NobleSMGianettiBAWitchleyJNCandida albicans cell-type switching and functional plasticity in the mammalian hostNat. Rev. Microbiol2017159610810.1038/nrmicro.2016.157
- FestaRAHelselMEFranzKJThieleDJExploiting innate immune cell activation of a copper-dependent antimicrobial agent during infectionChem. Biol.20142197798710.1016/j.chembiol.2014.06.0094170187
- LiCXCandida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutaseProc. Natl Acad. Sci. USA2015112E5336E534210.1073/pnas.15134471124586888
- BiswasSVan DijckPDattaAEnvironmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicansMicrobiol. Mol. Biol. Rev.20077134837610.1128/MMBR.00009-061899878
- ChowdharyANew clonal strain of Candida auris, Delhi, IndiaEmerg. Infect. Dis.2013191670167310.3201/eid1910.1303933810747
- AndersonJMSollDRUnique phenotype of opaque cells in the white-opaque transition of Candida albicansJ. Bacteriol.19871695579558810.1128/jb.169.12.5579-5588.1987213989
- Borman, A. M., Szekely, A. & Johnson, E. M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida auris and Other Key Pathogenic Candida Species. mSphere1, 10.1128/mSphere.00189-16 (2016).
- KathuriaSMultidrug-Resistant Candida auris Misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA Sequencing and Its Antifungal Susceptibility Profile Variability by Vitek 2, CLSI Broth Microdilution, and Etest MethodJ. Clin. Microbiol2015531823183010.1128/JCM.00367-154432077
- KeanRSurface disinfection challenges for Candida auris: an in-vitro studyJ. Hosp. Infect.20189843343610.1016/j.jhin.2017.11.015
- Fakhim, H. et al. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses, 10.1111/myc.12754 (2018).
- LiuHKohlerJFinkGRSuppression of hyphal formation in Candida albicans by mutation of a STE12 homologScience19942661723172610.1126/science.7992058
- HuangGN-acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicansPLoS Pathog.20106e100080610.1371/journal.ppat.10008062837409
- Xie, J. et al. White-opaque switching in natural MTLa/alpha isolates of Candida albicans: evolutionary implications for roles in host adaptation, pathogenesis, and sex. PLoS Biol.11, e1001525, 10.1371/journal.pbio.1001525(2013).
- KatohKStandleyDMMAFFT multiple sequence alignment software version 7: improvements in performance and usabilityMol. Biol. Evol.20133077278010.1093/molbev/mst0103603318
- StamatakisARAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed modelsBioinformatics2006222688269010.1093/bioinformatics/btl446
- WangHXDouglasLMAimaniandaVLatgeJPKonopkaJBThe Candida albicans Sur7 protein is needed for proper synthesis of the fibrillar component of the cell wall that confers strengthEukaryot. Cell201110728010.1128/EC.00167-103019807
- DuHRoles of Candida albicans Gat2, a GATA-type zinc finger transcription factor, in biofilm formation, filamentous growth and virulencePloS One20127e2970710.1371/journal.pone.00297073261855
- TaoLDiscovery of a “white-gray-opaque” tristable phenotypic switching system in candida albicans: roles of non-genetic diversity in host adaptationPLoS Biol.201412e100183010.1371/journal.pbio.10018303972085
- JacobsenIDGalleria mellonella as a model host to study virulence of CandidaVirulence2014523723910.4161/viru.274343956497