Abstract
Background: Academic detailing (AD) is a defined form of educational outreach that can be deployed to intrinsically motivate practitioners towards improving quality of care. This paper describes the design of the ADVOCATE Field Studies. This proof of concept study aims to evaluate the feasibility, acceptability and usefulness of AD, reinforced with feedback information to promote prevention-oriented, patient-centred and evidence-based oral healthcare delivery by general dental practitioners (GDPs).
Methods: Six groups of GDPs will be recruited; two groups of six to eight GDPs in each of three countries – the Netherlands, Germany and Denmark. GDPs will meet for four Academic Detailing Group (ADG) meetings for open discussions using comparative feedback data to stimulate debate about their dental practice performance and care delivery. Group meetings will be moderated using the AD methodology. Qualitative data will be collected through focus group interviews, an online discussion forum, field notes and debriefs of ADG meetings and analysed by conventional content analysis using MaxQDA software.
Discussion: The results of the study will provide novel information on the feasibility, perceived acceptability and usefulness of AD and feedback data for GDPs to improve oral healthcare delivery.
Background
Historically, the delivery of oral care has focussed largely on the treatment of acute diseases and malformations of the teeth, gums and oral cavity [Citation1]. Whilst excellent restorative outcomes remain a cornerstone of professional dental practice, new quality of care challenges has emerged. First of all, evaluation of effectiveness has fallen short with rapid developments in restorative care and in prevention and screening of future diseases. Hence, evidence for the comparative effectiveness of many contemporary preventive and restorative interventions is limited [Citation2–4]. Second, meeting the needs and expectations of patients on their oral healthcare have become key features of care quality [Citation1]. Communication and empathy, easy access to care and participation in decision making, including self-care support, influence how patients perceive care quality [Citation5]. Nowadays, whilst several legitimate but preference sensitive options exist for the management of most oral conditions, dental restorative and preventive care has a discretionary or elective nature [Citation6]. Therefore, adequately informing patients whilst taking their personal values and preferences into account, and conversations based on trust and partnership between patients and healthcare professionals are cornerstones for informed choices and appropriate decision making [Citation7].
Variation exists in oral healthcare delivery amongst general dental practitioners (GDPs) [Citation8–11]. There are two important reasons underlying such variation. First, because of the low level of familiarity with evidence based practice amongst GDPs [Citation4,Citation12–15], decision making on care delivery is largely dominated by personal experience, anecdote, theory, opinion or untested hypothesis that might or might not prove true when tested or scrutinized. Second, there is asymmetry of information between patients and healthcare professionals, and many patients delegate decision-making to healthcare professionals [Citation16]. Patients assume that the professional will accurately understand their values and preferences, and rely on an assumption that they will recommend the appropriate treatment for them. Yet, studies in medicine show that when patients are fully informed about their options, they often make very different choices than their physicians [Citation6].
Reducing unwarranted variation in preference-sensitive care for discretionary treatments requires a better evidence base, a better understanding of the current evidence base, transparency of care delivery and changes in the way conversations with patients are undertaken and decisions are reached. Academic detailing (AD) is an approach which has the potential to achieve these aims [Citation17]. As originally described, AD is a university or non-commercial-based educational outreach which involves face-to-face education of healthcare practitioners by other trained healthcare professionals, often peers. It can be used to improve the dissemination and uptake of evidence-based practice, the aim being improved patient care, reduced unwarranted variation and possibly reduced healthcare costs [Citation18,Citation19].
AD traditionally has three components: (i) the evidence for one or more clinical interventions is independently collated, (ii) the results of that evidence review are attractively summarized and packaged and (iii) clinicians are trained to serve as academic detailers. Earlier research using AD shows that by comparing a clinicians own data with national or local data, the performance is likely to improve [Citation20]. It is the collaborative and supportive nature of communication in AD that creates a relationship of trust between the detailer and the clinician. Creating a safe place to discuss variation in care and demonstrate the need for improvement. Experienced practitioners with excellent interpersonal skills are recruited as detailers. Training consists of ensuring the detailers have a solid grasp of the clinical issues and the foundations of clinical decision making. This is followed by establishing proficiency in conducting an interactive educational exchange that explores the knowledge, attitudes and skills which underpin clinical practice [Citation21,Citation22].
Educational outreach, including AD, alone or when combined with other multi-faceted approaches, including the provision of performance feedback, can be effective in improving practice [Citation23]. Simpler approaches, such as the sole provision of feedback reports and prompts in electronic health records are appealing because they require comparably little effort and resource use. However, they may not convey the depth of the information that is required to change practice [Citation24]. Yet, feedback data should not be used to form normative assessments of competence [Citation25]. AD therefore offers practitioners a methodology which enables discussion and reflection on their current practice compared with evidence-based practice or peers, potentially stimulating the intrinsic motivation to change approaches to care delivery. AD has the potential to play an important role in reducing unwarranted variation in oral health and oral healthcare. This approach has been shown effective in medicine [Citation8]. However, it has not been widely used in dentistry.
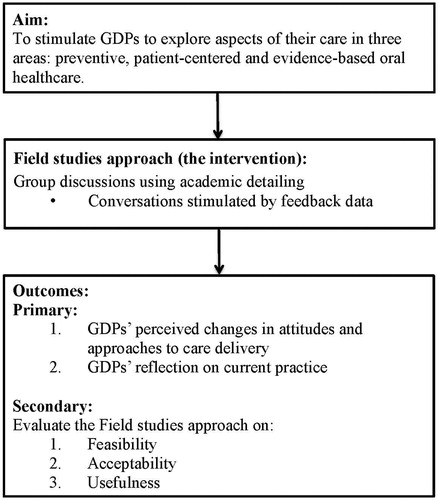
In May 2015, the ADVOCATE project (‘Added Value for Oral Care’) was launched, funded by the EU Commission’s Horizon 2020 programme [Citation26]. The project builds upon identifying strategies that appeal to the extrinsic and intrinsic motivation of GDPs to adopt a more preventive oral healthcare approach. The ADVOCATE Field Studies is a proof of concept study which is part of the ADVOCATE project. The aim of the Field Studies is to stimulate GDPs to explore aspects of their care in three areas: prevention-oriented, patient-centred and evidence-based oral healthcare. The Field Studies approach (the intervention) consists of group discussions amongst GDPs by using AD. The group discussions are supported by structured feedback data which is provided to GDPs [Citation27]. The outcomes of the intervention are GDPs’ perceived changes in attitudes and approaches in care delivery, as they reflect on their current practice in group discussions. The Field Studies approach will be evaluated for feasibility, acceptability and usefulness (see ). The Field Studies is the first attempt to provide evidence on the applicability of this approach in dental care. The hypothesis is that increasing awareness of variation in dental practice amongst GDPs provides a basis to stimulate debate on quality of care and consensus on best practice. This paper sets out the design of the ADVOCATE Field Studies.
Methods
The ADVOCATE Field Studies uses AD, reinforced with feedback information, in the setting of groups of GDPs to improve prevention-oriented, patient-centred, and evidence-based oral healthcare delivery. The feedback data consist of both patient-reported data and claims data. Using mainly qualitative methods, the Field Studies will evaluate the feasibility, GDPs’ perceived acceptability and usefulness of AD with data feedback. Local groups of GDPs will be brought together for open discussions, using feedback information to stimulate debate about their dental practice performance and care delivery (further referred to as ‘Academic Detailing Groups (ADGs)).
Study population
The Field Studies will be conducted in The Netherlands, Germany and Denmark. In each country, there will be two academic detailers recruited, each with their own ADG. Every ADG will consist of six to eight GDPs who will be recruited by the academic detailer.
As the Field Studies concerns a proof of concept study, no a priori hypotheses were put forward on the required sample size. Descriptive qualitative analyses will be used in this study to explore the feasibility, acceptability and usefulness of the tested approach; therefore, no power calculations were performed.
Academic detailers: Stewards
In the Field Studies, the term for the academic detailer will be ‘Steward’. Stewards will be GDPs with some current or prior experience of education with undergraduate or postgraduate dentists, and with good interpersonal skills and expertise in evidence based practice. Dental hygienists and specialized dental professionals will not be recruited as Stewards. Specialized dental professional are dentists with a postgraduate residency training (e.g. periodontology, endodontology, pedodontology). Stewards will be recruited from the network of the ADVOCATE research team, by pro-actively searching within the researchers’ extended networks for colleagues who meet the inclusion criteria and who are enthusiastic about participation. Individuals expressing an interest in becoming an ADVOCATE Steward will be further informed about the project through a face-to-face meeting with a research team member and a written information letter. The Steward’s role will be to recruit six to eight GDPs for their ADG, to support GDPs with the collection of feedback data in their dental practice, to organize and moderate group discussions using the AD methodology, and to support data collection for the evaluation of the Field Studies. Stewards will receive training and support from the ADVOCATE research team. In the context of this study, Stewards will be offered an honorarium (around 3000 Euros) and reimbursement of travel expenses for their role over 19 months.
General dental practitioners
GDPs will be eligible to participate in the Field Studies if they are working in dental practice at the time of the study. Dental hygienists and specialized dental professionals will not be recruited for participation in the ADGs. GDPs will be recruited from the networks of Stewards. For recruitment, the Stewards will provide the GDPs with oral and written information about the Field Studies and the ADVOCATE project. Participation for GDPs will entail supporting the collection of feedback data in the dental practice, and attendance at ADG meetings. In the context of this study, GDPs will not receive an honorarium for their participation, but travel expenses can be reimbursed.
Intervention
Academic detailing – Stewards will be trained as academic detailers according to the principles defined by Soumerai and Avorn [Citation19]. This will equip Stewards with an understanding of how to interpret the feedback data which describes GDPs’ dental care delivery and patient outcomes, and with skills to conduct interactive group discussions with GDPs in a reflective manner, focused on the processes of clinical decision making in oral healthcare.
During an ADG meeting, the Steward will begin by highlighting the importance of a safe environment where GDPs can have open, non-judgmental and confidential discussions. Based on the original principals of AD, the important educational techniques include (i) establishing baseline knowledge and motivations for current clinical practice, (ii) discussing underlying evidence and presenting both sides of controversial issues, (iii) stimulating active participation in group discussions, (iv) using concise graphical materials, (v) identifying action points for improvement of clinical practice and (vi) providing positive reinforcement of improved practices in follow-up visits [Citation19].
Feedback information
Feedback information will be used to stimulate conversations amongst participating GDPs. The feedback information will comprise routinely collected oral healthcare data on GDPs’ dental practice performance and patient oral health outcomes, comparative to their peers. Data sources for feedback information will be administrative health insurance (claims) data retrieved from health insurers in each participating country, and patient self-reported data collected through an online questionnaire application administered in the dental practice. The acquisition of claims data as part of the overall ADVOCATE project has been described elsewhere [Citation28,Citation29]. An earlier study, using an extensive four-stage approach, which has been conducted as part of the ADVOCATE project, has defined measures that are considered relevant, important and useful for feedback information by oral healthcare stakeholders [Citation30] (Supplementary Appendix 1). These measures will be used to compromise the patient self-reported online questionnaire.
The online questionnaire application will be used to collect feedback data directly from patients in the dental practices of the participating GDPs prior to the ADG meetings. The questionnaire was originally developed in English (Supplementary Appendix 2) and has been forward and back translated to Dutch, German and Danish. When patients arrive at the dental practice they will receive a flyer to inform them about the study. After their dental appointment, patients will be requested to provide informed consent and to complete the online patient feedback questionnaire on a tablet device provided at the dental practice. Patients may of course decline to participate, and the patient questionnaire is fully anonymous and contains no patient-identifiable data.
Dashboard
Aggregated summaries of feedback data will be presented in an electronic dashboard to members of the ADGs prior to meetings, and will be used to facilitate discussions. The dashboard provides a secure environment for storage of data and allows a variety of possibilities to visualize the feedback data. It facilitates comparison of data between GDPs within the same ADG, between ADGs, and across countries. According to the preferences of the GDPs, the data can be visualized in graphs, tables or text (Supplementary Appendix 3). All GDP comparison data will be presented anonymously as the default for the dashboard. However, GDPs within an ADG can disclose their identities by mutual consent if that is their preference.
Training of Stewards
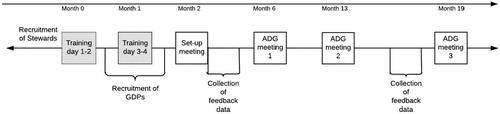
The Stewards will undertake two training sessions, each consisting of two days, prior to the first ADG meeting. The training will include interactive lectures on basic educational theory and practice, group dynamics, feedback techniques, consultation skills, decision making in clinical practice, evidence-based practice and performance measures. Mock meetings will be conducted to practice moderating and AD skills using role-play. The principles of evidence-based practice and how to find it form part of the training of the Stewards. They will use that evidence in their discussions with the GDPs, utilizing not only their knowledge of the evidence but also the collective wisdom of the group in discussion. In addition, the Stewards will become familiar with the dashboard, the patient questionnaire application and the organizational aspects of running ADG-meetings. The training will be led by an expert in AD (NM) and two researchers from ACTA (FB and DD). Materials discussed and used during the training is available on http://oralhealthfieldstudies.com; the website will function as a repository for the Stewards.
ADG meetings
The six ADGs in the Field Studies will each organize four group meetings led by a Steward – a set-up meeting, and three ADG meetings to reflect on feedback data and discuss clinical practice. The set-up meeting will introduce GDPs within one ADG to the project and to each other, and will provide the GDPs with information and materials needed to collect patient-derived feedback data in their dental practice. This includes issues of information provision to patients and consent, and practical issues involving the tablet device which will be used for the online patient feedback questionnaire, together with login instructions to access the online questionnaire and dashboard. After the set-up meeting, GDPs will start collecting patient-derived feedback data with the online questionnaire for a period of two months.
ADG Meeting 1 will be scheduled three months after the set-up meeting, and will use aggregated patient-derived feedback data collected in the preceding months. Stewards will make an initial selection of measures for discussion based on the discriminative value of the collected data, modified by discussion within the ADG. During preparatory meetings with the research team, the initial selection of measures will be discussed with the Stewards. Furthermore, Stewards will facilitate the identification of action points for improvement of dental practice for individual GDPs, and these action points will be revisited in subsequent meetings.
ADG Meeting 2 will be scheduled three months after ADG Meeting 1. Discussion on care delivery will be continued using patient-derived feedback data supplemented with claims data. It is expected that discussions will cover any changes in thinking about and/or actual care delivery since Meeting 1 and revisiting action points set up in Meeting 1. New action points could be devised, and feedback on the utility of claims data compared with patient-derived feedback data will be obtained.
ADG Meeting 3 will be held six months after Meeting 2. Prior to ADG Meeting 3, there will be a second data collection period of two months using the patient questionnaire application. During this meeting, GDPs and the Steward will discuss the new data and possibly any changes in feedback data that have occurred during the course of the Field Studies. Any action points from previous meetings will be discussed and reflected on.
Timeline
The full timeline of the Field Studies, including the two training sessions and four ADG-meetings with intermittent periods of data collection runs for 19 months; see .
Support of Stewards
Throughout the Field Studies, the Stewards will receive ongoing support from the research team. For this, a learning community involving the Stewards will be established. This learning community will consist of online resources, including the website http://oralhealthfieldstudies.com and an email discussion group. Prior to each ADG meeting, the Stewards will have a preparatory telephone meeting with the research team to discuss feedback data in the dashboard, and to discuss the selection of measures and clinical topics, and the educational plan for each meeting. The research team will also provide a telephone debriefing with Steward after each meeting to facilitate reflection and future planning.
Outcomes of the study
The Field Studies will evaluate the Field Studies approach. The primary outcomes for the impact of this approach on intrinsic motivation of GDPs are GDPs’ perceived changes in attitudes or approaches to care delivery. Secondary outcomes concern the feasibility, acceptability and usefulness of this approach, and will be derived from evaluating experiences and satisfaction of GDPs and ADs with the organizational aspects of the ADGs, the process of AD, and experiences by the patients with the app, the patient questionnaire application and the dashboard (see ).
Table 1. Overview of data collection.
Data collection
The main source of data used to evaluate this proof of concept study will be focus group interviews held with GDPs in ADG Meeting 3, at the end of the study. These data will be supplemented with additional data collected from evaluation forms after each ADG meeting, field notes (including the discussion forum and teleconferences) and debriefing interviews with Stewards. In addition, descriptive data will be collected throughout the study period.
At the end of ADG Meeting 3, a focus group interview will be conducted with GDPs within each ADG, using a semi-structured interview-guide containing open-ended questions and probes (Supplementary Appendix 4a). The focus group interview will cover the following topics: experiences with and perceived usefulness of the AD approach and the feedback data, actual changes made to clinical practice, or precursors of change such as reported changes in attitudes or approaches to care delivery. The focus group interviews will be undertaken for every ADG by trained external researchers from the three participating countries. A detailed protocol for running the focus group interviews will be provided to focus group moderators in order to standardize focus group moderation in the different countries. Focus group interviews will take place in the local language and will be transcribed and translated prior to analysis. In addition, GDPs will be asked to complete an evaluation form after each ADG meeting about their perceived usefulness of the dashboard, their satisfaction with the content and organizational aspects of the meeting, their views on the relevance of the meeting for their work and the action points they specified for improvement (Supplementary Appendix 4b).
Throughout the study, the research team will be in regular contact with the Stewards via telephone conferences and the online discussion forum to collect contemporary information on Stewards’ perceived experiences, challenges and problems with recruiting GDPs and preparing, organizing and moderating ADG meetings. Stewards will be asked if they received feedback from GDPs about their experiences and obstacles with the collection of feedback information in dental practice and the use of the dashboard. Detailed contemporaneous notes of all these discussions will be collected as field notes. Field notes include data from the email discussion forum, notes from the teleconferences between the research team and the Stewards and brief structured notes taken by the Steward during or just after the ADG meetings (Supplementary Appendix 4c). Furthermore, after each ADG meeting a debriefing telephone interview with the Stewards and the research team will be conducted using a structured questionnaire (Supplementary Appendix 4d). This questionnaire will be used to collect descriptive information on the ADG meetings including the number of GDPs attending, the duration of the meeting, which measures were prior selected and which were actually discussed during the meeting.
Descriptive background information
At the set-up meeting, GDPs will complete a questionnaire to obtain demographic background information, such as age, gender, single or group practice, year of graduation and any current areas of specialization (Supplementary Appendix 4e).
Data analysis
For a proof of concept study, a descriptive approach to analysing collected data is considered appropriate. Qualitative data collected will be processed using the ‘Maximised Qualitative Data Analysis program’ (Verbi-software MAXQDA 10). Conventional content analysis will be used to analyse the data for both primary and secondary outcomes, derived from the focus groups. Focus groups will include questions to assess whether and which changes in GDPs attitudes and approaches to care have occurred. Also questions on the usefulness and the experiences on all aspects of the Field Study approach will be discussed. First, focus groups transcripts will be read repeatedly, so the researcher is familiar with the breadth and depth of the data. Then, data will be read word by word to derive open codes, which will then be labelled to obtain the initial coding scheme. A coding guideline will be developed that will define the categories, identify the coding rules and will provide anchors. The categories will be derived from the relationship between the different codes. Preconceived categories or theories will be avoided when analysing the data; instead categories will flow from the data [Citation31]. Then, to conceptually extend, confirm or potentially refute the framework derived from the focus group, content analysis will be used as a basis for a directed content analysis approach of the additional data from the evaluation forms, field notes and debriefings. Data from these additional data sources will be coded using the predetermined codes from the focus group analysis. Any remaining text that cannot be categorized according to the initial coding scheme will be given a new code, and incorporated into the revised framework. In addition, quantitative data from the evaluation forms, e.g. questions with Likert-scale responses, will be analysed using descriptive analysis.
Ethics and data protection
The VU medical ethical committee in the Netherlands, the Heidelberg ethics committee in Germany and the Copenhagen Videnskabsetiske Komiteer and the data protection agency in Denmark have provided ethical approval for this study. Written informed consent will be obtained from the Stewards and GDPs prior to their participation in the ADVOCATE Field Studies. Consent for participation by patients will be obtained for all patients within the patient questionnaire application. To ensure relationships between the project and the Stewards are transparent, a formal contract will be established, including a Memorandum of Understanding between the Steward’s employer, the Steward and the research team, together with a code of conduct for the Stewards.
Discussion
Academic detailing with feedback data is a well-established technique to change clinical practice [Citation23]. However, it has not been widely used in dentistry. This proof of concept study will be the first to explore its use, particularly in relation to patient-centred, evidence-based and preventative oral healthcare.
The ADVOCATE Field Studies are health services research. The intervention is complex rather than simple; it consists of a number of components including the novel implementation of an application to collect feedback data from patients, challenges in the acquisition and use of the routinely available claims data and the inherent complexity of an educational intervention (AD). These components are interdependent and will be introduced as a package into a real world clinical environment. In contrast to randomized controlled clinical trials, subjects and the environment in which they function cannot be controlled in this type of study. It is therefore anticipated that challenges and learning will occur during the course of the study. The design of the study, data collection and data analysis reflect the need for a flexible approach in this type of research. Such adaptations will in themselves form part of the important findings of this study.
This is a short-term study with a limited number of participants. It is well established that significant changes in clinical behaviour of practitioners usually takes longer than the period of this study to embed. Previous research indicates that reinforcement of AD aids memory retention and promotes behavioural change [Citation32]. Reinforcement of messages about care delivery is necessary to initiate sustainable changes in behaviour and further diffuse the approach within their social system. Since the ADVOCATE Field Studies is a proof of concept pilot study, long-term impact evaluation of the approach on actual clinical practice is beyond its scope. Rather, this study is a necessary precursor to an impact evaluation. It is first necessary to establish the feasibility, perceived acceptability and usefulness of the approach, including each individual component, on a small scale. If appropriate and after potential adaptation, a follow-up study including more countries and more ADG groups will study the scalability and replicability of the approach.
Convenience sampling will be used to recruit GDPs and Stewards; achieving a representative sample is not the goal of this study. It is likely that there will be different levels of engagement with the different components of the interventions, for example: some GDPs are likely to be more enthusiastic advocates of collecting feedback data from patients than others and being open for changes in care delivery. However, it is recognized that the participants recruited are more likely to be early adopters of change [Citation33]. This is not a limitation of the study; rather the approach provides an appropriate sample for a proof of concept study. Similarly, the patient-derived data are also not intended to be representative of an individual clinical dental practice. The availability of the feedback data is to stimulate discussion about clinical practice, and not to develop a normative and judgmental approach [Citation34]. In addition, perceived changes in attitudes and behaviours of GDPs will be qualitatively measured using focus groups, which creates a risk of socially desirable answers. However, this will be mitigated by having separate focus groups in different countries, independently facilitated.
The results of the study will provide new and crucial information on the feasibility of delivering AD to GDPs. This study may demonstrate an effective approach to stimulate the intrinsic motivation of GDPs towards patient-centred, evidence-based and prevention-oriented care; thereby, progressing towards the needs and expectations of twenty-first century oral healthcare.
Appendix__design_paper_met_aanpassingen_versie_2.docx
Download MS Word (331.6 KB)Acknowledgements
We would like to thank the contributors to the ADVOCATE project: the ADVOCATE Scientific Advisory Board – Stephen Birch, Martin Chalkley, Roger Ellwood, Ekatarina Fabrikant, Jeffery Fellows, Christopher Fox, Frank Fox, Dympna Kavanagh, John Lavis, Roger Matthews, Mariano Sanz, Paula Vassalo and Sandra White; the ADVOCATE General Assembly – Barry Egberts, Lisa Bøge Christensen, Gail Douglas, Kenneth Eaton, Gerard Gavin, Geert van der Heijden, Jochem Walker, Stefan Listl, Gabor Nagy, Karen O’Hanlon, Andrew Taylor, Helen Whelton, Noel Woods; the ADVOCATE Ethics Advisory Board – Mary Donnelly, Eckert Feifel, Jon Fistein, Evert-Ben van Veen and Agnes Zana; the ADVOCATE project coordinator, Anita Blakeston; and the coworkers of the ADVOCATE project. We specially thank Kasper Rosing and Olivier Kalmus in liaising with the local ethics committee in Germany and Denmark.
Ethics approval: This study was approved by the VU medical ethical committee in the Netherlands, the Heidelberg ethics committee in Germany and the Copenhagen Videnskabsetiske Komiteer in Denmark and the Danish data protection agency.
Availability of data and materials: Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Disclosure statement
The authors declare they have no potential conflicts of interest.
Additional information
Funding
References
- Baâdoudi F, Maskrey N, Listl S, et al. Improving oral healthcare: towards measurement? Br Dent J. 2016;221:3–4.
- Yusof ZYM, Han LJ, San PP, et al. Evidence-based practice among a group of Malaysian dental practitioners. J Dent Educ. 2008;72:1333–1342.
- Straub-Morarend CL, Marshall TA, Holmes DC, et al. Toward defining dentists' evidence-based practice: influence of decade of dental school graduation and scope of practice on implementation and perceived obstacles. J Dent Educ. 2013;77:137–145.
- Iqbal A, Glenny A-M. General dental practitioners' knowledge of and attitudes towards evidence based practice. Br Dent J. 2002;193:587–591.
- Silva DDd, Bamber J. Improving quality in locum general practice. BMJ. 2014;317:7150:2a–2a.
- Atlas D. Preference-sensitive care. Dartmouth Atlas. Center for the Evaluative Clinical Sciences; 2007. p. 603.
- Maskrey N, Gordon A. Shared understanding with patients. JAMA Intern Med. 2017;177:1247.
- Hummel R, Bruers J, van der Galiën O, et al. Outcome measures for oral health based on clinical assessments and claims data: feasibility evaluation in practice. BMC Oral Health. 2017;17:1–18.
- Gezondheidsraad. De mondzorg van morgen. Den Haag. de Gezondheidsraad; 2012. Available from: www.gr.nl.
- Rosing K, Hede B, Christensen LB. A register-based study of variations in services received among dental care attenders. Acta Odontol Scand. 2016;74:14–35.
- Eaton KA, Ramsdale M, Leggett H, et al. Variations in the provision and cost of oral healthcare in 11 European countries: a case study. Int Dent J. 2018. DOI:10.1111/idj.12437
- Madhavji A, Araujo EA, Kim KB, et al. Attitudes, awareness, and barriers toward evidence-based practice in orthodontics. Am J Orthod Dentofac Orthop. 2011;140:309–316.e2.
- McColl A, Smith H, White P, et al. General practitioner's perceptions of the route to evidence based medicine: a questionnaire survey. BMJ. 1998;316:361–365.
- Solomons NM, Spross JA. Evidence-based practice barriers and facilitators from a continuous quality improvement perspective: an integrative review. J Nurs Manag. 2011;19:109–120.
- Rousseau DM, Gunia BC. Evidence-based practice: the psychology of EBP implementation. Annu Rev Psychol. 2016;67:667–692.
- Birch S, Listl S. The economics of oral health and health care. SSRN Electron J. 2015. DOI:10.2139/ssrn.2611060.
- Kisuule F, Wright S, Barreto J, et al. Improving antibiotic utilization among hospitalists: a pilot academic detailing project with a public health approach. J Hosp Med. 2008;3:64–70.
- Oxman AD, Thomson MA, Davis DA, et al. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ. 1995;153:1423–1431.
- Soumerai SB, Avorn J. Principles of educational outreach ('academic detailing') to improve clinical decision making. JAMA. 1990;263:549–556.
- Patel BB. Back to school: quality improvement through academic detailing. Am Heal Drug Benefits. 2011;4:455–459.
- Fischer MA, Avorn J. Academic detailing can play a key role in assessing and implementing comparative effectiveness research findings. Health Aff. 2012;31:2206–2212.
- Avorn J. Academic detailing: “marketing” the best evidence to clinicians. JAMA. 2017;317:361.
- O’Brien MA, Rogers S, Jamtvedt G, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane database systematic review. 2007;(4):CD000409.
- Fischer MA. Academic detailing in diabetes: using outreach education to improve the quality of care. Curr Diab Rep. 2016;16:98.
- Powell A, Davies H, Thomson R. Using routine comparative data to assess the quality of health care: understanding and avoiding common pitfalls. Qual Saf Heal Care. 2003;12:122–129.
- Leggett H, Duijster D, Douglas GVA, et al. Toward more patient-centered and prevention-oriented oral health care: the ADVOCATE project. JDR Clin Transl Res. 2017;2:5–9.
- DePasque S, Tricomi E. Effects of intrinsic motivation on feedback processing during learning. Neuroimage. 2015;119:175–186.
- Haux C, Rosing K, Kalmus O, et al. Acquiring routinely collected claims data from multiple European health insurances for dental research: Lessons learned. 62. Jahrestagung der Deutschen Gesellschaft für Medizinische Informatik, Biometrie und Epidemiologie eV (GMDS) Oldenburg, 17–21 September 2017. DOI:10.3205/17gmds167
- Gabel F, O'Hanlon K, Brankin P, et al. Linkage of health care claims data and apps data: the ADVOCATE oral health care dashboard. Int J Popul Data Sci. 2017. DOI:10.23889/ijpds.v1i1.251
- Baâdoudi F, Trescher A, Duijster D, et al. A consensus-based set of measures for oral health care. J Dent Res. 2017;96:881–887.
- Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–1288.
- Simon SR, Majumdar SR, Prosser LA, et al. Group versus individual academic detailing to improve the use of antihypertensive medications in primary care: a cluster-randomized controlled trial. Am J Med. 2005;118:521–528.
- Rogers EM. Diffusion of innovations. New York: Free Press; 1995.
- Berwick D. Era 3 for medicine and health care. JAMA. 2016;315:1–2.