Abstract
Background and aims
There is no clear guideline nor protocol for macroscopic examination of the gallbladder, leaving surgeons extemporaneous in regard of gallbladder examination in selective histopathologic policy. The purpose of this article is to describe a surgical approach for adequate macroscopic inspection of the gallbladder.
Materials and methods
The described practical method was developed in collaboration between surgeons and pathologists. This method was introduced in 2011 and implemented in 2012. We retrospectively reviewed the number of cholecystectomies and number of histopathologic examinations between 2006 and 2017, using our own patient database. We used the Netherlands Cancer Registry (NCR) to examine the incidence of gallbladder cancer patients before and after implementation of the selective policy in our hospital. In addition to the method, we depict several frequent macroscopic abnormalities in order to provide some examples for surgical colleagues.
Results
Since implementation of the selective policy, 2271 surgical macroscopic gallbladder examinations were performed. As a result, we observed a significant decrease from 83% in 2012 to 38% in 2017, in histopathologic examination of the gallbladder following cholecystectomy. We observed a stable trend of gallbladder carcinoma in the same period (0.17%, n = 4 during 2006–2011 and 0.26%, n = 6 during 2012–2017).
Conclusion
A simple, valid and easy method is described for future macroscopic analysis by the surgeon following a cholecystectomy.
Introduction
The introduction of evidence-based medicine aimed at a safer, more consistent and more cost effective care. The paradigm shift included many clinical guidelines, protocols and standard operating procedures and therefore achieved many successes in daily clinical practice [Citation1]. Despite the continuous flux of new published guidelines, there still remain knowledge gaps in daily clinical practice. One of these gaps is a method for gallbladder examination after cholecystectomy to macroscopically assess whether a gallbladder specimen needs histopathologic evaluation.
Historically, all gallbladders are examined by a pathologist to search for the presence of malignancy. In an attempt to reduce the numbers of unnecessary pathologic examinations a selective histopathologic (Sel-HP) policy has been introduced. This resulted in two options after cholecystectomy: either routine histopathologic (Rout-HP) or Sel-HP examination of the gallbladder. Several hospitals already implemented a Sel-HP policy. To this extent, the Dutch national guideline regarding gallbladder policy changed in 2016 favouring a Sel-HP over Rout-HP policy [Citation2–4]. This leads to a shift in the macroscopic examination of the gallbladder from the pathologist to the surgeon. Due to this change in the Netherlands, the surgeon should now perform a macroscopic examination of the gallbladder, and decide whether additional histopathologic assessment is warranted.
To the best of our knowledge, up to this date, there is no publication, clear guideline nor standard operating procedure which explains how to perform a proper clinical macroscopic examination of a surgically removed gallbladder. Without a clear protocol the surgeons are left extemporaneous to perform a safe and consistent gallbladder examination. The aim of this study was to suggest a simple, valid and easy method to perform a macroscopic examination of the gallbladder by the surgeon following a cholecystectomy.
Materials and methods
The method of surgical gallbladder examination used in our hospital was developed in close collaboration with the department of pathology. After evaluation of the existing literature the department of surgery decided to change to a Sel-HP examination of the gallbladders antecedently to any change in national guidelines. As of 2010, all gallbladders were macroscopically examined according to a standard protocol following a cholecystectomy, by the surgeon prior to sending it to the pathologist for Rout-HP examination. We prospectively evaluated the agreement between surgeon and pathologist concerning the macroscopic examination in 319 gallbladder specimens prior to implementing the policy change. [Citation5]. Based on this analysis we changed the hospitals’ policy in 2012 to a Sel-HP examination where the surgeon macroscopically examines the gallbladder and consequently decides whether further histopathologic examination is warranted.
To investigate the implementation of the surgical method we retrospectively reviewed the patient database between January 2006 and January 2018. All patients undergoing a cholecystectomy at Máxima Medical Centre (MMC) Veldhoven/Eindhoven, the Netherlands, were included in this study. MMC is a large teaching hospital serving a population of 350,000 patients in a semi-rural area in the south-eastern part of the country. The number of cholecystectomies and the number of histologically examined specimens per year prior to, and after the change in policy were determined. The Netherlands Cancer Registry (NCR) of the ‘Netherlands Comprehensive Cancer Organisation’ (IKNL) was used for identifying the number of GBC during this time period. All data were analyzed using IBM SPSS Statistics version 24 (IBM Corp., Armonk, NY). A descriptive analysis of the cases is presented.
Macroscopic examination by the surgeon
The technique used in our hospital is illustrated in and demonstrated in the supplementary video (appendix). The macroscopic examination was conducted immediately after the cholecystectomy, and prior to sending the gallbladder specimen to the pathology department. Usually, during laparoscopic surgery, the gallbladder is removed with or without the use of an endobag by one of the trocar incisions. First, the gallbladder is removed from the endobag () and externally irrigated with saline solution, to remove debris and adhesive tissue from the gallbladder (). Thereafter, the surface is observed closely in search of any abnormalities (). This includes; ulcers, polyps, perforations, masses, indurations, calcifications or (focal) wall thickening. Secondly, the surgeon incises the gallbladder longitudinally along the serosal surface preserving the cystic duct and gallbladder bed to the liver margins (), followed by irrigation of the mucosa with saline solution (). Any stones or debris are hereby removed allowing a meticulous inspection of the mucosal wall. The serosa and mucosa are observed and palpated thoroughly in search of any of the aforementioned abnormalities ().
Figure 1. (a–f) Sequential steps of macroscopic examination of the gallbladder by the surgeon. Full video is available in the Supplementary appendix.
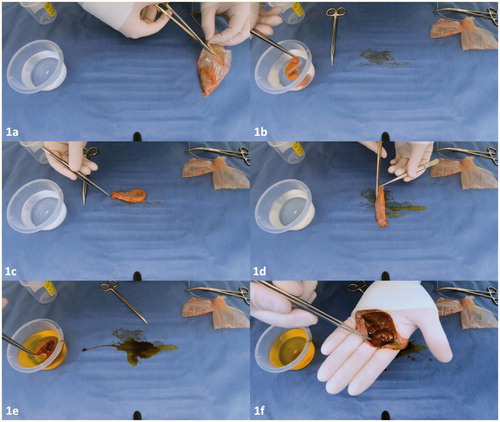
After inspection the surgeon records his/her conclusions in the operating report and has to decide whether further histopathologic examination by the pathologist is necessary. Reasons that warrant additional histopathologic examination are outlined in . Above all, if there is any doubt regarding the macroscopic analysis or if the surgeon does not feel adequate to inspect the gallbladder, additional histopathologic examination is advised.
Table 1. Reasons for additional histopathologic examination.
For this procedure, merely surgical gloves, surgical scissors, surgical forceps and some saline solution are required. These materials are already present and used during the previously performed cholecystectomy. The surgical examination and the administrative procedures are performed in less than 5 min. It is usually performed following placement of the final suture and the sign-out procedure of the surgical team.
If there is an indication for histopathologic examination, the gallbladder is placed in formaldehyde and sent to the department of pathology for further evaluation. Observed abnormalities are described in the pathologic application form. The pathologist or lab technician re-examines the gallbladder and samples are taken either routinely or selectively from any macroscopic abnormalities and from the cystic duct.
Macroscopic gallbladder appearance and gross abnormalities with high risk of GBC
The gallbladder is a sac-like organ that is divided into the fundus, body, infundibulum (also known as Hartmann pouch), neck and cystic duct. An endoluminal transition surface zone from the neck to the cystic duct may contain undulating folds or valves known as ‘spiral valves of Heister’ [Citation6,Citation7]. A normal gallbladder has a smooth and glistening serosa and is endoluminally lined with folded mucosa [Citation8–11] (). If intact, the gallbladder is usually filled with green-brown bile. Discolored bile may be indicative of a gallbladder outlet obstruction, either by stones or a tumor. Acute cholecystitis may present as an enlarged and distended gallbladder, with thickened wall with edema and hemorrhage (), congested vessels, serosal and or mucosal exudate, ulcers with blood clots and or pus. In case of an acute cholecystitis or cholangitis an enlarged lymph node may be situated within the triangle of Calot, known as Mascagni’s or Lund’s node and sometimes erroneously referred to as Calot’s node [Citation12].
Figure 2. (a, b) Macroscopic images of a normal appearance of the gallbladder mucosa. Please notice the smooth and glistening serosa and folded mucosa. The numerous spots are cholesterolosis, which is accumulation of lipoma filled macrophages in the mucosa. (c, d) Macroscopic image of acute cholecystitis gallbladder.
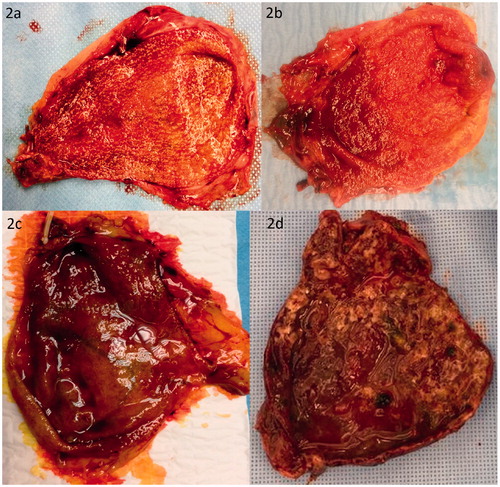
Although several cases of incidental gallbladder cancers present initially as an acute cholecystitis, evidence for routine histopathologic examination of all acute cholecystitis cases is lacking. Due to the disturbed serosal and mucosal architecture in acute cholecystitis, macroscopic examination is not thought to be reliable enough to exclude underlying malignancy. Therefore, routine pathology of the acute cholecystitis specimens is currently still advised.
There are a few benign gallbladder morphologies which may present macroscopic abnormalities. Some gallbladders present with Rokitansky-Aschoff sinuses also known as adenomyomatosis (), which is a benign condition and is characterised by pseudodiverticula of the gallbladder reaching into lamina muscularis as a result of hyperplasia and herniation into the gallbladder lumen [Citation13]. Distinguishing between these sinuses and a possible (pre-)malignancy can be challenging.
Figure 3. (a) Macroscopic image of a 1.8 cm large adenomyoma of the gallbladder at the hepatic side after 24 h of formaldehyde fixation. (b, c) Macroscopic image of a hyperplastic polyp is indicated with arrow (◄). (d, e) Macroscopic image of a multiple cholesterol gallbladder polyps indicated with arrows (◄).

Gallbladder polyps are found in 0.3–12% of healthy individuals [Citation14,Citation15], and may present as either a benign or malignant aetiology. Gallbladder polyps are usually preoperatively discovered by imaging and form a potential indication for cholecystectomy, due to the possible malignant aetiology. Surgical treatment for gallbladder polyps is primarily based on size, and cholecystectomy is advised for all polyps ≥ 10 mm. Nonetheless, polyps are mostly pseudopolyps rather than true polyps. True polyps arise from increased proliferation of epithelium, and can be divided into 2 main groups: adenomas and hyperplastic polyps (). Pseudopolyps do not arise from increased proliferated epithelium but rather as a result of inflammation (inflammation polyp), cholesterol depositions (cholesterol polyp, ) or hyperplastic muscle with diverticula (adenomyoma, as described above). In theory, pseudopolyps can be neoplastic based on proliferation of fat tissue (lipoma), smooth muscle cells (leiomyoma) and (peri)neural cells (neurofibroma/schwannoma) [Citation16]. Recommendations regarding routine or selective histopathologic examination in case of gallbladder polyps are lacking, but in view of the possible malignant transformation it is sensible that all gallbladders containing polyps should undergo histopathologic examination by the pathologist.
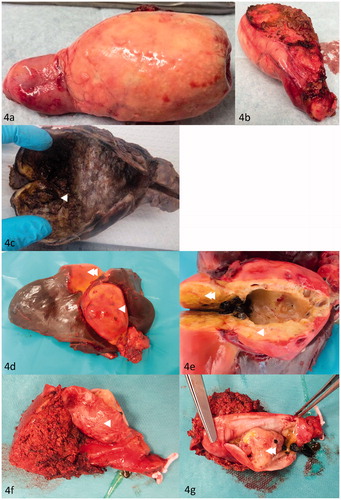
There are several abnormalities associated with an increased risk for gallbladder cancer (GBC). Primary Sclerosing Cholangitis (PSC) has been associated with increased risk of developing gallbladder cancer [Citation17]. The estimated lifetime incidence of GBC in patients with PSC is 3–14% [Citation18, Citation19]. Some studies show that 5–28% of patients with initial presentations of Mirizzi-syndrome turned out to contain GBC [Citation20,Citation21]. Other macroscopic gallbladder abnormalities include: porcelain gallbladder (), metastasis or the very rare neuroendocrine neoplasm of the gallbladder. The latter two are macroscopically indistinguishable from gallbladder carcinoma (). All above mentioned abnormalities should undergo additional histopathology in search of malignancy.
Figure 4. (a, b) Macroscopic image of a porcelain gallbladder. (c) Macroscopic image of gallbladder containing T2 GBC in the fundus of the gallbladder. Mucosal irregularity containing GBC is indicated with arrow (◄). (d, e) Macroscopic image of gallbladder containing T3 GBC in the fundus of the gallbladder (◄) and liver metastasis (◄◄). (f) Macroscopic image of gallbladder with liver resection (◄). (g) Macroscopic image of intracholecystic papillary neoplasm with highgrade dysplasia (◄◄).
Results
During the study period (2006–2017), a total of 4605 consecutive patients underwent a cholecystectomy in MMC for several indications. Results are depicted in for total cholecystectomies and the number of histopathologic examinations. In the first period (2006–2011, n = 2334) gallbladders were routinely sent to the pathologist. In the second period (2012–2017, n = 2271) gallbladders were macroscopic examination by the surgeon of which 47.7% (n = 1083) were selectively sent to the pathologist. The number of pathology assessments decreased from 83% in 2012 to 38% in 2017. In the period (2006–2011) during Rout-HP examination the incidence of GBC was 0.17% (n = 4). Once the macroscopic examination of the gallbladder was introduced in 2012 the number of GBC was 0.26% (n = 6 cumulative over the years 2012–2017).
Table 2. Number of cholecystectomies per year and the number of histopathologic gallbladder specimens sent to department of pathology.
Discussion
This report demonstrates an easy and valid way to perform macroscopic examination of the gallbladder by the surgeon. This simple technique was developed in collaboration between the departments of surgery and pathology. This was based on initial experience and current practice where a lab technician or pathologist inspects the mucosa and routinely or selectively takes samples from the specimen. Similar methods have not previously been described for clinical purpose, only in research setting [Citation22]. Currently, we have over 6 years’ experience with a Sel-HP and performed over 2000 surgical macroscopic examinations. By implementation of the method, we greatly reduced the number of gallbladder specimens sent to the pathologist, without a decline in finding GBC cases.
An analysis of the current Dutch guidelines in all Dutch hospitals learns that half of the hospitals have implemented a Sel-HP [Citation23]. Still 65% of gallbladders are sent for histopathologic examination while this number could be reduced to as low as 17–23% [Citation2,Citation23]. To date, adjustment of the Dutch guideline did not result in the expected decline of histopathologic gallbladder examinations nationwide [Citation24]. Most surgeons are open to switch to a Sel-HP, but feel the evidence is lacking to do so safely. An important reason why individual doctors or clinics have not introduced a Sel-HP could be the lack of a standardized method for gallbladder examination. On the other hand, it could also be caused by the fear of missing an occult carcinoma or a force of habit to submit any removed specimen for additional histopathologic examination.
Historically, in the pathology laboratory the lab technician examines and palpates the gallbladder and takes samples of abnormalities [Citation6]. By means of palpation lab technician is able to discover small tumours in the tissue with a diameter of greater than 0.5 to 1 cm. If there are no abnormalities, the lab technician randomly takes samples throughout the gallbladder and one from the section of the cystic duct. The histological assessment is done by the pathologist. The number of specimens taken randomly throughout the gallbladder varies, usually being 3 samples. The likelihood of finding a non-palpable and thus very small malignancy through randomly taken samples is low and is probably based on coincidence. It is intuitively clear that random samples through the gallbladder tissue have a limited likelihood of hitting a malignant target. Increased histopathologic sampling of gallbladder specimens results in a higher yield of precursor lesions, but does not necessarily result in a greater gallbladder malignancy detection [Citation25].
The only clinically relevant conclusion of histopathologic examination is the exclusion of malignancy, because these findings could result in a change of treatment plan. Current consensus meetings and guidelines advise to pursue an R0 resection [Citation26]. In case of a Tis or a T1a, a cholecystectomy by itself would already suffice. An additional surgical intervention of the liver and lymph nodes is deemed necessary in case of a malignancy of T1b or deeper tumour penetration. Smaller tumours (< T1b) might be missed by manual palpation, nevertheless the finding of such tumours would not change the clinical treatment plan.
Further research might yield an all-encompassing risk stratification model to identify all patients with occult gallbladder cancer. Pitt et al. found that surgeons should be suspicious for incidental GBC in case of conversion to open cholecystectomy, older or female patients, Asian or African American patients and in cases of elevated alkaline phosphate levels [Citation27]. This might be the first step towards a preoperative screening model. A multivariable analysis by Muszynska et al. produced a preoperative prediction model for incidental gallbladder cancer [Citation28]. A total of five independent predictors were identified: age, sex, previous cholecystitis, acute cholecystitis, jaundice without acute cholecystitis. This proposed prediction model will undoubtedly result in a decrease in pathologic workload. However, it remains unclear which amount of pathologic reduction will be achieved after incorporating this model in daily practice. Further studies may provide additional evidence concerning this question.
We believe that Sel-HP examination is a safe policy. After the incorporation of the surgical gallbladder examination in 2012, over 2000 cholecystectomies were performed in our institute. Sel-HP examination resulted in a reduction of roughly 60% of pathologic gallbladder assessments and still continues to decline. Since that introduction, we did not observe a drop in incidence of clinical gallbladder cancer cases. In a related study, we found that the surgeon is very well capable to identify macroscopic anomalies of the gallbladder following this macroscopic screening method [Citation5].
The current study is one of few studies to evaluate a Sel-HP examination in a clinical setting [Citation29]. Moreover, the described technique is simple and reliable. It does not require any additional materials and only needs some saline solution and a few minutes to execute. With this method we hope that surgeons feel competent to safely execute a macroscopic examination of the gallbladder. However, if there is the slightest doubt in the macroscopic analysis, we suggest further histopathologic examination. Nevertheless, the a priori chance of finding gallbladder cancer is extremely low in normal appearing gallbladders, especially in low-incidence countries [Citation30].
In conclusion, an easy and valid best practice method is described for future macroscopic analysis by the surgeon following a cholecystectomy. We encourage all incentives to contribute to a better practice and understanding of surgical macroscopic examination of the gallbladder.
| Abbreviations | ||
| Sel-HP | = | selective histopathologic |
| Rout-HP | = | routine histopathologic |
| GBC | = | Gall Bladder Cancer |
| PSC | = | Primary Sclerosing Cholangitis |
| MMC | = | Máxima Medical Centre |
| NCR | = | Netherlands Cancer Registry (NCR) |
| IKNL | = | Netherlands Comprehensive Cancer Organisation |
Supplemental Material
Download Zip (78.1 MB)Acknowledgements
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice. Furthermore the authors acknowledge the Visual Media Department at for assistance with creation of the video and figures that accompany this manuscript. Last but not least the authors extend their gratitude to all surgeons and residents in their supply of macroscopic images of the gallbladders directly following a cholecystectomy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Greenhalgh T, Howick J, Maskrey N; Evidence Based Medicine Renaissance Group. Evidence based medicine: a movement in crisis? BMJ. 2014;348:g3725.
- van Vliet JLP, van Gulik TM, Verbeek P. Is it necessary to send gallbladder specimens for routine histopathological examination after cholecystectomy? The use of macroscopic examination. Dig Surg. 2013;30:472–475.
- Deng YL, Xiong XZ, Zhou Y, et al. Selective histology of cholecystectomy specimens-is it justified? J Surg Res. 2015;193:196–201.
- Swank HA, Mulder IM, Hop WC, et al. Routine histopathology for carcinoma in cholecystectomy specimens not evidence based: a systematic review. Surg Endosc. 2013;27:4439–4448.
- Corten B, Alexander S, van Zwam PH, et al. Outcome of surgical inspection of the gallbladder in relation to final pathology. J Gastrointest Surg. 2019;23:1130–1134.
- Lester SC. Manual of surgical pathology. 2nd ed. Philadelphia (PA): Elsevier; 2006.
- Pina LN, Samoilovich F, Urrutia S, et al. Surgical considerations of the cystic duct and heister valves. Surg J. 2015;01:e23.
- Feakins R, Campbell F, Mears L, et al. Tissue pathways for gastrointestinal and pancreatobiliary pathology. London (UK): The Royal College of Pathologists; 2009.
- Allen DC, Cameron RI. Histopathology specimens: clinical, pathological and laboratory aspects. London (UK): Springer-Verlag; 2012.
- Odze RD, Goldblum J. Surgical pathology of the GI tract, liver, biliary tract and pancreas. Philadelphia (PA): Saunders Elsevier; 2009.
- Ishak KG, Goodman ZD, Stocker JT. Atlas of tumour pathology. Tumours of the liver and intrahepatic bile ducts. 3rd series, Fascicle 31. Washington: Armed Forces Institute of Pathology; 2001.
- Nagral S. Anatomy relevant to cholecystectomy. J Minim Access Surg. 2005;1:53–58.
- Golse N, Lewin M, Rode A, et al. Gallbladder adenomyomatosis: diagnosis and management. J Visc Surg. 2017;154:345–353.
- Lee KF, Wong J, Li JC, et al. Polypoid lesions of the gallbladder. Am J Surg. 2004;188:186–190.
- Cha BH, Hwang JH, Lee SH, et al. Pre-operative factors that can predict neoplastic polypoid lesions of the gallbladder. WJG. 2011;17:2216–2222.
- Zielinski MD, Atwell TD, Davis PW, et al. Comparison of surgically resected polypoid lesions of the gallbladder to their pre-operative ultrasound characteristics. J Gastrointest Surg. 2009;13:19–25.
- Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54:1842–1852.
- Tabibian JH, Ali AH, Lindor KD. Primary Sclerosing Cholangitis, Part 2: Cancer Risk, Prevention, and Surveillance. Journal of Gastroenterology and Hepatology. 2018;14:427–432.
- Yamamoto T, Uki K, Takeuchi K, et al. Early gallbladder carcinoma associated with primary sclerosing cholangitis and ulcerative colitis. J Gastroenterol. 2003;38:704–706.
- Prasad TL, Kumar A, Sikora SS, et al. Mirizzi syndrome and gallbladder cancer. J Hepatobiliary Pancreat Surg. 2006;13:323–326.
- Redaelli CA, Buchler MW, Schilling MK, et al. High coincidence of Mirizzi syndrome and gallbladder carcinoma. Surgery. 1997;121:58–63.
- Romero-González RJ, Garza-Flores A, Martínez-Pérez Maldonado L, et al. Gallbladder selection for histopathological analysis based on a simple method: a prospective comparative study. Ann R Coll Surg Engl. 2012;94:159–164.
- Corten B, Leclercq WKG, Dejong CHC, et al. Selective histological examination after cholecystectomy: an analysis of current daily practice in The Netherlands. World J Surg. 2019;43:2561–2570.
- Olthof PB, Metman MJH, de Krijger RR, et al. Routine pathology and postoperative follow-up are not cost-effective in cholecystectomy for benign gallbladder disease. World J Surg. 2018;42:3165–3170.
- Ozgur T, Toprak S, Koyuncuer A, et al. Do histopathologic findings improve by increasing the sample size in cholecystectomies? World J Surg Onc. 2013;11:245.
- Aloia TA, Járufe N, Javle M, et al. Gallbladder cancer: expert consensus statement. HPB (Oxford). 2015;17:681–690.
- Pitt SC, Jin LX, Hall BL, et al. Incidental gallbladder cancer at cholecystectomy: when should the surgeon be suspicious? Ann Surg. 2014;260:128–133.
- Muszynska C, Lundgren L, Lindell G, et al. Predictors of incidental gallbladder cancer in patients undergoing cholecystectomy for benign gallbladder disease: Results from a population-based gallstone surgery registry. Surgery. 2017;162:256–263.
- Lundgren L, Muszynska C, Ros A, et al. Are incidental gallbladder cancers missed with a selective approach of gallbladder histology at cholecystectomy? World J Surg. 2018;42:1092–1099.
- Jamal K, Ratansingham K, Siddique M, et al. Routine histological analysis of a macroscopically normal gallbladder – a review of the literature. Int J Surg. 2014;12:958–962.
