Abstract
Background: Postoperative ileus (POI) and anastomotic leakage (AL) following colorectal surgery severely increase healthcare costs and decrease quality of life. This study evaluates the effects of reducing POI and AL via perioperative gum chewing compared to placebo (control) on in-hospital costs, health-related quality of life (HRQoL), and assesses cost-effectiveness.
Methods: In patients undergoing elective, open colorectal surgery, changes in HRQoL were assessed using EORTC-QLQ-C30 questionnaires and costs were estimated from a hospital perspective. Incremental cost-effectiveness ratios were estimated.
Results: In 112 patients, mean costs for ward stay were significantly lower in the gum chewing group when compared to control (€3522 (95% CI €3034–€4010) versus €4893 (95% CI €3843–€5942), respectively, p = .020). No differences were observed in mean overall in-hospital costs, or in mean change in any of the HRQoL scores or utilities. Gum chewing was dominant (less costly and more effective) compared to the control in more than 50% of the simulations for both POI and AL.
Conclusion: Reducing POI and AL via gum chewing reduced costs for ward stay, but did not affect overall in-hospital costs, HRQoL, or mapped utilities. More studies with adequate sample sizes using validated questionnaires at standardized time points are needed.
Introduction
Postoperative outcomes following colorectal surgery have markedly improved since the implementation of fast-track protocols [Citation1]. However, the incidence of severe complications including anastomotic leakage (AL) (up to 19%) and postoperative ileus (POI) (up to 45%) remains substantial [Citation1–4]. Postoperative complications strongly increase healthcare costs and negatively impact both short- and long-term quality of life (QoL) [Citation5–12].
In a recent randomized trial, perioperative gum chewing significantly reduced POI (14/52 patients versus 29/60 patients) and AL (2/52 patients versus 8/60 patients) when compared to placebo [Citation4]. However, given the current trend of rising healthcare expenditures, new interventions cannot be implemented in routine care based on clinical efficacy alone. Economic evaluations are warranted to determine the value for money of an intervention [Citation13]. While perioperative gum chewing had a clear beneficial effect on clinical outcomes [Citation4], the effects on in-hospital costs and QoL are unknown.
This study evaluates the effects of reducing POI and AL via perioperative gum chewing compared to control on in-hospital costs and health-related quality of life (HRQoL), and estimates cost-effectiveness in patients undergoing colorectal cancer surgery.
Materials and methods
This is a substudy from a previous multicenter, single-blind, randomized controlled trial in two large Dutch tertiary referral hospitals (Catharina Hospital and Orbis Medical Center) [Citation4]. The original trial was conducted according to the Declaration of Helsinki, and was approved by the local Medical Ethics Committee on 19 November 2008 (No 08-T-70). All the patients signed informed consent prior to participation.
Patient population
Patients were included in the original trial as described elsewhere [Citation4]. Briefly, patients were eligible for inclusion if aged 18 or older and undergoing elective, open colorectal surgery. Patients were excluded in case of the presence of peritoneal carcinomatosis, inflammatory bowel disease, a history of gastric or esophageal surgery, a disturbance of acetylcholine metabolism owing to neurological disease or depression, pre-existing ileostoma, allergy to mint, or if using agents influencing gut motility (including opioids) or acetylcholine metabolism.
Interventions
Patients were randomly allocated to the intervention or placebo group. Patients in the intervention group started chewing gum at least three hours prior to the start of the surgery, and again three hours after end of the surgery. Patients in the control group received a placebo dermal patch three hours before the start of the surgery and were instructed to not chew gum. Both the intervention and control were discontinued when patients started an oral diet [Citation4]. Further details on the intervention and control are described in the original trial report [Citation4].
Clinical outcomes
The primary outcome of the original study was length of stay. Secondary outcome measures included POI and AL [Citation14].
Health-related quality of life and utilities
Health-related quality of life was a secondary endpoint in the original trial [Citation4] and was assessed preoperatively and postoperatively using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (EORTC QLQ-C30 version 3.0) [Citation15]. The time point for completion of the postoperative QLQ-C30 questionnaire was not standardized. Missing data were imputed according to the EORTC guidelines [Citation15].
Utilities are preference weights for different health states in which more preferred health states receive more weight. Utility scores range between 0 (death) and 1 (perfect health). Mapping was done to compute utility scores from HRQoL-scores [Citation16]. The differences between the pre- and post-operative HRQoL and utility scores were reported as changes in mean.
Costs
Costs were calculated from the hospital perspective. In-hospital costs were determined by retrospectively extracting financial data from the electronic patient registration system. Additionally, costs for readmission within 1 year after surgery due to complications stemming from the primary operation were included. Costs were estimated using a bottom-up approach. Only the standardized unit costs of the Catharina Hospital were available; consequently these were also used for units of care from the Orbis Medical Centre. Costs were categorized into fees for the primary operation, pathology, laboratory tests, radiological examinations, intramural consults, therapeutic interventions under local anesthesia, re-operations, and admission costs for staying in the surgical ward and/or intensive care unit. Monetary units are expressed in Euros (€) and were indexed for the year 2011, as this was the year in which most patients were included in the original trial [Citation4].
Cost-effectiveness evaluation
The implementation of a new treatment depends on the maximum amount of money that society is willing to pay for a gain in effectiveness, which is termed the ‘threshold’ or ‘ceiling-ratio’. The gain in effectiveness is most commonly expressed in quality-adjusted life years (QALYs) [Citation17]. However, it was considered inappropriate to estimate QALYs from the available data, since the time point for postoperative QoL assessment was not standardized, and the required utility scores needed to be mapped from the EORTC QLQ-C30 scores. Instead, cost-effectiveness was assessed using the two clinical outcomes that were significantly reduced by perioperative gum chewing in the original trial (POI and AL) [Citation4]. A cost-effectiveness analysis was performed by determining the incremental cost-effectiveness ratio (ICER) of gum chewing in comparison with the control. ICERs were calculated by dividing the incremental costs by the incremental effects; the sample uncertainty concerning the ICERs was quantified by conducting 5000 bootstrapping replications. The ICERs are presented on a cost-effectiveness plane, which is divided into four quadrants. When the intervention is more effective and less costly than the comparator, the ICERs lie in the southeast quadrant and the intervention is considered dominant. Conversely, when the intervention is less effective and more costly, the ICERs lie in the northwest quadrant and the intervention is considered inferior. When the intervention is more costly and yet more effective, the ICERs lie in the northeast quadrant and when intervention is less costly and less effective, the ICERs lie in the southwest quadrant. A treatment is cost-effective when it is dominant.
As there is uncertainty surrounding the threshold per avoided incident of POI or AL, cost-effectiveness acceptability curves (CEAC) were used to present the results of the bootstrapping. A CEAC is a graphic representation of the uncertainty in differences in cost and effect between the two groups, showing the probability of an intervention being cost-effective for a wide range of threshold values.
Statistical analysis
Normally distributed data are presented as means (standard deviation) and were tested using the unpaired t-test, while non-parametric data are presented as median [range] and were tested using the Mann–Whitney U test. Categorical variables were tested with the Χ2 test. Cost data are presented as means, medians and 95% confidence intervals, as is recommended for cost-analysis studies [Citation18]. Cost means are compared using the unpaired t-test.
As perioperative gum chewing was hypothesized to affect only the postoperative course and not the type of operation and subsequent pathology analysis of the resected tissue, a sensitivity analysis was performed in which only postoperative costs were included.
Furthermore, as the original study was not powered to detect changes in HRQoL or costs, a post hoc power analysis was performed using an α equal to 0.05 to assess the statistical power of the current analysis. The statistical power (mean ± S.D.) of preoperative, postoperative, and delta change HRQOL scores were 0.29 ± 0.26, 0.12 ± 0.2 and 0.07 ± 0.06 respectively. The statistical power analysis for total costs, total admission costs and total readmission costs showed a power of 0.04, 0.05 and 0.05 respectively.
Results
Patient characteristics
A total of 120 patients were included in the original study, of which 58 patients were randomized to the intervention group and 62 patients to the control group. Groups were similar at baseline with regard to demographic variables, co-morbidities, and operative details (, adapted with permission from Heijkant et al. [Citation4]). Eight patients were excluded after randomization due to technical reasons [Citation4] and were not included in any analysis in this paper.
Table 1. Baseline characteristics.
Clinical outcomes
As noted in the original study report, incidence of POI was significantly lower in the gum chewing group compared to the control group (14/52 patients versus 29/60 patients, respectively, p = .02) [Citation4]. Furthermore, fewer patients in the gum chewing group experienced AL, in comparison with the control group (2/52 patients versus 8/60 patients, respectively, p = .03) [Citation4].
Health-related quality of life and utilities
Out of 112 patients, 95 patients completed the preoperative baseline questionnaire and 69 patients completed the postoperative questionnaire. A total of 66 patients completed both the pre- and postoperative questionnaires. The time point of completing the postoperative questionnaire was similar in both groups: median postoperative day 6 (range 2–39) in the gum chewing group and median postoperative day 8 (range 2–70) in the control group (p = .19). On average, 0.31 out of 30 questions were imputed per questionnaire. Pre- and postoperative HRQoL and mapped utility scores, and the change in scores between the two time points, are summarized in . The pre- and postoperative emotional functioning scale showed a significantly higher mean score in the gum chewing group (p < .01 and p = .02, respectively). The preoperative fatigue scale showed a significantly lower score in the gum chewing group (p = .03). Preoperatively, patients in the control group had significantly more appetite loss in comparison with the patients in the gum chewing group (p = .02). No other significant differences were seen between the groups.
Table 2. Health-related quality of life and mapped utility scores in gum chewing versus control.
At baseline, patients in the gum-chewing group showed a higher score in utilities (p = .01); however, postoperative utilities and change in utilities between the two time points were similar between the groups (p = .43 and p = .53, respectively).
Costs
In-hospital costs are presented in . Total costs for admission (p = .71), total costs for readmission (p = .78), and total costs for admission and readmission combined (p = .85) were not statistically different between the groups. However, mean costs for ward stay were lower in the gum-chewing group when compared to the control group (p = .02). In the sensitivity analysis, which excluded costs for primary operation and pathology analysis, there were no differences in postoperative admission costs, or in the combined postoperative admission and readmission costs (p = .67 and p = .82, respectively).
Table 3. In-hospital costs.
Cost-effectiveness analysis
Incremental costs, effects, and ICERs relating POI and AL were estimated using the total admission and readmission costs combined. The incremental mean costs divided by the incremental mean effects resulted in an ICER of −8450 per AL and −2414 per POI (). Both ICERs were negative, indicating that the gum-chewing group dominated the control group due to lesser costs and positive effect (i.e. reduction in POI or AL).
Table 4. Incremental costs, effects and ICERs of the outcomes of the study.
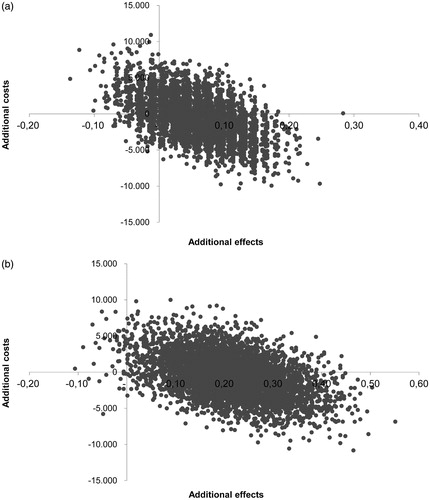
The uncertainty analyses of the ICERs representing 5000 bootstrap replications are presented in the cost-effectiveness plane (). For AL, 56% of the bootstrapped replications were located in the southeast quadrant, indicating the dominance of gum chewing (i.e. positive effect at lesser costs), while 9% of the bootstrapped replications were located in the northwest quadrant, indicating inferiority (). For POI, the cost-effectiveness plane shows that 59% of the replications were located in the southeast quadrant, indicating dominance, while 1% of the replications were located in the northwest quadrant, indicating inferiority ().
Figure 1. (a) Cost-effectiveness plane of gum chewing versus placebo expressing costs per avoided incident of anastomotic leakage. (b) Cost-effectiveness plane of gum chewing versus placebo expressing costs per avoided incident of postoperative ileus.
represents the CEAC for AL and POI. The probability of gum chewing being cost-effective in reducing POI and AL is >50% regardless of the applied threshold for willingness to pay. The sensitivity analyses for the cost-effectiveness analyses including only the postoperative costs are summarized in . Excluding costs for the primary operation and pathology analysis increased the probability of gum chewing being cost-effective for both POI and AL ( and ).
Figure 2. Cost-effectiveness acceptability curves of postoperative ileus (POI) and anastomotic leakage (AL).
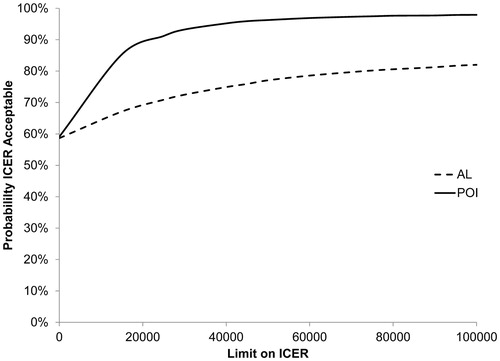
Figure 3. Sensitivity analysis of cost-effectiveness acceptability curves for postoperative admission costs (AL: anastomotic leakage; POI: postoperative ileus).
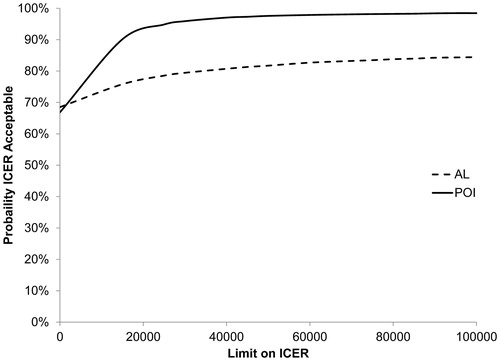
Figure 4. Sensitivity analysis of cost-effectiveness acceptability curves for postoperative admission + readmission costs (AL: anastomotic leakage; POI: postoperative ileus).
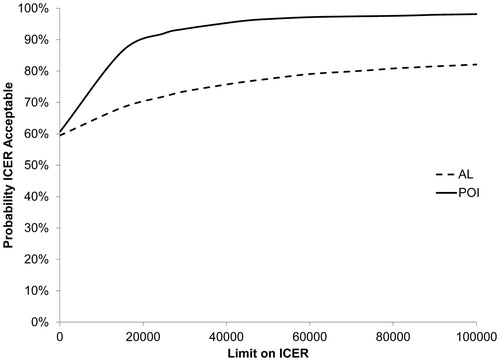
Table 5. Sensitivity analysis of cost-effectiveness analysis.
Discussion
This study aimed to estimate the cost-effectiveness of perioperative gum chewing in colorectal cancer patients and to assess the effects on HRQoL, utilities, and in-hospital costs. Based on our data, gum chewing may be cost-effective in reducing POI, and to a lesser extent in reducing AL, while no clear benefit of gum chewing was observed on HRQoL, mapped utilities, and overall in-hospital costs. However, gum chewing was associated with a significant reduction in costs for ward stay.
The probability of gum chewing being dominant over the control was >50% for both POI and AL, regardless of the threshold value. In a recent randomized controlled trial comparing postoperative gum chewing (starting on day 1 after surgery) with standard care, Atkinson et al. found no differences in net monetary benefit between the groups and concluded that gum chewing is unlikely to be cost-effective [Citation19]. However, comparing the study by Atkinson et al. with our findings is inappropriate due to important differences between studies regarding (A) timing of gum chewing (postoperative [Citation19] versus perioperative [Citation4]), (B) clinical outcomes (no benefits of gum chewing [Citation19] versus a reduction of POI and AL [Citation4]), (C) cost perspective (societal [Citation19] versus hospital [Citation4]), and (D) measurement of effects in cost-effectiveness analysis (QALY [Citation19] versus POI/AL [Citation4]). Further studies are thus needed to compare the results of the current economic evaluation.
Perioperative gum chewing reduced costs for ward stay, but did not reduce overall costs. We hypothesize that the reduction in ward stay costs in the gum chewing group is directly linked to the reduction of POI and therefore, shorter length of stay [Citation4]. Both POI and AL are known to severely increase healthcare costs [Citation5–7], as was visible in our data set when comparing total costs between patients with and without POI or AL (data not shown). As gum chewing conferred a reduction of both POI and AL [Citation4], the lack of a significant difference between the groups in mean overall costs may be explained by the fact that the original study [Citation4] was not powered to detect differences in costs. In addition, as opposed to cost-effectiveness analyses, the crude comparison between groups using p-values may not be the optimal method for detecting any cost-reducing effects of an intervention [Citation18].
Surgery for colorectal cancer has been shown to improve quality of life 3 months after surgery [Citation20]. Conversely, in our cohort, mean HRQoL scores and mapped utilities deteriorated in both groups after surgery. This may be explained by the fact that most patients completed the postoperative questionnaire within 10 days after surgery while still recovering from the surgical trauma regardless of the complications. In general, QoL may not return to preoperative levels within the first 3 weeks after surgery even in the absence of complications [Citation21], while postoperative complications can have both short- and long-term negative effects on QoL [Citation9–12]. Reducing postoperative complications may limit the decrease in QoL shortly after surgery; however, this could not be demonstrated in our data. Future studies with repeated QoL assessments using validated questionnaires at standardized time points are needed to provide more insight into the effects on postoperative QoL by interventions that reduce complications.
Several limitations are present in the current study. As noted, the original trial [Citation4] was not powered to detect differences in in-hospital costs or quality of life, but rather on clinical outcomes. Moreover, there was a significant number of missing QLQ-C30 questionnaires and consequently the results were based on a limited sample size. The estimation of QALYs was inappropriate given the non-standardized time point for postoperative QoL assessment and the necessity of mapping the HRQoL scores to the required utility scores. A cost-utility analysis, as is recommended for current economic evaluation studies, could therefore not be performed. Furthermore, the obtained costs were from a hospital perspective only and productivity costs were not included. Lastly, this study was conducted as a trial-based economic evaluation, which may limit the generalizability of the results [Citation22].
Overall, our data suggest that reducing POI and AL via perioperative gum chewing reduces costs for ward stay, but does not reduce overall in-hospital costs or confer a beneficial effect on HRQoL or mapped utilities. Although the CEAC curves suggest that gum chewing may be cost-effective for a wide range of threshold values, we cannot conclude with certainty that gum chewing is cost-effective in reducing POI or AL due to the limitations of our data. We recommend future studies with appropriate sample sizes to incorporate repeated assessments of QoL and utilities using validated questionnaires (e.g. EORTC QLQ C-30, EQ-5D-5L) at standardized time points. In addition, the use of a productivity cost questionnaire and medical cost questionnaire is recommended in order to include a societal perspective in cost-analyses.
Acknowledgements
The authors thank Tim Brouwers, Anja Verdonschot and Ruud Schurgers for their contributions in obtaining the financial data.
Disclosure statement
The authors report no conflicts of interest.
Funding
The authors declare no funding associated with this manuscript.
References
- Spanjersberg WR, Reurings J, Keus F, et al. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. 2011;CD007635.
- Daams F, Luyer M, Lange JF. Colorectal anastomotic leakage: aspects of prevention, detection and treatment. World J Gastroenterol. 2013;19:2293–2297.
- McDermott FD, Heeney A, Kelly ME, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102:462–479.
- van den Heijkant TC, Costes LM, van der Lee DG, et al. Randomized clinical trial of the effect of gum chewing on postoperative ileus and inflammation in colorectal surgery. Br J Surg. 2015;102:202–211.
- Ashraf SQ, Burns EM, Jani A, et al. The economic impact of anastomotic leakage after anterior resections in English NHS hospitals: are we adequately remunerating them? Colorectal Dis. 2013; 15:e190–e198.
- Iyer S, Saunders WB, Stemkowski S. Economic burden of postoperative ileus associated with colectomy in the United States. J Managed Care Pharm 2009;15:485–494.
- Govaert JA, Fiocco M, van Dijk WA, et al. Costs of complications after colorectal cancer surgery in the Netherlands: building the business case for hospitals. Eur J Surg Oncol 2015;41:1059–1067.
- Barletta JF, Senagore AJ. Reducing the burden of postoperative ileus: evaluating and implementing an evidence-based strategy. World J Surg. 2014;38: 1966–1977.
- Brown SR, Mathew R, Keding A, et al. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg. 2014;259:916–923.
- Bloemen JG, Visschers RG, Truin W, et al. Long-term quality of life in patients with rectal cancer: association with severe postoperative complications and presence of a stoma. Dis Colon Rectum. 2009;52: 1251–1258.
- Bosma E, Pullens MJ, de Vries J, et al. The impact of complications on quality of life following colorectal surgery: a prospective cohort study to evaluate the Clavien-Dindo classification system. Colorectal Dis. 2016;18:594–602.
- Marinatou A, Theodoropoulos GE, Karanika S, et al. Do anastomotic leaks impair postoperative health-related quality of life after rectal cancer surgery? A case-matched study. Dis Colon Rectum. 2014;57:158–166.
- Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996.
- Vather R, Trivedi S, Bissett I. Defining postoperative ileus: results of a systematic review and global survey. J Gastrointest Surg. 2013;17:962–972.
- Fayers PMAN, Bjordal K, Groenvold M, et al. on, Group. botEQoL. The EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer; 2001.
- Versteegh MM, Leunis A, Luime JJ, et al. Mapping QLQ-C30, HAQ, and MSIS-29 on EQ-5D. Med Decis Mak. 2012;32:554–568.
- Bleichrodt H, Quiggin J. Life-cycle preferences over consumption and health: when is cost-effectiveness analysis equivalent to cost-benefit analysis? J Health Econ. 1999;18:681–708.
- Mani K, Lundkvist J, Holmberg L, et al. Challenges in analysis and interpretation of cost data in vascular surgery. J Vasc Surg. 2010;51:148–154.
- Atkinson C, Penfold CM, Ness AR, et al. Randomized clinical trial of postoperative chewing gum versus standard care after colorectal resection. Br J Surg. 2016;103:962–970.
- Ronning B, Wyller TB, Nesbakken A, et al. Quality of life in older and frail patients after surgery for colorectal cancer – a follow-up study. J Geriatric Oncol. 2016;7:195–200.
- Dowson HM, Ballard K, Gage H, et al. Quality of life in the first 6 weeks following laparoscopic and open colorectal surgery. Value Health. 2013;16: 367–372.
- Drummond M, Manca A, Sculpher M. Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int J Technol Assess Health Care. 2005;21:165–171.
